Abstract
Purpose
An ex vivo intact toe model was developed to assess two different applicator designs for iontophoretic delivery of terbinafine into the nail only or the nail and surrounding skin.
Methods
Iontophoretic permeation studies were carried out on intact cadaver toes using nail-only and nail/skin applicators with a current dose of 10 mA*min (0.5 mA for 20 min).
Results
Iontophoresis enhanced drug permeation and tissue loading with both applicators tested. Greater drug delivery was observed with the nail/skin applicator due to the additional terbinafine being delivered directly through the lower impedance skin area surrounding the nail. The concentration of drug loaded into the contact area of the nail with the nail-only and nail/skin applicator was ~13 and ~7 fold higher than their respective passive delivery levels but equivalent from each other in total drug mass delivered over the whole nail plate. In vitro release of drug from the iontophoretically loaded nails into agar suggests that a single treatment could have a prolonged effect (>50 days).
Conclusions
This study demonstrates that the ex vivo toe model was useful in assessing the functionality of the different applicator designs. These results suggest that iontophoresis can significantly enhance the delivery of drugs to both the hard and soft tissues of the toe for the treatment of onychomycosis and other nail disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Existing treatment modalities for onychomycosis and recalcitrant dermatophytosis include oral and topical therapies, which are used alone or in combination (1,2). The main limitations in oral therapy include long treatment period (typically months) because of the low drug bioavailability due to low perfusion of the nail apparatus. Moreover, oral therapy can also lead to potential gastrointestinal side effects, drug-drug interactions and systemic toxicity. The clinical and mycological cure rate with newer oral antifungal agents is only about 70% at best, and treatment failure and recurrence is frequent (3–5). However, topical treatment of nail infections has several advantages over oral therapy, including greater proximity to the infected site, better patient compliance and minimal systemic adverse effects (6,7). However, the existing topical formulations are unable to deliver the required therapeutic amount of drug to the deeper infected site because of the hard and impermeable keratinized nail plate (8). There has been intensive effort to increase drug penetration across the nail through the use of chemical enhancers, but that has resulted in limited success (9–14). Normal human nail plates or nail clippings are most commonly used in the trans-ungual permeation studies. Reports in the literature suggest that the nail permeability of diseased nail and the healthy nails are comparable (15). The concept of trans-ungual drug delivery using iontophoresis to deliver a potent antifungal agent, terbinafine, across the hard keratinized nail plate at levels exceeding the effective therapeutic concentration was reported recently (16,17). The in vitro experiments carried out in Franz diffusion cells (FDC) demonstrated the ability of iontophoresis to rapidly deliver therapeutic levels of drug into and through the human nail plate. Given its ability to physically drive the drug molecules across the hard keratinized nail plate, iontophoresis appears to be a promising technique that could be developed as a potential method of treatment for onychomycosis and other nail diseases.
In order to transfer the trans-ungual iontophoretic drug delivery technology to clinical use, a suitable applicator system must be developed. The applicator must be able to hold the formulation, conform to the topography of the hard and soft tissues which make up the nail apparatus and concurrently deliver the drug from the reservoir upon application of electric current. The differences in size and shape of the nail plates among fingers and toes, including the disfigurement of the nail that can occur in more severe disease, make the applicator design a significant challenge. Unfortunately, there is a lack of suitable in vitro, ex vivo or in vivo models to evaluate and assess the utility of such applicators. To address this issue, we developed a novel, physiologically relevant ex vivo model using intact cadaver toes which has the potential to mimic the in vivo condition and allow pre-clinical evaluation of nail delivery. This model was used to assess two prototype applicators for the iontophoretic delivery of terbinafine for the treatment of onychomycosis: a “nail-only” applicator which delivers drug only into a part of the nail plate and a “nail/skin” applicator which delivers drug into both the whole nail plate and surrounding soft tissues.
