Abstract
Purpose
To compare the pulmonary pharmacokinetics and relative bioavailability of salmon calcitonin delivered as aqueous droplets, pH 6.6 and pH 4.8 with that of a spray dried powder in healthy volunteers.
Methods
Spray dried powders (1.6 μm [GSD 2.1]) containing 5% by wt. sCal, 6.25% human serum albumin, 73.55% mannitol and 15% citric acid/sodium citrate were prepared using a Buchi model 190 spray drier. Aqueous solutions were prepared by dissolving the spray dried powder at a sCal concentration of 1.25 mg/ml, pH was adjusted using 21 mM sodium hydroxide. Aerosols were delivered as part of a 4 way cross-over study to 16 healthy volunteers. The Nektar pulmonary delivery device was used to deliver the dry powder aerosol. A Salter nebulizer controlled by a Rosenthal dosimeter was used to deliver the aqueous aerosols. Miacalcin™ injection was used as the subcutaneous control. Dose delivered to the lung was estimated by gamma scintigraphy. Plasma concentrations of sCal were measured using a radioimmunoassay.
Results
Aerosol size distributions were matched, 3.3 μm MMAD and ∼2.2 GSD. Inhaled flow rates were similar, although not equal, 5.8 and ∼9.8 l/min respectively for dry powder and liquid inhalations. Lung doses of sCal ranged from 53 to 88 μgm, peripheral lung doses from 25 to 51 μgm. Pharmacokinetic profiles and lung bioavailability relative to subcutaneous injection for all formulations were similar (not statistically significantly different p > 0.05), relative lung bioavailability ranged from 11% to 18%, estimates of relative bioavailability based on peripheral lung dose ranged from 20% to 33%.
Conclusion
The study showed no difference in pharmacokinetic profiles between the various aerosol dosage forms. pH of the aqueous solutions did not affect kinetics or relative bioavailability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The pulmonary absorption of peptides and proteins has been studied extensively over the last decade. The main tool for investigating absorption has been animal models (1–5). Pulmonary insulin, Exubera™, a 6 kDa protein, has been approved for marketing in both Europe and the USA (6). However, despite over a decade of work and an approved product, the mechanisms and factors affecting the rate and extent of pulmonary systemic absorption of peptides and proteins are still poorly understood. The techniques used in animals to study the effects of formulation variables range from liquid and powder instillation to “true” liquid or dry powder aerosol delivery. However, while the reported studies have compared many different molecules, a variety of physicochemical properties, such as pH and tonicity and the effects of a large number of absorption enhancers, their relevance to aerosol delivery in man is questionable. There are also few reports comparing the absorption kinetics and bioavailability of liquid and powder formulations of the same molecule. This is of interest as it would be useful to understand the influence of dissolution of powder formulations on absorption. The data presented in this report compares the absorption kinetics (rate and extent of absorption) of two liquid formulations of pH 4.8 and pH 6.6 with a powder formulation of salmon calcitonin (sCalcitonin) in human volunteers. Gamma scintigraphy was used to quantify dose delivered to the lung and lung periphery (non-ciliated airways) and a radioimmunoassay was used to quantify plasma concentrations following absorption for 6 h post delivery. Because absorption can be affected by the deposition pattern within the airways [established dogma suggests delivery to the non-ciliated alveolar spaces is required for absorption (7)], attempts were made to ensure that the aerosols had similar aerosol particle size distributions and that they were inhaled at similar flow rates (in the event the flow rates were not matched as well as was hoped for). The time course of absorption (plasma kinetics) and the relative bioavailabilities of the inhaled sCalcitonin from the whole lung and lung periphery were compared to subcutaneous injection.
