Abstract
Purpose
To compare the short-term effect of treatment with atorvastatin and rosuvastatin on levels of serum lipids, inflammatory markers and adiponectin in patients with hypercholesterolemia.
Methods
Sixty-nine patients with hypercholesterolemia were randomly assigned to receive 10 mg/day of atorvastatin or rosuvastatin for 12 weeks. Inflammatory biomarkers, including highsensitivity C-reactive protein (hs-CRP), tumor necrosis factor (TNF)-alpha, matrix metalloproteinase-9 (MMP-9), and endothelin (ET-1), plasminogen activator inhibitor type 1 (PAI-1) and plasma tissue plasminogen activator (tPA), adiponectin, and lipid profiles were measured before and after statin therapy.
Results
Atorvastatin and rosuvastatin both lowered levels of hs-CRP, MMP-9, PAI-1, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) from baseline values, with rosuvastatin lowering TC and LDL-C to a greater extent than atorvastatin (P < 0.05). Adiponectin level increase was 15% higher than that at baseline with atorvastatin (P > 0.05) but 67% higher with rosuvastatin (P < 0.05).
Conclusions
Therapy with both statins not only significantly improved lipid profiles but also decreased levels of vascular biomarkers hs-CRP, MMP-9, and PAI-1; however, only rosuvastatin increased serum adiponectin levels significantly in patients with hypercholesterolemia, which could imply a beneficial effect in coronary artery disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Dyslipidemia, a key risk factor for cardiovascular diseases and one of the most serious threats to public health, can be accompanied by low adiponectin level and vasomotor dysfunction (1). Adiponectin plays an important role in modulating glucose and lipid metabolism, vascular biology, and energy homeostasis (2,3). In animal studies, recombinant adiponectin improved insulin sensitivity, inhibited inflammatory responses, and reversed diet-induced lipid abnormalities (4). Statins, such as atorvastatin and puvastatin, can decrease serum lipid levels and increase adiponectin levels in patients with ischemic heart disease (5,6). They can also stabilize and even retard the progression of atherosclerotic plaque (7), thereby decreasing the incidence of cardiovascular events.
Rosuvastatin, a relatively new statin, has powerful lipid-lowering effects. However, its effect on serum adiponectin levels in hypercholesterolemic patients has not been recorded. We aimed to compare the short-term effect of rosuvastatin and atorvastatin on level of serum lipids, inflammatory markers and adiponectin, as well as to explore their effect on vasomotor function in patients with hypercholesterolemia.
MATERIALS AND METHODS
Study Population and Design
The study was approved by the Ethics Committee of Shandong University Qilu Hospital. All participants gave their written, informed consent.
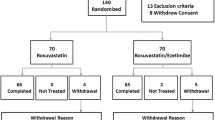
A total of 69 consecutive outpatients (36 males) with primary hypercholesterolemia [low-density lipoprotein cholesterol (LDL-C) level 130 to 250 mg/dl (3.36–6.3 mmol/L)] were recruited from the Cardiology Department of Qilu Hospital between February 2005 and June 2007. Patients were excluded if they had liver disease or transaminase levels > 1.5 times the upper normal limit at inclusion, creatine kinase (CK) level > 1.5 times the upper normal limit, atrioventricular block and sinus bradycardia, acute or chronic renal failure, electrolyte disturbances, acute cerebrovascular disease or myocardial infarction within the preceding three months, or evidence of alcohol abuse.
After a 2-week wash-out period, patients were randomly assigned to receive rosuvastatin (10 mg) (35 patients, mean age 59.2 ± 9.3 years) or atorvastatin (10 mg) (34 patients, mean age 57.7 ± 11.1 years) once daily for 12 weeks. Patients were observed at 14-day intervals or more frequently during the study. To monitor side effects, we measured levels of serum asparate aminotransferase (AST), alanine aminotransferase (ALT), CK, blood urea nitrogen, and creatinine (Cr) before and after therapy.
Laboratory Assays
Blood samples were obtained at 8:00 a.m. after an overnight fast before and after treatment. Samples were immediately coded so that investigators performing laboratory assays were blinded to subject identity or study sequence. Assays for AST, ALT, CK, Cr, triglycerides (TGs), total cholesterol (TC), LDL-C, and high-density lipoprotein cholesterol (HDL-C), apolipoprotein A (Apo A) and apolipoprotein B (Apo B) involved use of a Hitachi 7107A automatic biochemistry analyzer (Hitachi, Japan). Non-HDL-c was calculated by TC minus HDL-c.
