Abstract
Purpose
To investigate the anomalous phenomenon of particle size shift during post-milling storage.
Materials and Methods
Crystallised and ball-milled adipic acid were stored under different humidity conditions. Analyses were carried out to characterise changes in particle size distribution (laser diffraction), morphology (SEM), bulk flow properties (annular shear tester), surface adhesion forces (AFM) and crystallinity (PXRD and DVS).
Results
It was observed that the particle size distribution of milled adipic acid can shift to finer fractions, remain unchanged, or even shift to coarser fractions depending on storage conditions. SEM analysis showed that milled adipic acid is composed of agglomerates, which can undergo de-aggregation or further agglomeration via re-crystallisation. Empirical analysis ruled out the effects of electrostatic charges on the particle size shift. In addition, an improvement in powder flow in terms of bulk tensile strength was seen for milled adipic acid stored under high relative humidity but not under low humidity.
Conclusions
Storage of milled adipic acid below the critical relative humidity led to localised disintegration from the agglomerate surface and particle size reduction, which was not influenced by moisture sorption or loss. This evidence supports that “stress relaxation” mechanism behind particle breakage of post-milled particles. Appropriate storage conditions are important in maintaining the stability of milled powders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Although milling or particle size reduction processes form an integral part of pharmaceutical secondary manufacturing, problems are still frequently encountered in controlling consistent particle size during post-milling storage. The milled powders tend to undergo an undesired material-dependent particle size enlargement or reduction during storage. Controlling the particle size is crucial to the formulability and the bioavailability of a drug product. It governs for instance, dissolution rates of oral dosage forms, sedimentation and flocculation rates in suspensions, good powder homogeneity in tablets and therapeutic effect of dry powder inhalations. A reduced particle size can, in some cases, even double the bioavailability of a product (1). Presently, a “black-box” solution has often been adopted by simply storing the milled material until no further changes in particle size take place. The storage period is material-dependent and needs to be empirically determined for every new material, which can take weeks or even months. Though such a practice is both scientifically and economically unsatisfactory, it still forms an important part of the manufacturing process as there is insufficient understanding to develop a good scientific solution.
There are conflicting literature reports on the effect of storage on particle size shift of post-milled powders. Particle growth was reported for milled revatropate hydrobromide (2) and salbutamol sulfate (3–5) while particle size reduction was observed for micronised budesonide (6) and also recently, for a commercial inhalant active by the authors. Pfeiffer et al. (4,5) proposed that the particle size enlargement of salbutamol sulfate resulted from the moisture sorption-mediated re-crystallisation of amorphous surfaces leading to agglomeration. These amorphous regions exist as disorders within the crystalline structure, which are created by the mechanical and thermal stresses during milling (1,7–9). SEM analyses provided supporting evidence of agglomeration showing that particle growth was accompanied by the formation of new inter-particle bridges (2–3). In contrast, to account for the reported particle size reduction, Joshi et al. (6) postulated that internal stresses were created and stored inside the particles during the milling process. Subsequent relaxation of these stresses during storage led to crack formation, propagation and particle breakage. No further work to understand the breakage pattern or the effect of storage conditions has been reported. Two main patterns of breakage of agglomerates have been described by (10). The first type of breakage termed as localised disintegration occurs when an agglomerate suffer only localised damage, with the disintegration of the damaged zone into very fine debris. In the second type termed as fragmentation, large cracks are propagated into the body of the agglomerate, which give rise to two or more large fragments. Fragmentation may be accompanied by localised disintegration.
The aim of this paper is to investigate the anomalous phenomenon of particle size shift during post-milling storage. An experimental study was conducted to study the effect of storage conditions in terms of relative humidity and temperature on the particle size distribution (PSD) of milled adipic acid. Accompanying changes in particle morphology were observed under SEM to provide further understanding how particle agglomeration and breakage occur. To analyse the particle size reduction process, the magnitude of binding forces within the particles was theoretically calculated, so as to better understand the nature of bonds being broken during storage. Besides PSD, two other key particle characteristics, bulk powder flow and surface adhesion forces, were also studied as a function of storage humidity conditions. It is hoped that providing an in-depth understanding behind the particle size shift phenomenon could encourage new solutions to be devised, in order to either avoid the particle size shift or to accelerate the completion of the undesired process.