MATERIALS AND METHODS
Formulations and Delivery
The dry powder formulations consisted of 5% by weight sCalcitonin (Peninsula Lab, San Carlos, CA, USA), 6.25% human serum albumin (Biocon, California, USA), 73.55% mannitol USP and 15% citric acid/sodium citrate, with a solution of pH 4.8 prior to spray drying. A 0.75% aqueous formulation was spray dried using a Buchi Model 190 spray drier (Buchi, Switzerland) which generated dry particles with a mass median diameter of 1.6 μm and a geometric standard deviation of 2.1 as determined by centrifugal photosedimentation (Horiba Industries, Tokyo, Japan). Powder aerosol delivery was facilitated using the Nektar Pulmonary Delivery System (6,8) (Nektar Therapeutics, San Carlos, CA, USA). The PDS is an active inhaler which uses a bolus of compressed air, created by compressing a handle in the base of the device, to generate an aerosol cloud that is then stored in a 250 ml holding chamber prior to inhalation by the volunteer. The active nature of the device ensures that the volunteer’s inhalation effort does not affect the particle size distribution of the delivered aerosol. An inhalation of approximately 500 ml, twice the chamber volume, is required to completely extract the aerosol cloud (9). Approximately 6 mg of the spray dried/radiolabeled sCalcitonin powder was accurately weighed and sealed into pre-formed foil-foil blisters under low humidity conditions. The Nektar pulmonary delivery system (PDS), the system used for Exubera, was used to disperse the powder using a small compressed air bolus. The aerosol was captured in the integral 250 ml chamber prior to inhalation. Delivery was achieved by the volunteers inhaling the aerosol dose from the holding chamber at a target flow rate of 10 l/min. The inhalation patterns were measured using a Respitrace respiratory inductive plethysmograph (Respitrace Corporation, Ardsley, NY, USA).
The nebulizer solution consisted of the spray dried powder described above dissolved at a concentration of 1.25 mg/ml sCalcitonin in water for injection. Two pH formulations were tested, nominally pH 4.8 and pH 6.6. The natural pH of the reconstituted spray dried salmon calcitonin solution was pH 4.8, 0.2% 21 mM sodium hydroxide was used to adjust the pH of the neutral formulation. Delivery of the salmon calcitonin solution was achieved using a Salter nebulizer (Salter Labs, Carlsbad, CA, USA) driven at 60 psig via a Rosenthal dosimeter (Rosenthal, Munich, Germany). The Rosenthal dosimeter synchronized actuation of the nebulizer with the volunteer’s inhalation via a small pressure sensor. The time over which the aerosol was delivered during each breath was controlled by the dosimeter. The Rosenthal settings chosen for the study were a 300 ms delay in nebulization after the start of inhalation, a 3s dose duration and sample timer control (delay before next breath) of 7s. The volunteers were trained, again using inductive plethysmography, to inhale from residual volume at a flow rate of 10 l/min with the intent of matching the breathing pattern used for the powder delivery (it should be noted that in the event, as will be seen below, flow rates for the two delivery systems were not matched as well as would have been expected).
Miacalcin™ subcutaneous injection, 200 IU per ml, (Novartis Pharmaceutical Corporation, East Hanover, NJ, USA) injected into the abdomen was used as a subcutaneous control.
Aerosol Characterization
As stated above one of the major factors affecting deposition profiles within the lung is the size distribution of the inhaled aerosol. The challenge of “matching” the aerodynamic size distributions of a liquid and powder aerosol ultimately reduces to accurately determining their size distributions. This challenge revolves around accurately accounting for particle shape and density for the powder aerosol and controlling evaporation for the liquid aerosol. When an impactor, the preferred method of aerodynamic size analysis, is used to size aqueous droplets, errors occur due to evaporation of the droplets as they pass through the impactor stages. This forces either very careful control of temperature and humidity during the characterization (10) or the use of an alternative technique such as laser diffraction (11), which “sizes” the aerosol as it emanates from the mouthpiece before any appreciable evaporation can take place. However, if laser light scattering is used to characterize a dry powder aerosol, particle density and shape are not accounted for correctly and hence a more appropriate technique is cascade impaction. Thus, despite the limitations, the optimum aerodynamic sizing technique was used for each type of aerosol; laser diffraction for the liquids and cascade impaction for the dry powder.
An Andersen cascade impactor (ACI) (MSP Inc., Minneapolis, MN, USA) without a pre-separator and equipped with a USP inlet throat was used for the powder aerosol characterization. Aerosol was “evacuated” from the PDS chamber at a flow rate of 28 l/min using a volume of 4 l. Gravimetric determination of the size distribution was carried out by weighing filter paper placed on each stage of the impactor before and after aerosol delivery. Since the spray dried powder contains a homogenous mixture of protein and excipients this technique was considered acceptable. Mass balance between blister fill weights and recovered aerosol on the filter papers indicated that less than 6% of the aerosol was retained in the inlet throat or as wall losses within the impactor confirming the acceptability of the gravimetric approach.
The particle size distribution of the labeled powder aerosol was obtained gravimetrically and was then compared with the activity distribution of the radiolabel determined by counting the filter papers. Separately the activity retained on the inlet throat and stages of the impactor were measured to ensure that wall losses and deposition in the inlet bend were small and similar for the label and unlabeled powder.