Serum Adiponectin Level and Inflammatory Markers
Enzyme-linked immunosorbent assay (ELISA) was used to measure the following according to the manufacturer’s instructions: total circulating serum adiponectin level (BPB Biomedicals, America) measured in duplicate; endothelin 1 (ET-1) (R&D Systems Inc, America); total matrix metalloproteinase-9 (MMP-9) (active MMP-9 plus pro-MMP-9, Quantikine MMP-9 kit); plasminogen activator inhibitor type 1 (PAI-1) antigen and plasma tissue plasminogen activator (tPA) antigen (both Diagnostica Stago, France). Plasma level of high-sensitivity C-reactive protein (hs-CRP) and tumor necrosis factor (TNF)-alpha were measured by chemiluminescent immunoassay (Both Immulite DPC, America). The inter- and intra-assay coefficients of variation were <6%.
Endothelium-Dependent Vasodilatation of Brachial Artery
Before and after treatment, after a 10-min rest, patients underwent imaging studies of the right brachial artery with use of a Sonos 7500 ultrasound scanner (Philips; The Netherlands) equipped with a 4–10-MHz linear-array transducer as described (8). Briefly, the artery proximal to the antecubital fossa was imaged longitudinally, with the center of the artery identified by the clearest visualization of the anterior and posterior intimal layers. After baseline measurements of the diameter in the brachial artery, a blood-pressure cuff placed around the forearm was inflated with a pressure of 50 mmHg higher than systolic pressure (at least 240 mmHg) for 5 min, then the cuff was deflated. The diameter was measured again in 15–90 s after cuff deflation. All images were coded and recorded on VHS videotape for subsequent analysis; from tape images, brachial arterial diameters were measured with use of ultrasonic calipers by two observers who were blinded to the protocols of the study and treatment groups. Measurements were taken from the anterior to the posterior interface between the media and adventitia at end diastole, coincident with the R wave, on a continuously recorded electrocardiogram. Diameters at four cardiac cycles for each scan were analyzed, and the measurements were averaged. The response of the vessel diameter to reactive hyperemia was expressed as percent change relative to that just before cuff inflation.
Statistical Analysis
Data for continuous variables are expressed as mean ± SEM; paired and unpaired Student’s t test was used for comparisons between and within the two treatment groups, respectively. Chi-square test was used for analysis of categorical variables. For each treatment, data for four different times were analyzed by one-way ANOVA. SPSS 10.0 (Chicago, IL) was used for all data analysis. A value of P < 0.05 was considered statistically significant.
RESULTS
Clinical data for all 69 patients were available for analysis. No side effects related to the two agents were observed. Baseline characteristics and anthropometric parameters are summarized in Table I.
Effect of Statins on Lipid Levels
The treatment groups did not differ in baseline levels of TGs, TC, LDL-C, HDL-C or adiponectin (P > 0.05) (Table II). Both atorvastatin and rosuvastatin treatment significantly decreased TC, LDL-C, and Apo B at 4weeks, and the effects continued throughout 8 weeks of the treatment. Treatment with rosuvastatin, but not atorvastatin, decreased Non-HDL-C at 4 and 8 weeks significantly (P < 0.05) (Table III) (Table IV). After 12 weeks, treatment with both atorvastatin and rosuvastatin significantly reduced serum levels of TC (by 29.3% and 35.2%, P < 0.05 and P < 0.01, respectively), LDL-C (36.1% and 47.5% respectively, P < 0.01), apolipoprotein B and Non-HDL-C (both P < 0.05) as compared with at baseline. Only rosuvastatin increased HDL-C level significantly from baseline (P < 0.05). The reduction in TC, LDL-C, and non-HDL-C levels was greater with rosuvastatin than with atorvastatin (P < 0.05).
Serum Adiponectin Levels
Serum adiponectin levels were higher with both atorvastatin and rosuvastatin treatment than at baseline [increase from 11.74 ± 7.82 to 13.55 ± 8.91 μg/mL and 9.82 ± 5.71 to 16.46 ± 7.10 μg/mL (P < 0.05), respectively], the increase with rosuvastatin being significantly higher than that with atorvastatin (67.6% vs. 15.4%; P < 0.05) (Table II, Fig. 1).