Adipic acid (C6H10O4) is selected because it was reported as a single polymorph (11) and is a common pharmaceutical excipient, which is used as an acidulent in effervescent tablets or lubricant in tablets. Being a single polymorph, the need to consider possible influence of polymorphic transformation on particle size shift can be avoided. Adipic acid possesses another advantage in having a high critical relative humidity (CRH) or hygroscopic point of 85% RH at 27 °C. The influence of moisture adsorption/desorption on particle size shift can be investigated below the CRH without the need to consider other influences like surface wetting, caking and agglomeration. Ball-milling, which is the most commonly used type of tumbling mill in pharmacy and most often used at the preformulation stage, is selected as the process for particle size reduction (12).
MATERIALS AND METHODS
Materials
Crystallisation of Adipic Acid
Crystallised adipic acid is used both as the starting material for milling and also as an “unmilled” control. To prepare crystallised adipic acid, 99% pure adipic acid purchased from Sigma-Aldrich was re-crystallised in ultra-pure water using a batch cooling crystallisation method (13). The crystals were vacuum filtered, subsequently dried for 24 h in oven at a temperature of 55 °C and then stored for 24 h in a dessicator using phosphorus pentoxide. The relative humidity was kept at between 20 and 25% RH and monitored using a thermohygrometer.
Milling of Adipic Acid
A planetary ball mill (Fritsch Pulverisette 5, Germany) with stainless steel jar and balls (diameter 10 mm) was used to mill crystallised adipic acid. The mass ratio of ball to sample was kept at 50:1. The rotation speed was set at 400 rpm and a milling duration of 30 min was used for all samples.
Methods
Sieving
To obtain uniform particle size for storage experiments, crystallised and milled adipic acid were sieved using a sonic sifter (L3P Sonic Sifter Separator, USA). The sieving conditions were set at amplitude of 6, duration of 2 min and in sift-pulse mode. The sieve fraction between 250 and 500 μm was used in all experiments.
Sample Storage
Crystallised and milled adipic acid were placed in separate desiccators at room temperature of approximately 22 °C for 7 days. Storage humidity conditions of 25, 34, 58 and 90% were prepared using phosphorus pentoxide or standard hygrostatic salt solutions (14). Storage at 98% RH and 35 °C was used to investigate if substantial changes in the physicochemical properties would occur above the CRH. The samples were also kept heated at 110 °C (m.p. ~152 °C) inside an oven to check for any changes at a temperature about 70% of the melting point.
Particle Size Analysis
The particle size distribution was measured using laser diffraction (Malvern Mastersizer MS-2000, UK) with n-hexane as the wet dispersion medium and at a pump rate of 800 rpm. Measurements were done in triplicate.
Scanning Electron Microscopy
The particle morphology was examined by high resolution scanning electron microscopy (SEM, JSM-6700F, JEOL, Japan) operating at 2 kV. Prior analysis, the samples were sputter coated with platinum for 1 min by a sputter coater (Cressington Sputter Coater 208HR, UK).
Electrostatic Measurement
The electrostatic charges were measured using a Faraday cage (Model TR8031) attached to an electrometer (Keithley 6517A, USA). The electrometer was used to measure the charge in the pail and all tests took place in a low relative humidity (<20%) environment.
Dynamic Vapour Sorption
Sorption/desorption isotherms of crystallised and milled adipic acid were measured using dynamic vapour sorption (DVS) (Advantage, Surface Measurement Systems, UK). The humidity range was varied from 0 to 90% RH in steps of 10% RH at 22 °C. The instrument was run in dm/dt mode to decide when equilibrium was reached, with a dm/dt set at 0.002% per min within an interval of 5 min. All sample weights were approximately 20–25 mg. Microscopic images of samples were captured during DVS runs via an optical probe.
Karl Fischer Titration
The moisture content of the milled and crystallised adipic acid was measured using the Karl Fischer titration method without diaphragm (Metrohm 831 KF Coulometer, Switzerland). The reagent used was Hydranal-Coulomat AG. The sample mass for each run used was 0.1 g. Measurements were done in triplicate.