MATERIALS
In order to maximize iontophoretic delivery, the concentration of terbinafine in the formulation was 4% w/w. Given that the drug is applied for a short duration, any irritation and/or toxicity would be minimal or nonexistent (as verified by toxicology testing and initial clinical testing; data not shown). The composition (w/w %) of the terbinafine formulation (TPI-DF-507; pH 3.2) used in the current study was terbinafine HCl (4%) [MW = 327.90 Da, aqueous solubility = 1 mg/mL], 95% ethanol (21%), polysorbate 80 (5%), glycerin (40%), hydroxy ethyl cellulose (0.3%), benzoic acid (0.2%), butylated hydroxyl toluene (0.01%), disodium EDTA (0.01%), and water (up to 100%), and was obtained from Transport Pharmaceuticals, Inc. (Framingham, MA). Fresh human cadaver healthy intact toes (only the hallux; total of 40 toes) both male and female, aged between 54–78 years, were procured from Science care (Phoenix, AZ) and were stored at −20°C and were used within a week. All of the toes used had normal clear nails and were not indicative of diseased or discolored. In all the experiments, the age and sex were matched in the control experiments.
METHODS
Analytical Method
The amount of terbinafine in the tissues was quantified by high performance liquid chromatography (HPLC) system (Waters, 1525) with an autosampler (Waters, 717 plus) consisting of a Phenomenex C18 (2) 100 R analytical column (4.6 × 150 mm, Luna, 5.0 µm) and a variable wavelength dual λ absorbance detector (Waters, 2487). Mobile phase consisted of aqueous solution (0.096 M triethyl amine, 0.183 M orthophosphoric acid) and acetonitrile (60:40) adjusted to pH 2 with orthophosphoric acid. Elution was performed isocratically at 32°C at a flow rate of 1.0 mL/min. Injection volume was 20 µL and the column effluent was monitored at 224 nm. The method was validated by determination of linearity, precision, and accuracy. The range for the calibration curve was 2–1000 ng/mL (R2 = 0.98). The coefficient of variation and the relative error ranged 0.75–6.74% and −0.37–−7.68%, respectively.
Applicators for Terbinafine Delivery
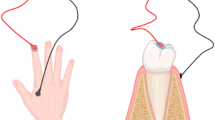
Two newly designed prototype applicators (i.e., ‘nail-only applicator’ and ‘nail/skin applicator’) are composed of an adhesive backing with a snap joint to which polyurethane foam with an active electrode is adhered. The 1 mm thickness polyurethane foam (Rynel, Wiscasset, ME) acts as a reservoir capable of being loaded with the liquid gel formulation. The electrical circuit is completed by placing a counter electrode on the bottom of the toe. The nail-only applicator was intended to deliver the drug into a selected area of the nail (no drug delivery into the skin), while the nail/skin applicator covers the whole nail plate and the adjacent skin (lateral and proximal folds). In both the applicators the reservoir area can be varied, based on the nail plate area or the size of the toe. The reservoir assembly was constructed using polyurethane foam which provides homogenous and wrinkle free contact with the tissue, which is crucial for uniform current distribution. The reservoir capacity of the applicators is 100 µL/cm2, and the drug is loaded in the whole applicator. For the nail-only applicator, the mask restricts the contact between the applicator and the nail to the opening in the mask. The mask used in the nail-only applicator was made of ~2 mm thick polyvinyl chloride foam tape (3 M, St Paul, MN). Figs. 1 and 2 show the prototype nail-only and nail/skin applicator, respectively, and their function during use in an intact toe model.
Schematic representation of the nail/skin applicator (left) and a picture demonstrating the application in the ex vivo intact toe model for ungual and trans-ungual iontophoretic delivery of terbinafine. The nail/skin applicator differs from the nail-only applicator with respect to the mask (which is not present).
Unlike the nail-only applicator, the nail/skin applicator was designed to deliver drug into the whole nail and adjacent skin. The nail/skin applicator is identical to the nail-only applicator except for the mask (associated with the nail-only applicator), which limits drug delivery to the central portion of the nail plate (approximately 40–50% of the total nail area). However, in the nail/skin applicator, the area of the whole nail plate and surrounding skin exposed to the drug reservoir remains similar (~3 cm2).