Effect of atorvastatin and rosuvastatin therapy on serum adiponectin levels. Twelve weeks of rosuvastatin treatment significantly increased adiponectin levels. No significant difference was found with atorvastatin therapy. The increase of adiponectin levels with rosuvastatin was significantly higher than that with atorvastatin.
Effect of Statins on Levels of hs-CRP, TNF-α, MMP-9 and Markers of Fibrinolysis
Plasma level of hs-CRP was significantly lower with atorvastatin and rosuvastatin treatment, by 24% and 40% (P < 0.05, P < 0.01, respectively), than at baseline, the reduction greater with rosuvastatin than with atorvastatin (40% vs. 24%; P < 0.05) (Fig. 2). Treatment with neither atorvastatin nor rosuvastatin statin significantly changed plasma levels of tPA, TNF- and ET-1, but with both PAI-1 level was significantly lowered, by 23.1% and 33.6%, as was total MMP-9 level, by 28% and 31% (P < 0.05), respectively, than that at baseline.
Effect of Statins on Vasomotor Function
With atorvastatin and rosuvastatin treatment, the percent change of the brachial diameter with reactive hyperemia was higher after 12-week treatment (8.79 ± 0.48% vs. 15.75 ± 2.02% and 9.43% ± 0.54% vs. 18.76 ± 1.62%, respectively, P < 0.05).
DISCUSSION
Hypercholesterolemia clearly constitutes a major risk factor for CAD, and lowering high levels of LDL-C is a key target for reducing cardiovascular events. High levels of LDL-C can increase lipid oxidation, the inflammatory process, and migration of monocytes/macrophages and their transformation into foam cells (9,10). Drug therapies such as 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) can substantially reduce LDL-C level and CAD risk (11,12). Atorvastatin has more powerful anti-inflammatory and lipid-lowering effects than simvastatin, pravastatin, and lovastatin (13). However, rosuvastatin, a relatively new HMG-CoA reductase inhibitor, has a number of favorable characteristics, including low lipophilicity, high hepatocyte selectivity, minimal metabolism, and a low propensity for cytochrome P450 drug interactions (14). The present study comparing the two statins found both with favorable effects on lipid modulation, but 10 mg rosuvastatin in particular lowered serum TC and LDL-C levels to a greater extent in patients with hypercholesterolemia.
Our results from study of Chinese patients are similar to those of a previous study showing 6-week rosuvastatin treatment producing significantly greater LDL cholesterol reduction than atorvastatin in a Western population (15), and the safety was similar for both groups. We also showed that 10 mg rosuvastatin results in greater and earlier reduction in non-HDL cholesterol level than 10 mg atorvastatin, which is of clinical importance because this measure is recommended as a secondary goal for patients with high TG levels.
HDLs have long been considered as an important endogenous factor that protects against atherosclerosis and are thus an attractive therapeutic target. The most well-established mechanism by which HDLs protect against atherosclerosis is by promoting cholesterol efflux from macrophages and transporting the cholesterol to the liver, a process termed reverse cholesterol transport (16). In addition, HDLs have various other properties that could contribute to their antiatherogenic properties. Previous study proved that a high level of HDL cholesterol is inversely related to plaque growth (17). In our study, rosuvastatin treatment produced significant increase in HDL cholesterol level, and hence might be valuable in decreasing the atherosclerotic risk.
In addition to lipid lowering, statins have a wide range of biologic effects, such as anti-inflammatory and anti-thrombotic effects (18–21). They can stabilize and even lead to the regression of atherosclerotic plaque, which cannot be achieved with other lipid-modulating agents (22,23). Our results also showed statin therapy still reducing the plasma levels of the inflammatory markers hs-CRP, PAI-1 and MMP-9 in hypercholesterolemic patients, but the level of hs-CRP was reduced particularly with rosuvastatin, presenting an important implication for the treatment of coronary artery disease.
Endothelial dysfunction is characterized by an imbalance between vasodilating and vasoconstricting substances, with an impairment of vasodilators such as nitric oxide (NO) and prostacyclin (PGI2) and a predominance of vasoconstrictors such as endothelin-1 (ET-1) and angiotensin II (Ang II) (24). In patients with established atherosclerosis, disturbed vasomotion associated with endothelial dysfunction may contribute to transient myocardial ischemia and angina pectoris. It is also associated with changes in plaque composition and biology, which may influence plaque stability (25). In a large population of young adults (The Cardiovascular Risk in Young Finns Study), a strong inverse relationship has been shown between endothelial-dependent flow-mediated dilatation (FMD) and structural arterial disease (by carotid intima media thickness) after multivariable adjustment for traditional risk factors (26). Thus, endothelial dysfunction is a process fundamental to atherosclerotic lesion initiation, progression, and destabilization.