The size distribution of the liquid nebulized aerosol was determined at the exit of the Salter mouthpiece by laser diffraction (Helios Spraytech, Munich, Germany) using methods previously described (11). In brief, the spray emanating from the mouthpiece of the Salter nebulizer was directed across the laser sensing zone of the Spraytech and the instrument then converted the resultant far field diffraction pattern into the size distribution of the droplets. A model independent fitting solution was used. This technique inherently measures the volume distribution of the droplet. Since the droplets are spherical, conversion to an aerodynamic size distribution merely required multiplication by 1 (1.25 mg/ml aqueous solutions of salmon calcitonin have a density close to 1 gm cm−3).
In order to try and match the dose to the lungs for each formulation in vitro delivered dose measurements were performed on both the powder and liquid aerosol clouds in addition to the particle size distribution (PSD) measurements. The delivered dose from the PDS was determined by summing the stages of the ACI and carrying out delivered dose determinations using the United States Pharmacopeia (USP) dose content uniformity (DCU) test apparatus (12), n = 3. The delivered dose from the Salter/Rosenthal dosimeter was determined gravimetrically and by chemical assay, using the USP DCU apparatus (12), n = 3.
Biochemical Stability
Reversed phase HPLC (RP-HPLC) was employed to monitor chemical stability of sCalcitonin. The chromatographic system consisted of a Waters 2690 HPLC module equipped with a photo diode array detector. Samples containing sCalcitonin (100 μl) were loaded onto a Vydac C18, 250 × 4.6 mm reversed phase analytical column. The elution of sCalcitonin and related substances was accomplished by 1 ml/min flow of mobile phase buffer A (0.1% trifluoroacetic acid in 30%:70% acetonitrile [CAN]/water) and mobile phase B (0.1% trifluoroacetic acid in 50%50% CAN/water). Upon injection, after 5 min elution with 100% mobile phase A, a linear gradient of 0–50% mobile phase B was performed over 40 min. The sample temperature was maintained at 5°C. The column temperature was maintained at 40°C, and the eluent absorbance was monitored at 210 nm.
Powder stability was tested over a period of 3 months which adequately covered the duration of the clinical study. The sCalcitonin nebulizer solution was prepared just prior to use. Its stability to nebulization was assessed by the reverse phase HPLC assay described above. The aerosol was collected on a membrane filter which was dried and weighted and then washed, using HPLC mobile phase to recover the sCalcitonin. The recovered solution was then subjected to HPLC to determined impurities. Recovery was estimated by comparing the HPLC salmon calcitonin assay values to that expected based on the filter weights, allowing for the excipient content of the solution. Recovery was close to 98%. Solutions of sCalcitonin sampled from the nebulizer before and after nebulization were also compared. Four determinations were carried out for each formulation (pH 4.8 and pH 6.6).
Radiolabeling for Gamma Scintigraphy
Since it would have been difficult to rely on in vitro “dosimetry” (particle size and delivered dose determinations) to accurately predict either inhaled dose or lung dose in volunteers, gamma scintigraphy was used to quantitate delivered dose to various locations within the respiratory tract. A 99mTc pertechnetate label was added to both the powder and nebulizer formulations just prior to delivery to the volunteers (13). For the dry powder formulation individual blisters were prepared by accurately weighing labeled powder directly into the blisters, for the nebulizer a small quantity of 99mTc pertechnetate solution was added directly to the nebulizer solution. Quantitative imaging was then used to estimate lung doses and lung distribution. The method of addition, and the subsequent performance of the dry powder aerosol, was validated using the particle size and dose characterization techniques described above. In the case of the nebulizer solution the addition of the small amount of 99mTc to the formulation was considered to constitute a negligible change and since it was in solution it was felt that no direct validation of the correspondence of the droplet distribution and activity distribution was necessary (14). The dose of sCalcitonin delivered to the lungs and the lung periphery (defined by regions of interest were determined using a modification of a previously reported gamma scintigraphy techniques (15). See below.
Serum sCalcitonin Concentrations and PK Analysis
Quantification of the sCalcitonin reaching the systemic circulation was achieved by radioimmunoassay of serum samples taken over 6 h post dose (“Ultrasensitive Radioimmunoassy kit for the quantitative determination of Salmon Calcitonin in serum and plasma”, Diagnostic Laboratories Inc., Vienna, VA, USA). Samples were collected in heparinized tubes, centrifuged at 10,000 rpm for 10 min. The plasma phase was separated and stored at −70°C until analysis.