Permeation Studies
Permeation Studies Using Intact Toe
The applicator polyurethane foam was trimmed (~6.25 cm2, 2.5 × 2.5 cm, based on the nail area) and fixed to the backing layer, and the foam was loaded with 4% w/w terbinafine formulation (TPI-DF-507, 100 µL/cm2) using a micropipette to cover the entire area of the foam. The intact toe, up to the first joint, was soaked in saline for 1 h and wiped with a Kimwipe® to remove the surface water. For the nail-only applicator, the mask (exposed area ~1.5 cm2) was secured over the toe, such that the opening in the mask was centered on the nail. For both applicators, the drug loaded polyurethane foam along with the backing was draped over the nail and the surrounding tissues. The counter electrode was adhered to the bottom of the toe. A constant current (0.5 mA for 20 min) was applied using a custom made dose controller (0–1 mA, 500 V capacity; Model Labion 003; Transport Pharmaceuticals, Inc. Framingham, MA). The anode was connected to the snap joint on the applicator and the cathode to the counter electrode. Similarly, passive permeation experiments were run in parallel (0 mA for 20 min) for comparison.
After the permeation study, the toe surface was cleaned using a standardized protocol to avoid the washout of drug permeated into the skin while removing surface drug. In brief, the skin surface was scrubbed with cotton soaked with 95% alcohol and blotted using a Kimwipe®. This procedure was repeated twice again using alcohol and 5 times with water (pH adjusted to 3). The toenail was separated from the intact toe using tissue forceps and scalpel. Similarly, the skin surrounding the nail plate (~5 mm from exposed edge of nail plate; lateral nail fold, distal nail fold, proximal nail fold, nail bed, and nail under cuticle) were separated from the intact toe using scalpel and scissors. The drug which had permeated into the surrounding tissues (lateral, distal, and proximal folds) and the nail bed was then extracted using the standardized protocol and analyzed for the drug content. The schematic representation of various regions of the toe is presented in Fig. 3.
pH Measurement
The pH of the nail plate and the surrounding tissues was measured before and after the permeation experiments for both iontophoresis and passive delivery. Briefly, the skin surface was wiped using a Kimwipe®, and the pH of the skin lateral, proximal, and distal to the nail, and the counter electrode area (bottom region of the toe) was recorded using a flat surface electrode (Orion 4 Star Benchtop, Thermo Fisher Scientific Inc., Waltham, MA).
Amount of Drug in Nail
Following permeation studies the nail surface was separated from the intact toe and was cleaned using a cotton swab soaked in 95% ethanol and rinsed with 1 mL ethanol (95%) — this alcohol washing procedure was repeated twice and 3 times with water (pH adjusted to 3). The nail surface was wiped with Kimwipe®. The use of 95% alcohol ensured that all surface drug was removed. For the nail-only applicator, the nail region corresponding to the active diffusion area (nail directly exposed to the applicator) and the peripheral nail area (nail not directly exposed to the applicator) were separated using a metric punch. Similarly, in the case of the nail/skin applicator, the nail region corresponding to the active diffusion area (nail directly exposed to the applicator) and the nail under the proximal fold (nail not directly exposed to the applicator but within the path of iontophoresis) were separated. Nail sections were weighed and dissolved in 1 M sodium hydroxide (1.5 mL) by constant overnight stirring. Extraction of drug was carried out by a slight modification of the method described by Dykes et al. (18). Briefly, after dissolving the tissues in the vials, 200 µL of 5 M hydrochloric acid was added to neutralize the mixture. Terbinafine was extracted by adding hexane (3 mL) to the vial and shaking manually for 30 min. The mixtures were transferred into centrifuge tubes and centrifuged at 4000 rpm for 10 min. The hexane layer was collected, 1 mL of 0.5 M sulphuric acid- isopropyl alcohol mixture (85:15) was added, and the mixture was shaken vigorously for 30 min. The lower acidic aqueous layer, which holds the majority of the terbinafine, was collected separately, and the amount of drug in the skin was determined. This extraction procedure was validated by spiking different drug amounts (2 ng to 20 µg) into a sodium hydroxide solution in which nail was previously dissolved. The recovery was found to be 84 ± 7%, and the limit of detection was 2 ng/mL.
Extraction of Terbinafine from Skin
The skin extraction procedure was validated using tissue from the lateral and distal nail fold area of the human cadaver toe (1 sq. cm and ~2 mm thickness), which was cut and separated from the intact toe and weighed. Terbinafine solutions containing 2 ng to 20 µg of terbinafine in 20 µL of water (pH adjusted to 3) were injected into each part separately and kept aside for 1 h and then placed in screw cap pyrex vials. Sodium hydroxide (1.5 mL of 1 M) was added and the vials were incubated for 24 h in a shaker water bath at room temperature to allow the skin to dissolve. Extraction of drug was carried out in a manner similar to that described for the nail extraction method above. The recovery was found to be 79 ± 5%. This validated procedure was used for extraction and measurement of drug in the cadaver toe skin subjected to trans-ungual iontophoresis using the applicators.