Previous studies already proved that some statins can improve endothelial function (27,28). However, controversy persists regarding whether rosuvastatin can lead to the restoration of endothelial function in different circumstances (29,30). In our study, rosuvastatin treatment produced higher percent change of the brachial diameter with reactive hyperemia (9.43% vs 18.76%). Since alteration in endothelial function precedes the development of morphological atherosclerotic changes and can also contribute to lesion development and later clinical complications (31), so the restoration of endothelial function most likely can postpone the atherosclerotic disease process in patients with hypercholesterolemia.
Long-term prospective studies have shown lower serum adiponectin level associated with an increased chronic heart disease risk profile, as well as insulin resistance and risk of type 2 diabetes (32). The adipocytokine adiponectin is highly specific for adipose tissue. It improves insulin resistance, regulates lipid metabolism such as stimulating fatty-acid oxidation and has anti-inflammatory properties (33–36), considered independent risk factors for cardiovascular disease (37). In the vascular endothelium, adiponectin decreases monocyte adhesion to endothelium, suppresses macrophage-to-foam cell transformation, and inhibits vascular smooth muscle cell proliferation and migration (3). It can also stimulate production of nitric oxide from vascular endothelial cells (38). Adiponectin can influence thrombus formation and platelet aggregation in mouse models, and adiponectin deficiency leads to enhanced thrombus formation and platelet aggregation (39). Therefore, adiponectin may influence myocardial infarction through thrombotic processes as well as atherosclerotic processes (40).
Several studies reported the effects of different statins on adiponectin levels. Simvastatin was reported failed to affect adiponectin levels or improve insulin sensitivity in subjects with the metabolic syndrome and in healthy men (41,42), while pravastatin exhibits beneficial effects on glucose metabolism especially in the postprandial state associated with increasing plasma adiponectin levels in CAD patients with IGT (43). Atorvastatin has direct effects on the differentiation, apoptosis and endocrine function of adipocytes and can inhibit insulin-induced glucose uptake in differentiated white adipocytes as well as elevate serum adiponectin levels in CAD patients (5,44). It can even decrease PAI-1, suggesting that it may have an antithrombotic effect (45). Treatment with rosiglitazone, then atorvastatin not only improved lipid profiles but also further increased adiponectin level by 124% in patients with type 2 diabetes mellitus (46). In our study, serum adiponectin level was higher with rosuvastatin, which suggests that rosuvastatin has a more favorable effect on regulating adiponectin in patients with hypercholesterolemia.
The exact mechanisms by which rosuvastatin affects adiponectin levels is unknown and further studies are needed to explore the underlying mechanisms.
LIMITATIONS
One issue should be kept in mind when reviewing the results of the study. That is, in this study we only observed the short-term effect of both statins based on a fix dose of 10 mg, while 10 mg of rosuvastatin is a relatively high starting dose in Asian patients. Further studies are needed to investigate the long-term effects of a titrate-to-goal regimen of atorvastatin and rosuvastatin on these biomarkers and clinical outcomes.
CONCLUSIONS
The present study demonstrate that 12-week treatment with 10 mg rosuvastatin led to more favorable effects than 10 mg atorvastatin on modulating lipid and adiponectin levels in subjects with hypercholesterolemia, which suggests promise in clinical practice.
References
M. Matsubara, S. Maruoka, and S. Katayose. Decreased plasma adiponectin concentrations in women with dyslipidemia. J. Clin. Endocrinol. Metab. 87:2764–2769 (2002). doi:10.1210/jc.87.6.2764.
P.J. Havel. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 53(suppl 1):S143–151 (2004). doi:10.2337/diabetes.53.2007.S143.
M. Chandran, S. A. Phillips, T. Ciaraldi, and R. R. Henry. Adiponectin: more than just another fat cell hormone? Diabetes Care. 26:2442–2450 (2003). doi:10.2337/diacare.26.8.2442.
T. Kadowaki, and T. Yamauchi. Adiponectin and adiponectin receptors. Endocr. Rev. 26:439–451 (2005). doi:10.1210/er.2005-0005.