Relative “bioavailability” was estimated by comparing dose corrected AUC (area under the curve) for total lung dose, peripheral lung dose (see discussion below) with subcutaneous injection.
Statistical Analysis
Statistical analysis of both the scintigraphy deposition data and the AUC values was performed using a Wilcoxon matched pairs signed ranks test. The Wilcoxon test is a non-parametric test appropriate for small sample size. A p value of <0.05 was considered to be significant,
CLINICAL STUDY DESIGN
The clinical study was an open label four-way crossover in 16 healthy volunteers, mean age 32 years, with an equal number of males and females. The study was approved by the Quorn Research Review Committee (investigational review board) and was performed at Pharmaceuticals Profiles plc, Nottingham, UK. The study was performed over a 4 week period with a one week washout between doses. Each of the 16 healthy volunteers received:
-
Regimen A:
sCalcitonin delivered as a powder aerosol from the Nektar PDS. A single inhaled dose of approximately 2.5 mg of powder (nominal blister dose 6 mg) containing 133 μg (790 IU) of sCalcitonin radiolabelled with 10 MBq 99mTc pertechnetate with a Mass Median Aerodynamic Diameter of 3.3 μm.
-
Regimen B:
sCalcitonin delivered as an aqueous aerosol from a Salter nebulizer controlled by a Rosenthal dosimeter. Four breaths inhaled over 2 min, each followed by a 5 s breath hold, of a 75 IU/ml (1.25 mg/ml), pH 7.6 aqueous solution delivering a total inhaled dose of 112 μg (670 IU) of sCalcitonin, radiolabelled with 10 MBq 99mTc pertechnetate. Mean Volume Median Diameter 3.3 μm.
-
Regimen C:
sCalcitonin delivered as an aqueous aerosol from a Salter nebulizer controlled by a Rosenthal dosimeter. Four breaths inhaled over 2 min, each followed by a 5 s breath hold, of a 75 IU/ml (1.25 mg/ml), pH 4.8 aqueous solution delivering a total inhaled dose of 112 μg (670 IU) of sCalcitonin, radiolabelled with 10 MBq 99mTc pertechnetate. Mean VMD 3.3 μm
-
Regimen D:
sCalcitonin administered as a subcutaneous injection. 12.5 μg (75 IU) Miacalcin injected subcutaneously in the abdomen.
Scintigraphy
Immediately following inhalation of the dose, two dimensional images of the anterior and posterior chest, lateral oropharynx, exhalation filter and delivery device (including the blister for the powder device) were obtained using a gamma camera (GE Maxicamera, Milwaukee, WI, USA). The dose delivered to each volunteer was calculated by assuming the activity at a given location was proportional to the dose of salmon calcitonin and that the total activity was equal to the total salmon calcitonin placed either in the dry powder blister or the nebulizer. Lung scintigraphs were compared to whole lung 81mKr ventilation scans obtained for each individual volunteer. Regions of interest were used to assess the dose delivered to each location. Attenuation and radioactive decay corrections were applied to the raw count data to estimate the fraction of the dose deposited in each location (16). Peripheral (P) to central (C) lung zones, constructed as previously described (17), were compared in order to estimate drug distribution within the lung. The correlation developed by Newman et al. (17) relating P/C ratio to 24 h clearance was used to estimate the dose of sCalcitonin reaching the peripheral, non-ciliated, airways. While at best, this method can only be expected to produce an estimate of alveolar deposition, in this type of crossover study it should give a reasonable comparison between the different formulations and it does allow computation of bioavailability based on a measure of peripheral dose. However, it should be made clear that bioavailabilities based on estimates of peripheral dose do not in themselves deny or confirm that the lung periphery is the major site of absorption. On the contrary the values are for reference only as an estimate of the ultimate bioavailability that can be obtained if the peripheral lung is the major site of absorption and if 100 percent peripheral deposition could be achieved.
Plasma Sampling
Venus blood samples for determination of plasma sCalcitonin concentration were collected at the following intervals; −5, 0,10, 20, 30, 40 60, 120 240 and 360 min after administration. As described above samples were centrifuged and the plasma phase was separated and stored in polypropylene tubes at −70°C until analysis.
Pulmonary Function Measurements
Pulmonary function values, (FVC) forced vital capacity; (FEV1) forced expiratory volume in 1 s, and vital signs, were recorded before, 1 and 6 h after dosing.