Drug Release Studies
Sabouraud Dextrose Agar (SDA) plates were prepared by dissolving 6 g of SDA in 100 mL of water by gentle heating until it became a clear solution and then pouring the mixture into petri dishes (~3 mm thickness). Nails on intact toes were loaded with terbinafine by passive (20 min) and iontophoretic (0.5 mA for 20 min) delivery using nail-only and nail/skin applicators. The nail surface was cleaned by the standard washing protocol. Each drug-loaded nail plate (~5 × 5 mm segment) was placed on an SDA plate with the dorsal side facing up and allowed to release terbinafine over 4 days at ambient temperature. At the end of every 4 days, the nail samples were transferred to new SDA plates and this procedure was followed until the release reached a plateau. The amount of terbinafine released into the agar was determined by extracting the drug from the agar plate (percentage recovery: 92 ± 2%). After each study period (4 days), the agar was transferred into a centrifuge tube, 10 mL of 0.01 M HCl was added, and the mixture was kept overnight at room temperature in the dark. Samples were vortexed (4 min) and centrifuged (4000 rpm for 10 min), and the supernatant analyzed by HPLC (17).
Data Analysis
Enhancement of drug delivery was calculated as the ratio of the iontophoresis value to its corresponding control. Statistical analysis was performed by one-way analysis of variance (ANOVA) and t-test using Graphpad Prism 5 (Graphpad software, Inc., San Diego, CA) to test the effects of various treatments. P value less than 0.05 was considered statistically significant. The data points provided in the graphs are an average of six trials unless otherwise noted. The error bars represent the standard deviation (s.d.).
RESULTS AND DISCUSSION
This is the first report on the feasibility of using the intact whole toe as a novel ex vivo model for the development of new trans-ungual drug delivery systems. We believe that this ex vivo model could serve an important role as a surrogate for in vivo nail delivery studies, especially given the lack of suitable animal models. This model system was used to assess two prototype applicators for iontophoretic drug delivery, a nail-only and a nail/skin applicator, for their drug delivery characteristics. This was especially critical for the nail/skin applicator, as there are no other models available in which to evaluate the relative current flow, and therefore drug delivery, between the skin and the nail. Ideally, for the treatment of onychomycosis, drug should be delivered to the nail plate, cuticle, nail bed, and surrounding tissues to ensure that all infected tissues receive efficacious levels of the antifungal drug. In addition, delivering drug into the newly formed nail in the nail matrix would help prevent infection of the new nail as it grows out.
The applicators were designed in such a way that an application of a low electric current for a short duration (ideally ≤30 min) would be sufficient to drive efficacious amounts of drug from the reservoir of the applicator into the nail and/or the surrounding skin (Figs. 1 and 2). The different parameters measured during the studies using iontophoretic and passive deliveries are shown in Table 1. As can be seen from the table, the reservoir area, nail plate area, and applicator fill volume were kept relatively constant to eliminate any effect on drug delivery due to changes in these parameters. As mentioned previously, the drug-loaded reservoir in the nail-only applicator was not in contact with the skin, while the reservoir in the nail/skin applicator was exposed to the both skin and nail. The mask used with the nail-only applicator prevents drug delivery into the skin surrounding the nail plate.