K. Miyagishima, S. Hiramitsu, and S. Kato. Efficacy of atorvastatin therapy in ischaemic heart disease-effects on oxidized low-density lipoprotein and adiponectin. J. Int. Med. Res. 35:534–539 (2007).
K. K. Koh, M. J. Quon, S. H. Han, W. J. Chung, J. Y. Ahn, Y. H. Seo, I. S. Choi, and E. K. Shin. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J. Am. Coll. Cardiol. 45:1649–1653 (2005). doi:10.1016/j.jacc.2005.02.052.
S. Wissen, T. J. Smilde, E. Groot, BA. Hutten, J. J. Kastelein, and A. F. Stalenhoef. The significance of femoral intima-media thickness and plaque scoring in the Atorvastatin versus Simvastatin on Atherosclerosis Progression (ASAP) study. Eur. J. Cardiovasc. Prev. Rehabil. 10:451–455 (2003). doi:10.1097/01.hjr.0000103277.02552.1e.
K. K. Koh, C. Cardillo, M. N. Bui, L. Hathaway, G. Csako, M. A. Waclawiw, J. A. Panza, and R. O. III Cannon. Vascular Effects of estrogen and cholesterol-lowering therapies on vascular function in hypercholesterolemic postmenopausal women. Circulation. 99:354–360 (1999).
R. C. Schlant, and R. W. Alexander. Hurst’s the heart: Arteries and veins. 8McGraw-Hill, New York, 1995.
A. Niemann-Jönsson, P. Dimayuga, S. Jovinge, F. Calara, M. P. S. Ares, G. N. Fredrikson, and J. Nilsson. Accumulation of LDL in rat arteries is associated with activation of tumor necrosis factor- expression. Arterioscler. Thromb. Vasc. Biol. 20:2205–2211 (2000).
K. K. Ray, C. P. Cannon, and P. Ganz. Beyond lipid lowering: What have we learned about the benefits of statins from the acute coronary syndromes trials? Am. J. Cardiol. 98:18P–25P (2006). doi:10.1016/j.amjcard.2006.09.016.
G. G. Schwartz, A. G. Olsson, M. D. Ezekowitz, P. Ganz, M. F. Oliver, D. Waters, A. Zeiher, B. R. Chaitman, S. Leslie, and T. Stern. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 285:1711–1718 (2001). doi:10.1001/jama.285.13.1711.
T. C. Andrews, C. M. Ballantgne, J. A. Hsia, and J. H. Kramer. Achieving and maintaining National Cholesterol Education Program Low-density lipoprotein cholesterol goals with five statins. Am. J. Med. 111:185–191 (2001). doi:10.1016/S0002-9343(01)00799-9.
A. Cheng-Lai. Rosuvastatin: A new HMG-CoA reductase inhibitor for the treatment of hypercholesterolemia. Heart Disease. 5:72–78 (2003). doi:10.1097/01.HDX.0000050417.89309.F8.
P. H. Jones, M. H. Davidson, E. A. Stein, H. E. Bays, J. M. McKenney, E. Miller, V. A. Cain, J. W. Blasetto, and STELLAR Study Group. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Am. J. Cardiol. 92:152–160 (2003). doi:10.1016/S0002-9149(03)00530-7.
P. Libby, R. O. Bonow, D. L. Mann, D. P. Zipes, and E. Braunwald. Braunwald’s heart disease: A textbook of cardiovascular medicine. 8Saunders, USA, 2007.
S. H. Johnsen, E. B. Mathiesen, E. Fosse, O. Joakimsen, E. Stensland-Bugge, I. Njølstad, and E. Arnesen. Elevated high-density lipoprotein cholesterol levels are protective against plaque progression. Circulation. 112:498–504 (2005). doi:10.1161/CIRCULATIONAHA.104.522706.
S. E. Nissen, E. M Tuzcu, P. Schoenhagen, T. Crowe, W. J. Sasiela, J. Tsai, J. Orazem, R. D. Magorien, C. O. 'Shaughnessy, P. Ganz, and the Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) Investigators. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N. Engl. J. Med. 352:29–38 (2005). doi:10.1056/NEJMoa042000.
G. Weitz-Schmidt, K. Welzenbach, V. Brinkmann, T. Kamata, J. Kallen, C. Bruns, S. Cottens, Y. Takada, and U. Hommel. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 7:687–692 (2001). doi:10.1038/89058.