RESULTS
Aerosol Characterization
Figure 1 presents the aerodynamic particle size distribution of the drug and radiolabel for the dry powder aerosol delivered via the PDS and that for the solution formulations delivered via the Salter nebulizer/dosimeter. The size distribution of the labeled powder aerosol was similar to that of the unlabled powder, however it is not presented here since it is not relevant to the intent of the study, i.e. to a comparison of the relative bioavailabilities of wet and dry aerosols (note: this study was not designed to compare the delivery efficiency of the dry powder inhaler with the nebulizer). It can be seen that the distributions match remarkably well, both in terms of mass median aerodynamic diameter (MMAD; 3.3 μm for liquid, powder and powder radiolabel) and geometric standard deviation (GSD; 2.28, 2.16 and 2.13 for the liquid, powder and powder radiolabel respectively). Also it can be seen that there is an excellent agreement between the dry powder drug distribution and that of the radiolabel, indicating that from an aerosol size, and hence aerodynamic behavior perspective, the label is a good surrogate for the drug and should be acceptable as a method of estimating the drug distribution and dose throughout the airways. Tables I and II summarize the delivered dose data for the dry powder and aqueous droplet aerosols. Delivered doses were determined gravimetrically and by HPLC. These data were used to confirm blister fill weights and dosing times from the nebulizer to ensure similar lung dose for each formulation. However, in vivo doses and dose distributions were determined using the activity distributions determined by scintigraphy, not by using these in vitro values.
A comparison of aerodynamic size distributions of sCalcitonin powder and the radiolabel dry powder delivered via the Nektar PDS, with sCalcitonin solution droplets delivered via the nebulizer/dosimeter. (1) Powder aerosol size distributions were measured by Andersen impactor at a flow rate of 28 l/min. Activity at each stage was measured by gamma camera, drug content was quantified gravimetrically (n = 3). (2) Nebulizer droplet size distributions were measured by Helios diffraction analyzer under free flow (∼15 l/min) at the mouthpiece exit with a nebulizing pressure of 60 psig. pH had no effect on droplet size distributions (n = 3). (3) Error bars represent 1 standard deviation. Bars to the left are for the gravimetric powder determination and were similar for the activity distribution. Bars to the right are for the nebulizer determinations.
Table III summarizes the stability of the sCalcitonin powder and Table IV stability of the pH 4.8 and 6.6 solutions following nebulization. No significant degradation was observed with either formulation.
Clinical Study Results
Inhaled Volumes and Flow Rates
Table V summarizes the breathing patterns for each of the three aerosol dosage regimens presenting mean inhaled flow rate and mean inhaled volumes. Inhalation times for each of the inhaled nebulizer doses were of the order of 20 s and those for the dry powder aerosol were slightly longer, reflecting the lower mean flow rate, and were of the order of 25 s. It should be noted that no feedback was given to the volunteers during the dosing sessions and this may have been responsible for the lower flow rates inhaled during powder dosing. As can be seen from the table the breathing profiles for each of the nebulized doses were reasonably similar. However, mean flow rates and inhaled volumes were higher for the nebulized doses than for the powder doses, despite training and the same target profiles for each.
It is interesting to note that despite the slightly higher average flow rate for the nebulized delivery the peripheral to central (P/C) deposition ratios, a measure of alveolar penetration, were lower for the powder than for the liquid, 1.4 ± 0.3 and 1.3 ± 0.3 for the pH 4.8 and pH 6.6 liquids respectively compared to 0.8 ± 0.3 for the dry powder (see below for discussion).
Pharmacokinetics
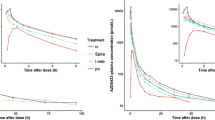
Mean non-dose corrected plasma kinetic profiles for the 3 inhaled doses and the subcutaneous dose are shown in Fig. 2. It can be seen that the profile for the two routes of administration and that for the different formulations are similar. T max values were in the range 20–40 min and terminal half lives on the order of 100 min. Table VI summarizes the mean dose delivered to each location together with calculated AUC values for each dosing regimen.
Mean pharmacokinetic profiles for inhalation of wet (nebulized), dry (Nektar PDS) aerosols and subcutaneous injection (Miaclacin) of sCalcitonin. For clarity the 1 standard deviation error bars are only shown for the subcutaneous injection and one nebulizer formulation. The three inhaled dose regimen error bars where of similar magnitude.