Jenike Shear Tester
Bulk flow properties in form of yield loci were measured using the annular shear tester based on the Jenike method (Dietmar-Schulze RST-XS, Germany). A standard shear cell with cross-sectional area of 24 cm2 and volume of 30 cm3 was used. The normal load at pre-shear was set at 3,000 Pa with the lowest shear point of 10%. The number of stress levels used was 5. As each test required significant quantity of sample, the test was only conducted on crystallised and milled samples prior storage and those stored at 25 and 75% RH.
Atomic Force Microscopy
Normal surface adhesion forces were measured using the Multimode AFM (Digital Instruments, Veeco, USA) with its Nanoscope IV SPM controller and E-type scanner. All measurements were recorded in air at ambient conditions (19 °C, 63–65% RH) using the contact mode AFM. A rectangular beam silicon nitride cantilever with a nominal spring constant of 0.39 N/m was cleaned with acetone solution before analysis. Its normal deflection sensitivity was calibrated and the normal adhesion force was measured with the bare cantilever tip (tip radius <20 nm). The surface force measurements were only done on the same samples that were subject to annular shear test in order to compare the results with their bulk flow properties.
X-ray Powder Diffraction
The X-ray diffraction patterns of crystallised and milled adipic acid were measured with an X-Ray powder diffractometer (D8 Advance, Bruker AXS GmbH, Germany). Measurements were performed with CuKα radiation over the range of 5 to 50° (2θ) with steps of 0.04° (t = 1 s/step). Crystal structures of the samples were verified by comparing to the standard reported in the Cambridge Structural Database.
Differential Scanning Calorimetry
Thermal analyses of the samples were determined using the Perkin-Elmer Diamond Series DSC (Perkin-Elmer Ltd., USA). Samples between 2 to 3 mg were sealed hermetically in aluminium pans and heated at ramp rates of 10 °C/min and 50 °C/min under dry nitrogen flow of 20 ml/min over a temperature range of 25–250 °C.
RESULTS AND DISCUSSION
Influence of Storage Conditions on PSD and Morphology
The particle size distribution (PSD) of milled adipic acid stored for 1 week under varying relative humidity and temperature conditions were measured using laser diffraction and plotted in Fig. 1. Figure 1(a) showed that the PSD before storage has a major primary peak at around 600 μm with only a very small secondary peak at 90 μm. After storage ranging from 25 to 90% RH and 22 °C, the particle size reduction resulted in a more obvious bimodal PSD, which is composed of a secondary peak in the finer fractions at around 50–60 μm together with intensity decrease and a shift of the primary peak from 600 to 500 μm. Storage at 98% RH and 35 °C resulted in no significant changes in both peak positions of the PSD as compared to that before storage. In contrast, after storage at 110 °C, the particle size enlargement resulted in a shift of the primary peak from 700 to 1,100 μm without a significant change in intensity of the secondary peak. In Fig. 1(b), a reduction in the particle size was observed after storage at conditions of 25–90% RH and 22 °C. At 98% RH and 35 °C, there was a slight increase in the particle size. At 110 °C, the particle size above d40 was larger than that before storage. As a control, the PSD of crystallised adipic acid that were stored under different humidity conditions did not show any significant changes.
To investigate the particle size shift phenomenon, particle morphologies of pre- and post-storage adipic acid were analysed using SEM. Figure 2(a) shows that crystallised adipic acid consists initially of discrete particles. During ball-milling, these particles were broken up into finer fragments which assembled into agglomerates consisting of primary particles ranging from 1 to 10 μm in size as shown in Figs. 2(b) and 3(b). After storage at 110 °C, observations showed that some of the primary particles on the agglomerate surfaces appeared larger (Fig. 2(f)) and the particle edges became more clearly defined (Fig. 3(d)) as compared to the more rounded edges prior storage (Fig. 3(b)). The morphological change suggests the occurrence of re-crystallisation, which is consistent with previous reports showing that particle size enlargement resulted from re-crystallisation and agglomeration during storage (2–5). Storing adipic acid at 110 °C, which has a melting point of 152 °C, probably led to surface softening and wetting, plasticisation, followed by the formation of solid bridges and larger agglomerates (15). Similarly, storage above CRH at 98% RH and 35 °C seems to bring about some morphological changes as shown in Fig. 3(c) as compared to that before storage (Fig. 3(b)). This might also be caused by surface wetting and re-crystallisation of the adipic acid particles above the CRH. However, the change is less apparent as compared to (Fig. 3(d)).