T. Bourcier, and P. Libby. HMG-CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20:556–562 (2000).
A. Undas, K. E. Brummel-Ziedins, and K. G. Mann. Statins and blood coagulation. Arterioscler. Thromb. Vasc. Biol. 25:287–294 (2005). doi:10.1161/01.ATV.0000151647.14923.ec.
L. O. Jensen, P. Thayssen, K. E. Pedersen, S. Stender, and T. Haghfelt. Regression of coronary atherosclerosis by simvastatin: a serial intravascular ultrasound study. Circulation. 110:265–270 (2004). doi:10.1161/01.CIR.0000135215.75876.41.
K. J. Molloy, M. M. Thompson, E. C. Schwalbe, P. R. Bell, A. R. Naylor, and I. M. Loftus. Comparison of levels of matrix metalloproteinases, tissue inhibitor of metalloproteinases, interleukins, and tissue necrosis factor in carotid endarterectomy specimens from patients on versus not on statins preoperatively. Am. J. Cardiol. 94:144–146 (2004). doi:10.1016/j.amjcard.2004.03.050.
J. Calles-Escandon, and M. Cipolla. Diabetes and endothelial dysfunction: A clinical perspective. Endocr. Rev. 22:36 (2001). doi:10.1210/er.22.1.36.
M. Crisby, G. Nordin-Fredriksson, P. K. Shah, J. Yano, J. Zhu, and J. Nilsson. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 103:926–933 (2001).
M. Juonala, J. S. Viikari, T. Laitinen, J. Marniemi, H. Helenius, T. Ronnemaa, and O. T. Raitakari. Interrelations between brachial endothelial function and carotid intima-media thickness in young adults: the Cardiovascular Risk in Young Finns study. Circulation. 110:2918–2923 (2004). doi:10.1161/01.CIR.0000147540.88559.00.
S. H. Wilson, R. D. Simari, P. J. Best, T. E. Peterson, L. O. Lerman, M. Aviram, K. A. Nath, D. R. Jr Holmes, and A. Lerman. Simvastatin preserves coronary endothelial function in hypercholesterolemia in the absence of lipid lowering. Arterioscler Thromb Vasc Biol. 21:546–554 (2001). doi:10.1161/hq1101.097805.
J. K. Williams, G. K. Sukhova, D. M. Herrington, and P. Libby. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J. Am. Coll. Cardiol. 31:684–691 (1998). doi:10.1016/S0735-1097(97)00537-8.
I. V. Sergienko, E. I. U. Samoĭlenko, V. P. Masenko, M. V. Ezhov, A. B. Sumarokov, G. A. Tkachev, O. A. Pogorelova, T. V. Balakhonova, and V. G. Naumov. Effect of therapy with rosuvastatin on lipid spectrum, factors of inflammation and endothelial function in patients with ischemic heart disease. Kardiologiia. 46:4–8 (2006).
E. Ter Avest, E. J. Abbink, S. Holewijn, J. de Graaf, C. J. Tack, and A. F. Stalenhoef. Effects of rosuvastatin on endothelial function in patients with familial combined hyperlipidaemia (FCH). Curr. Med. Res. Opin. 21:1469–1476 (2005). doi:10.1185/030079905X61910.
R. Ross. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362:801–809 (1993). doi:10.1038/362801a0.
D. M. Maahs, L. G. Ogden, G. L. Kinney, P. Wadwa, J. K. Snell-Bergeon, D. Dabelea, J. E. Hokanson, J. Ehrlich, R. H. Eckel, and M. Rewers. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 111:747–753 (2005). doi:10.1161/01.CIR.0000155251.03724.A5.
M. B. Schulze, E. B. Rimm, I. Shai, N. Rifai, and F. B. Hu. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 27:1680–1687 (2004). doi:10.2337/diacare.27.7.1680.
M. Cnop, P. J. Havel, K. M. Utzschneider, D. B. Carr, M. K. Sinha, E. J. Boyko, B. M. Retzlaff, R. H. Knopp, J. D. Brunzell, and S. E. Kahn. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 46:459–469 (2003).
T. Yamauchi, K. Hara, N. Kubota, Y. Terauchi, K. Tobe, P. Froguel, R. Nagai, and T. Kadowaki. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr. Drug Targets Immune. Endocr. Metabol. Disord. 3:243–254 (2003). doi:10.2174/1568008033340090.