Based on our initial in vitro permeation study using FDC (16), the protocol for iontophoresis was selected as 0.5 mA for 20 min (10 mA*min), which is generally considered as safe (19). Iontophoretic permeation studies were carried out using the terbinafine formulation (TPI-DF-507), and the amount of drug permeated through and loaded into the nail was measured. The permeation with the nail-only applicator represents the amount of drug that diffused into the nail bed, whereas with the nail/skin applicator it represents the amount of drug that diffused into the skin surrounding the nail (lateral nail folds, distal nail fold, proximal nail fold) as well as the nail bed. The schematic representation of various regions of the toe is represented in Fig. 3. Fig. 4 compares the permeation of terbinafine in the intact toe using the two applicator designs. For the nail-only applicator studies, drug permeation is expressed as the amount of terbinafine in the nail bed per weight of nail bed tissue. However, for the nail/skin applicator studies, drug permeation is expressed as the total amount of terbinafine in the nail bed and the surrounding tissues (lateral nail folds, distal nail fold, and proximal nail fold) per total weight of the tissues extracted. It is evident from these results that with the nail-only applicator iontophoresis delivered a higher amount of terbinafine (0.65 ± 0.21 µg/cm2 or 2.78 ± 1.23 µg/g) into the nail bed, which could be of great importance in onychomycosis therapy. In contrast, no drug was detected in the nail bed following passive delivery. For both passive and iontophoretic delivery using the nail-only applicator, no measurable drug diffusion into the surrounding skin area was observed, demonstrating the utility of the mask to prevent drug movement into the adjacent skin.
Amount of terbinafine permeated through the nail and skin during iontophoresis (0.5 mA) and passive studies after 20 min using the nail-only and nail/skin applicators. The permeation with the nail-only applicator indicates the amount of drug diffused into the nail bed, whereas with the nail/skin applicator it includes both the skin surrounding the nail as well as the nail bed. Each point is an average of six trials ± s.d.
Greater permeation into the surrounding skin was observed using the nail/skin applicator compared to the nail-only applicator, in both passive and iontophoretic delivery (Fig. 4). It is evident from Fig. 5 that more terbinafine permeated into the adjacent skin regions (lateral, distal, and proximal nail folds) using the nail/skin applicator. This is most likely due to the contact area with the skin, which has reduced impedance relative to the nail. From Fig. 5 it is also evident that a greater fraction of the drug that permeated through the nail and skin [9.86 ± 3.25 µg/g and 1.41 ± 0.57 µg/g (P = 0.001) with iontophoresis and passive delivery, respectively] was delivered into the surrounding skin with the nail/skin applicator, although the amount of drug transported via iontophoresis into the nail bed [2.78 ± 1.23 µg/g and 3.27 ± 1.96 µg/g (P = 0.615) for nail-only and nail/skin applicator, respectively] was comparable for both applicators. Although this similarity in nail bed permeation seems contradictory to the previous finding that permeation and current density were linearly dependent (16,17), it is difficult to apply the linear relationship observed between flux and current density in Franz cell studies to this study due to the complex nature of the toe model used. There may be other unknown variables (e.g., impact of drug delivered to the surrounding skin, static nature of the underlying nail bed that may potentially limit permeation) that may contribute to the comparable nail bed levels we observe between the two applicators. In addition, iontophoretic enhancement in drug permeation through nail and skin with the nail/skin applicator was found to be ~7 fold higher when compared to passive delivery. The voltage measured during iontophoresis was found to be much lower with the nail/skin applicator (17–32 V) as compared to the nail-only applicator (37–71 V). Moreover, the pH of the skin immediately after delivery did not vary significantly with delivery mode (iontophoresis or passive), area sampled (lateral, proximal, and distal nail folds, and the counter electrode area), or applicator used (nail-only and nail/skin), with the pH values in the range of 5.8–6.7 for all measurements collected. The overall results indicate that a short duration of iontophoresis is capable of effectively overcoming the impervious nail barrier to enhance drug permeation, which should result in a more successful treatment of onychomycosis.
Amount of terbinafine (µg/g) in different regions of the toe during iontophoresis (0.5 mA) and passive studies after 20 min using the nail/skin applicator. *Toe regions are represented in Fig. 3. Each point is an average of six trials ± s.d.