Figure 3 presents (left hand axis) the dose corrected AUC values based on total sCalcitonin dose deposited in the lung and lung periphery for each formulation. As described above, peripheral lung doses were estimated from the correlation between P/C ratio and 24 h clearance reported by Newman and co-workers (16). The underlying assumption in this analysis is that 24 h clearance represents non-ciliated airway and hence alveolar deposition. Despite being a rather crude estimate, this procedure does allow a bioavailability comparison against some measure of peripheral dose. However, as indicated above this representation of the data should not be taken as proof that the peripheral lung is the major site of absorption of this molecule. Doses at each location were calculated by multiplying the fraction of the total activity at each location by the total sCalcitonin dose placed in the blister or nebulizer. Also shown in Fig. 3 (right hand axis) are the relative bioavailabilities of the inhaled formulations based on lung dose and peripheral lung dose. Relative bioavailabilities were estimated by dividing the dose corrected AUCs by the dose correct AUC for the subcutaneous injection.
Mean dose corrected AUC data and Mean bioavailability relative to subcutaneous injection for dry powder and nebulized aerosols of sCalcitonin. (1) D dry powder delivered by PDS, W “wet” aerosol delivered by nebulizer, subscripts are nominal pH of formulations, (2) p values were calculated using a Wilcoxon matched pairs signed ranks test p < 0.05 considered significant.
Safety and Tolerability
Table VII summarizes the incidence and type of adverse events reported during the study. There were no adverse events that were considered serious or severe. By far the most common events were nausea and/or vomiting and mild diarrhea after subcutaneous dosing and cough after inhalation of the powder formulation. Of note is the fact that no cough or gagging incidents were reported with either of the nebulizer formulations and no nausea or vomiting was reported with any of the inhaled formulations. This latter finding is of interest because the serum profiles and peak levels were similar for all dosing groups (Fig. 2). There were no notable changes in lung function following administration of either subcutaneous or inhaled sCalcitonin.
DISCUSSION
Despite great efforts to ensure similar particle size distributions for the nebulized and dry powder aerosols and training to target similar inhalation flow rates, the lung distributions for the two aerosols, as measured by P/C ratio, were different. P/C ratios for the nebulized aerosols were higher (∼1.4) than for the powder (∼0.8) despite the fact that the inhaled flow rates were also higher (∼9.7 l/min liquid versus 5.8 l/min dry powder). Of course the lung distribution pattern can be affected by many factors beyond initial particle size and inhaled flow rate and it is interesting to speculate as to the cause of this observation. For example, hygroscopic growth might be partially responsible. The nebulized solutions were slightly hypertonic and would likely not experience significant hygroscopic effects, whereas the dry powder may have undergone growth during passage through the airways. The calculated equilibrium aerodynamic growth ratios for the liquid formulations are close to 0.75 (i.e. the droplets would shrink slightly when exposed to isotonic airway conditions). The growth ratio for the powder formulation was approximately 2.6, that is to say under isotonic equilibrium conditions the powder particles would grow to droplets 2.6 times the diameter of the original particles. Of course it remains speculation as to how rapidly the droplets and particles change size in the airways and hence whether this effect caused the observed differences in lung distribution or if other phenomena, such as the differences in flow profiles, were at work.
This study suggests that for this particular amorphous dry powder formulation of sCalcitonin, dissolution kinetics probably do not play a significant role in controlling absorption rates or overall lung bioavailability. Further no significant differences in bioavailability were observed between sCalcitonin solutions of neutral and acidic pH. The literature on both of these points is a little contradictory.
Komada et al. (18) using instillation in Wistar rats concluded that for a number of proteins, including calcitonin, drug absorption was slower for dry powder formulations than for solutions. In contrast Niven et al. (2) investigating insufflation of rhG-CSF in rabbits concluded that plasma concentration vs. time profiles were similar for insufflated solutions and powders. Although different formulation approaches were used in each of these studies, lyophilization and spray drying respectively, the resolution of these two data sets does not appear to lie in formulation differences as both powders would most likely have been amorphous. Rather the explanation for the contradiction may lie in the delivery techniques and lung distributions. While insufflation and instillation allow accurate determination of the dose reaching the lung (at least reaching the end of the intratracheal tube) the lung distribution of drug obtained with these techniques is far from representative of true aerosol delivery. For example in liquid instillation the volumes of solution instilled are markedly larger, relative to lung volume, than are similar dose delivered via aerosol. In addition, following instillation liquids can potentially distribute more readily within the lungs than powders.