C. -J. Li, H. -W. Sun, F. -L. Zhu, L. Chen, Y. -Y. Rong, Y. Zhang, and M. Zhang. Local Adiponectin treatment reduces atherosclerotic plaque size in rabbits. J. Endocrinol. 193:137–145 (2007). doi:10.1677/JOE-06-0173.
T. Pischon, C. J. Girman, G. S. Hotamisligil, N. Rifai, F. B. Hu, and E. B. Rimm. Plasma adiponectin levels and risk of myocardial infarction in men. J. Am. Med. Assoc. 291:1730–1737 (2004). doi:10.1001/jama.291.14.1730.
H. Chen, M. Montagnani, T. Funahashi, I. Shimomura, and M. J. Quon. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 278:45021–45026 (2003). doi:10.1074/jbc.M307878200.
H. Kato, H. Kashiwagi, M. Shiraga, S. Tadokoro, T. Kamae, H. Ujiie, S. Honda, S. Miyata, Y. Ijiri, J. Yamamoto, N. Maeda, T. Funahashi, Y. Kurata, I. Shimomura, Y. Tomiyama, and Y. Kanakura. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler. Thromb. Vasc. Biol. 26:224–230 (2006). doi:10.1161/01.ATV.0000194076.84568.81.
G. A. Laughlin, E. Barrett-Connor, S. May, and C. Langenberg. Association of Adiponectin with coronary heart disease and mortality: The Rancho Bernardo study. Am. J. Epidemiol. 165:164–174 (2007). doi:10.1093/aje/kwk001.
S. Devaraj, D. Siegel, and I. Jialal. Simvastatin (40 mg/day), adiponectin levels, and insulin sensitivity in subjects with the metabolic syndrome. Am. J. Cardiol. 100:1397–1399 (2007). doi:10.1016/j.amjcard.2007.06.028.
I. Gouni-Berthold, H. K. Berthold, J. P. Chamberland, W. Krone, and C. S. Mantzoros. Short-term treatment with ezetimibe, simvastatin or their combination does not alter circulating adiponectin, resistin or leptin levels in healthy men. Clin. Endocrinol. (Oxf). 68:536–541 (2008). doi:10.1111/j.1365-2265.2007.03080.x.
S. Sugiyama, H. Fukushima, K. Kugiyama, H. Maruyoshi, S. Kojima, T. Funahashi, T. Sakamoto, Y. Horibata, K. Watanabe, H. Koga, K. Sugamura, F. Otsuka, I. Shimomura, and H. Ogawa. Pravastatin improved glucose metabolism associated with increasing plasma adiponectin in patients with impaired glucose tolerance and coronary artery disease. Atherosclerosis. 194:e43–51 (2007). doi:10.1016/j.atherosclerosis.2006.08.023.
W. Mauser, N. Perwitz, B. Meier, M. Fasshauer, and J. Klein. Direct adipotropic actions of atorvastatin: differentiation state-dependent induction of apoptosis, modulation of endocrine function, and inhibition of glucose uptake. Eur. J. Pharmacol. 564:37–46 (2007). doi:10.1016/j.ejphar.2007.02.024.
J. Q. Li, S. P. Zhao, Q. Z. Li, Y. C. Cai, L. R. Wu, Y. Fang, and P. Li. Atorvastatin reduces plasminogen activator inhibitor-1 expression in adipose tissue of atherosclerotic rabbits. Clin. Chim. Acta. 370:57–62 (2006). doi:10.1016/j.cca.2006.01.024.
C. S. Chu, K. T. Lee, M. Y. Lee, H. M. Su, W. C. Voon, S. H. Sheu, and W. T. Lai. Effects of rosiglitazone alone and in combination with atorvastatin on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Am. J. Cardiol. 97:646–650 (2006). doi:10.1016/j.amjcard.2005.09.101.
Acknowledgements
This work was supported by The National Basic Research Program (973 Program) 2006CB503803 and HI-TECH Technique and Development Program of China (863 Program) 2007AA02Z448.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, Hy., Xiao, Yw., Jiang, Gh. et al. Effect of Atorvastatin Versus Rosuvastatin on Levels of Serum Lipids, Inflammatory Markers and Adiponectin in Patients with Hypercholesterolemia. Pharm Res 26, 958–964 (2009). https://doi.org/10.1007/s11095-008-9798-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9798-6