Fig. 6 represents the mean drug load detected in the active diffusion area of the nail plate (nail in contact with the drug reservoir) during iontophoresis and passive delivery using the nail-only and nail/skin applicators. The drug level achieved with a 10 mA*min electrical dose with the nail-only and nail/skin applicators was found to be ~13 and ~7 fold higher than their respective passive delivery values. The total amount of terbinafine loaded into the active diffusion area of the nail plate using iontophoresis was 138.89 ± 16.98 µg/g and 55.46 ± 14.07 µg/g for the nail-only and nail/skin applicators, respectively, while with passive delivery the values were 10.66 ± 3.54 µg/g and 7.77 ± 1.13 µg/g for the nail-only and nail/skin applicator, respectively. Although the concentration of drug loaded into the active diffusion area of the nail varied with the applicator (Fig. 6), the total mass of drug loaded into the whole nail was not statistically different (18.34 ± 3.43 µg and 13.86 ± 4.22 µg for the nail-only and nail/skin applicators, respectively; P = 0.071). The difference in the concentration of the drug loaded in the nail by the nail-only and nail/skin applicators is expected and is mainly because of the basic difference in the area (and thus mass) of the nail plate (active diffusion area) exposed to the reservoir applied (Table 1).
Separately, the amount of drug loaded into the nail area not directly in contact with the active diffusion area was also assessed. For the nail-only applicator, the peripheral nail area was collected separately from the active delivery region while nail under the proximal nail fold was collected separately for the nail/skin applicator. The nail under the proximal nail fold was not directly exposed to the nail/skin applicator but was instead under the skin that was directly exposed to the applicator. The drug loaded in the peripheral area with the nail-only applicator was 19.37 ± 8.65 µg/g and 1.64 ± 1.42 µg/g (P = 0.0006) for iontophoretic and passive delivery, respectively (Fig. 6). The diffusion of the drug into the nail adjacent to the active diffusion area is likely due to the flow of a low level of current into the peripheral nail plate. However, no drug was observed in the surrounding skin, suggesting effectiveness of the mask. Achieving peripheral area drug levels from the nail-only applicator can be considered a positive attribute of this applicator to maximize coverage in the nail plate. For the nail/skin applicator, a considerable amount of drug was delivered into the nail under the proximal fold during the short period of iontophoresis with the nail/skin applicator (33.78 ± 10.04 µg/g), although no drug was detected in that area with passive delivery (Fig. 6). To put these results in the context of clinical efficacy, the drug load in the nail plate immediately after topical iontophoresis (0.5 mA) for 20 min (assuming that the impact of clearance would be negligible in vivo if the samples were collected immediately after treatment) was compared with the drug level achieved after 28 days continuous oral Lamisil treatment. The drug load achieved in the nails by both types of applicators with a one time low coulombic dose (10 mA*min) iontophoretic delivery was found to be considerably higher (0.4 µg/g) than the oral Lamisil treatment (20) and well above the MIC of terbinafine for dermatophytes (0.003–0.006 µg/mL) (21). Achieving this level of drug delivery in the ex vivo toe model bodes well for a positive clinical outcome with this treatment approach. In addition, the extensive nail washing procedures used in this study effectively removed surface contaminated (16) drug and, if anything, may actually underestimate the amount delivered due to washing of the ventral surface of the nail which is not exposed in vivo. However, a key limitation to the ex vivo model is the lack of a functional circulatory system, so clearance of drug from the tissues is not accounted for in these studies.
As mentioned previously, the nail-only applicator was designed to deliver high amounts of drug into the nail (nail plate and bed) while the nail/skin applicator was intended to deliver drug to both the nail and nail folds (soft tissue adjacent to the nail). As expected, the total amount of drug delivered varied with the applicator design. The results show that the nail-only applicator could be utilized for drug delivery to the nail and nail bed. However, the nail/skin applicator delivered greater amounts of drug into the area surrounding the nail plate. This could be advantageous to ensure a more complete eradication of the fungal infection.
Earlier studies indicated that iontophoresis enhanced drug delivery into the nail, forming a drug depot in the nail plate (16,17). Drug release out of the nail into an agar plate substrate was assessed using both a quantitative assay (HPLC) as well as an antifungal assay (zone of inhibition study) to demonstrate sustained release of antifungal levels of drug over an extended period of time (> 50 days) (16,17). In the clinical setting, this depot could subsequently release effective levels of drug into the underlying and surrounding infected tissues over a prolonged period of time (16,17). In the current study, nails from intact toes iontophoretically loaded with terbinafine from both nail-only and nail/skin applicators also exhibited extended antifungal activity similar to a previous in vitro study (17), further demonstrating that the terbinafine released was active and sustained (data not shown). Furthermore, quantitative release studies were carried out for the ex vivo drug-loaded nails using agar plates as the drug receptor medium. The nails on intact toes were loaded with terbinafine by iontophoresis (0.5 mA for 20 min) or passive delivery (20 min) using the nail-only and nail/skin applicators. Loaded nails were then placed on agar plates and incubated at ambient temperature for 4 days. After 4 days, the nail samples which released measurable amounts of terbinafine were transferred to new agar plates and the process repeated. The drug was extracted from the agar and analyzed using HPLC.