However, support for the lack of impact of dissolution kinetics of protein and peptide powders on absorption kinetics can be found in the data reported on liquid and powder aerosol delivery of insulin in humans. Heinemann and Heise (19) summarized the available pharmacodynamic profiles for various forms of inhaled insulin. Their review compared two forms of liquid insulin (Novo Nordisk/Aradigm and Aerogen) with four powder formulations (Exubera (Pfizer/Nektar), Lilly/Alkermes, KOS and Mannkind). With the exception of the Mannkind formulation, which uses fumaryl diketopiperazine a novel excipient believed to be responsible for the more rapid absorption kinetics, the remaining data show no differences in rates of pharmacodynamic response. This observation indicates that dissolution of these amorphous insulin powders does not play a significant role in the control of absorption kinetics.
The literature is also somewhat equivocal on the role of pH in controlling the rate and extent of absorption of inhaled proteins and peptides. Again Komada et al. (18), amongst others [for example Okumura (20)], showed that more acidic pHs result in an increased rate and extent of absorption for a number of proteins and peptides. Studies in humans however, for example Farr et al. (21) with liquid insulin, seem to show no effect. (Note. while the intent of Farr’s experiment was to investigated hexameric versus monomeric absorption rates, the lack of an observed pH effect also supports the hypothesis in that in man, lightly buffered pH solutions cannot be used to enhance absorption). This contradiction between Komada, Farr and the current study may be explained by buffering capacity. Generally the formulations used in animal experiments use unacceptably high levels of buffering capacity. Similar approaches in humans could result in a high risk of bronchoconstriction hence both this study and published use pH adjustment without any major buffering capacity.
Finally it is worth commenting on the bioavailabilities observed in this study and putting the various measures used into context. To date the region from which the absorption of inhaled proteins and peptides actually takes place is not well defined. While the established dogma suggests it is the peripheral lung, where the surface area is large and the epithelium is thin [Patton et al. (7)], the data supporting this belief are rather sparse. In this study we chose to measure BAV against whole lung deposition and against an estimated peripheral deposition. Whole lung BAV measured against subcutaneous injection was 10% to 18%. BAV based on our estimate of peripheral deposition were in the range 20% to 30%. These values are consistent with other literature data for calcitonin, but they do indicate that the inhaled bioavailability in man is lower than that for inhaled insulin. The insulin data indicate 10% to 15% based on nominal dose (19). Allowing for device and deposition efficiency this would lead to a whole lung BAV of around 25% to 30%; twice that for sCalcitonin as observed in this study (22). Thus it would be expected that for an efficient delivery system similar to those used for pulmonary insulin delivery the ultimate bioavailability of sCalcitonin delivered by inhalation would be in the range of 5–7% based on nominal dose. It is interesting to speculate as to the mechanisms of elimination that control these values.
Abbreviations
- ACI:
-
Andersen impactor
- AUC:
-
area under the curve
- BAV:
-
bioavailability
- C:
-
central
- DCU:
-
dose content uniformity
- GSD:
-
geometric standard deviation
- HPLC:
-
high pressure liquid chromatograph
- MMAD:
-
mass median aerodynamic diameter
- P:
-
peripheral
- P/C:
-
ratio, peripheral to central ratio
- PDS:
-
Nektar pulmonary delivery system
- PSD:
-
particle size distribution
- sCal:
-
salmon calcitonin
- SD:
-
standard deviation
- USP:
-
united States Pharmacopeia
- VMD:
-
volume median diameter
References
H. Folkesson, B. Westrom, and B. Karlsson. Permeability of the respiratory tract to different sized macromolecules after intratracheal instillation in young and adult rats. Acta Physiol. Scand 139:347–354 (1990).
R. Niven, F. Lott, A. Ip, and J. Cribbs. Pulmonary delivery of powders and solutions containing recombinant human granulocyte colony-stimulating factor (rhG-CSF) to the rabbit. Pharm. Res 11(8):1101–1109 (1994).
S. Kobayashi, S. Kondo, and K. Juni. Pulmonary delivery of salmon calcitonin dry powders containing absorption enhancers in rats. Pharm. Res 13(1):80–83 (1996).
S. Kobayashi, S. Kondo, and K. Juni. Study on pulmonary delivery of salmon calcitonin in rats: effects of protease inhibitors and absorption enhancers. Pharm. Res 11(9):1239–1243 (1994).
T. Morita, A. Yamamoto, Y. Takaura, M. Hashida, and H. Sezaki. Improvement of pulmonary absorption of (Asu 1,7)—eel calcitonin by various protease inhibitors in rats. Pharm. Res 11(6):909–913 (1994).
S. White, D. B. Bennett, S. Cheu, S. P. Conley, D. B. Guzek, S. Gray, J. Howard, R. Malcolmson, J. M. Parker, P. Roberts, N. Sadrzadeh, J. D. Schumacher, S. Seshadri, G. W. Sluggett, C. L. Stevenson, and N. J. Harper. Exubera: pharmaceutical development of a novel product for pulmonary delivery of insulin. Diabetes Technol. Ther 7:896–906 (2005).
J. Patton. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev 19:3–36 (1996).
J. D. Burr, J. M. Anthony, G. S. Axford, J. W. Etter, and A. E. Smith. Apparatus and methods for dispersing dry powder medicaments. US Patent 6(089):228 (2000).
A. R. Clark, C. B. Chambers, D. Muir, M. T. Newhouse, S. Paboojian, and C. Schuler. The effect of biphasic flow profiles on the deposition and clearance of coarse (6.5 mm) bolus aerosols. J. Aerosol. Med 20(1):75–82 (2007).
E. Berg, J. Svensson, and L. Asking. Determination of nebulizer droplet size distribution: A method based on impactor refrigeration. J. Aerosol. Med 20(2):97–104 (2007).
A. R. Clark. The use of laser diffraction for the evaluation of the aerosol clouds generated by medical nebulizers. Int. J. Pharm 115:69–78 (1995).
USP/NF, Physical tests and determinations: Aerosols, Vol 20 US XVII. US Pharmacopeia, Rockville, MD: US Pharmacopeial conventions, Inc. 1992, pp. 3158–3178
G. R. Pitcairn, and S. P. Newman. Radiolabelling of dry powder formulations. In R. N. Dalby, P. R. Byron, and S. J. Farr (eds.), Respiratory drug delivery VI, Interpharm Press, Buffalo Grove, 1998, pp. 397–399.
M. T. Newhouse, P. Hirst, S. Duddu, Y. Walter, T. Tarara, A. Clark, and J. Weers. Inhalation of a dry powder tobramycin Pulmosphere formulation in healthy volunteers. Chest 124:360–366 (2003).
S. P. Newman, A. R. Clark, N. Talaee, and S. W. Clarke. Pressurised aerosol deposition in the human lung with and without an “open” spacer. Thorax 44:706–710 (1989).
G. R. Pitcairn, and S. P. Newman. Tissue attenuation corrections in gamma scintigraphy. J. Aerosol. Med 10:187–198 (1997).
S. P. Newman, P. R. Hirst, G. R. Pitcairn, and A. R. Clark. Understanding regional lung deposition in gamma scintigraphy. In R. N. Dalby, P. R. Byron, and S. J. Farr (eds.), Respiratory Drug Delivery VI, Interpharm Press, Buffalo Grove, 1998, pp. 9–15.
F. Komada, S. Iwakawa, N. Yamamoto, H. Sakakibara, and K. Okumura. Intratracheal delivery of peptide and protein agents: absorption from solution and dry powder by rat lung. J. Pharm. Sci 83(6):863–867 (1994).
L. Heinemann, and T. Heise. Current status of the development of inhaled insulin. Br. J. Diab. Vac. Dis 4(5):295–301 (2004).
K. Okumura, S. Iwaka, Y. Tsuguchika, S. Toshimitsu, and F. Komada. Intratracheal delivery of insulin absorption from solution an aerosol by rat. Int. J. Pharm 88:63–73 (1992).
S. J. Farr, I. Gonda, and V. Licko. Physicochemical and physiological factors influence the effectiveness of inhaled insulin. In R. N. Dalby, P. R. Byron, and S. J. Farr (eds.), Respiratory Drug Delivery VI, Interpharm Press, Buffalo Grove, 1998, pp. 25–33.
J. S. Patton, J. G. Bukar, and M. A. Eldon. Clinical pharmacokinetics and pharmacokinetics of inhaled insulin. Clin. Pharmacokinet 43(12):781–801 (2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, A., Kuo, Mc., Newman, S. et al. A Comparison of the Pulmonary Bioavailability of Powder and Liquid Aerosol Formulations of Salmon Calcitonin. Pharm Res 25, 1583–1590 (2008). https://doi.org/10.1007/s11095-008-9547-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9547-x