Iontophoretically loaded nails exhibited a 2-phase release, consisting of an initial rapid release phase (up to 20 days) which was followed by a slower release phase (up to ~60 days) (Fig. 7). The amount of drug released in 20 days from nails loaded using the nail-only and the nail/skin applicator was 3.19 ± 0.52 µg and 1.10 ± 0.19 µg (P = 0.003), respectively. In contrast, the passively loaded nails released much less drug in a single slow phase, which lasted for only 12 days (0.07 ± 0.01 µg and 0.05 ± 0.02 µg in nail-only applicator and nail/skin applicator loaded nails, respectively). It is also evident from the Fig. 7 that the amount of drug released was different between the nail-only and nail/skin applicators due to the difference in the concentration (and therefore initial mass) of drug loaded (138.89 ± 16.98 µg/g and 55.46 ± 14.07 µg/g for the nail-only and nail/skin applicators, respectively) into the nails by both the applicators. The total amount of drug delivered, when averaged over the entire nail, is similar for the two applicators, but the local concentrations are different because of the much smaller contact area with the nail-only applicator. Since identical nail plate sizes were tested, the initial total drug mass was higher for the nail plate loaded with the nail-only applicator due to the higher drug concentration.
The cumulative amount of terbinafine released at the end of the release study from nails loaded by iontophoresis using the nail-only and the nail/skin applicator was found to be 4.27 ± 0.89 µg (68 days) and 1.55 ± 0.31 µg (60 days), respectively (Fig. 7). The amount of drug retained in the nail after the release study with the nail-only applicator was 7.61 ± 2.35 µg/g and 59.22 ± 16.86 µg/g, for passive and iontophoresis processes, respectively. A similar trend was also observed with the nail/skin applicator (5.45 ± 1.27 µg/g and 23.48 ± 11.02 µg/g, for the passive and iontophoresis processes, respectively). The percentage of drug released in the iontophoretic-loaded nails was found to be 60.11 ± 7.03 % and 64.10 ± 6.16 % for the nail-only and nail/skin applicator, respectively. In contrast, the amount released from the passively loaded nails was 18.65 ± 4.42 % and 24.60 ± 7.92 % for nail-only and nail/skin applicator, respectively. These low drug loading and release values for passive nails are consistent with the previous finding (17) that the drug load in the passive delivery is mainly due to the localized drug in the dorsal layer. Further, the drug in the dorsal layer does not readily diffuse out of the matrix in the experimental setup where the ventral nail is in contact with the release medium (agar). These results indicate that iontophoretic delivery of drug using the nail-only and nail/skin applicator should result in a sustained release of drug to the site of the infection and therefore reduce the frequency of the treatments.
CONCLUSION
An ex vivo intact toe model was developed and used to assess two newly designed prototype applicators for the trans-ungual iontophoretic delivery of terbinafine. Application of iontophoresis using a low-level electric current (10 mA*Min) enhanced the trans-ungual delivery of terbinafine with both of the applicators tested. The nail/skin applicator was able to deliver considerable amounts of terbinafine into the lower impedance skin surrounding the nail while still delivering significant levels of drug to the high impedance nail. Both applicator types delivered high levels of terbinafine through and into the nail, achieving levels far above the MIC of terbinafine for dermatophytes (0.003–0.006 µg/mL). The drug release study indicates that the drug depot formed in the nail due to iontophoretic delivery using the newly designed applicators will release efficacious levels of drug into the underlying tissues for an extended period of time. These results further support the use of iontophoresis to enhance local drug delivery for the treatment of onychomycosis and other nail disorders.
REFERENCES
Effendy I. Therapeutic strategies in onychomycosis. J Eur Acad Dermatol Venereol. 1995;4:S3–S10.
Pierard GE, Arrese JE, Quatresooz P, Pierard-Franchimont C. Emerging therapeutics agents for onychomycosis. Expert Opin Emerging Drugs. 2007;12:345–353.
Arrese JE, Pierard GE. Treatment failures and relapses in onychomycosis; a stubborn clinical problem. Dermatol. 2003;207:255–260.
Baran R, Kaoukhov A. Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J Eur Acad Dermatol Venerol. 2005;19:21–29.
Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31–46.
Murdan S. Drug delivery to the nail following topical application. Int J Pharm. 2002;236:1–26.
Bodman MA, Feder L, Nace AM. Topical treatments for onychomycosis. J Am Podiatr Med Assoc. 2003;93:136–141.
Hui X, Shainhouse Z, Tanojo H, Anigbogu A, Markus GE, Maibach HI, et al. Enhanced human nail drug delivery: nail inner drug content assayed by new unique method. J Pharm Sci. 2002;91:189–195.
Van Hoogdalem EJ, Van den Hoven WE, Terpstra IJ, Van Zijtveld J, Verschoor JSC, Visser JN. Nail penetration of the antifungal agent oxiconazole after repeated topical application in healthy volunteers, and the effect of acetylcysteine. Eur J Pharm Sci. 1997;5:119–127.
Kobayashi Y, Miyamoto M, Sugibayashi K, Morimoto Y. Enhancing effect of Nacetyl-l-cysteine or 2-mercaptoethanol on the in vitro permeation of 5-fluorouracil or tolnaftate through the human nail plate. Chem Pharm Bull. 1998;46:1797–1802.
Malhotra GG, Zatz JL. Investigation of nail permeation enhancement by chemical modification using water as a probe. J Pharm Sci. 2002;91:312–323.
Hui X, Chan TCK, Barbadillo S, Lee C, Maibach HI, Wester RC. Enhanced econazole penetration into human nail by 2-n-nonyl-1, 3-dioxolane. J Pharm Sci. 2003;92:142–148.
Hao J, Smith KA, Li SK. Chemical method to enhance transungual transport and iontophoresis efficiency. Int J Pharm. 2008;357:61–69.
Murdan S. Enhancing the nail permeability of topically applied drugs. Expert Opin Drug Deliv. 2008;5:1267–1282.
Kobayashi Y, Komatsu T, Sumi M, Numajiri S, Miyamoto M, Kobayashi D, et al. In vitro permeation of several drugs through the human nail plate: relationship between physicochemical properties and nail permeability of drugs. Eur J Pharm Sci. 2004;21:471–477.
Nair AB, Vaka SRK, Sammeta SM, Kim HD, Friden PM, Bireswar C, et al. Trans-ungual iontophoretic delivery of terbinafine. J Pharm Sci. 2009;98:1788–1796.
Nair AB, Kim HD, Chakraborty B, Singh J, Zaman M, Gupta A, et al. Ungual and trans-ungual iontophoretic delivery of terbinafine for the treatment of onychomycosis (In press). J Pharm Sci. 2009b.
Dykes PJ, Thomas R, Finlay AY. Determination of terbinafine in nail samples during systemic treatment for onychomycoses. Br J Dermatol. 1990;123:481–486.
Singh P, Liu P, Dinh SM. Facilitated transdermal delivery by iontophoresis. In: Bronaugh RL, Maibach HI, editors. Percutaneous Absorption. New York: Marcel Dekker; 1999. p. 633–657.
Faergemann J, Zehender H, Denouel J, Millerioux L. Levels of terbinafine in plasma, stratum corneum, dermis-epidermis (without stratum corneum), sebum, hair, and nails during and after 250 mg terbinafine orally once per day for four weeks. Acta Derm Venereol. 1993;73:305–309.
Clayton YM. Relevance of broad-spectrum and fungicidal activity of antifungals in the treatment of dermatomycoses. Br J Dermatol. 1994;130:7–8.
ACKNOWLEDGEMENT
The authors would like to thank Transport Pharmaceuticals, Inc., Framingham, MA for providing financial support for this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, A.B., Kim, H.D., Davis, S.P. et al. An Ex Vivo Toe Model Used to Assess Applicators for the Iontophoretic Ungual Delivery of Terbinafine. Pharm Res 26, 2194–2201 (2009). https://doi.org/10.1007/s11095-009-9934-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9934-y