No change in surface morphology of milled particles was observed under storage conditions ranging from 25 to 90% RH and 22 °C. From 25 to 90% RH at 22 °C, small clusters of primary particles (fine fractions) appeared to have broken off from the agglomerate surfaces and are dispersed around the larger and fairly regularly shaped agglomerates as shown in Fig. 2(c) and (d). This observation tallied with the first breakage pattern of agglomerates termed as localised disintegration as illustrated in Fig. 4 (10). If the second breakage type of fragmentation were to occur, median or oblique crack lines would occur deep inside the body of agglomerates and lead to the formation of irregularly shaped and larger secondary fragments. Such large fragments were neither observed under SEM nor detected in the PSD results.
Breakage patterns of milled agglomerates (a) localised disintegration (b) fragmentation (10).
During the breakage process, the inter-particle bonding between primary particles needs to be overcome. Possible binding mechanisms between the primary particles are attraction forces between solids (electrostatic, Van-der-Waals forces), interlocking bonds and solid bridges. Magnetic or liquid-mediated adhesion forces and capillary pressure can be excluded in the absence of ferromagnetic materials and liquids. It is reported that at extremely small distances between individual particles, electrostatic forces can be very high and the tendency to agglomerate increases as the powders become finer (16). From electrostatic measurements, the crystallised and milled adipic acid are found to have charges of 1.523 × 10−9 C/g and 1.315 × 10−9 C/g, respectively. As compared to the reference values given in the British Standard 5958, adipic acid is less prone to electrostatic charging effects after milling (12). Based on the measured results, the electrostatic attraction force, F ec was calculated using Eq. 1 and the measured true particle density of 1,337 kg/m3 (17). The results showed that the electrostatic charges alone were insufficient to overcome the weight of primary particles larger than 0.22 μm in size and cannot bind them together as agglomerates. The observed primary particle size under SEM ranged between 1 and 10 μm.
where σ 1 and σ 2 are surface charge densities of the particles, D p is the particle diameter, and ε o is the dielectric constant of the medium.
Long-range attractive forces usually associated with electrostatic attraction were not observed during AFM measurements, which further supported the theoretical analysis. The contribution of Van-der-Waals adhesion forces, F vdw, to the binding mechanism was estimated using the Hamaker microscopic and Lifshitz macroscopic models according to Eqs. 2 and 3:
where H is the Hamaker constant with a value of approximately 10−19 to 10−18 J, hω is the Lifshitz–Van-der-Waals constant with a value of approximately 1.6 × 10−20 to 1.6 × 10−18 J, and a is the inter-particle distance (16).
Calculated results revealed that Van-der-Waals forces are sufficient in overcoming the weight of up to 14 μm-sized particles and bind them together as agglomerates. While the contribution of interlocking bridges and solid bridges cannot be calculated directly like attraction forces, it has been reported that these bonds can be formed by either partial melting and/or recombination bonding (16). Heat generated during the milling process could have partially melted the particle surfaces leading to the formation of liquid bridges, which solidify quickly into solid bridges due to the large heat sink provided by the solid phase. Recombination bonding can also occur when large newly cleaved surfaces with unsatisfied valences combine with each other. Therefore, Van-der-Waals forces, interlocking, solid bridges or a combination of them are likely the binding mechanisms that need to be overcome during the particle size reduction process.
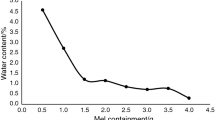
Moisture uptake or loss during storage has been shown to either weaken or strengthen such inter-particle bonding depending on the material type (18,19). The sorption isotherm of milled adipic acid was measured using DVS over the same humidity range of 0 to 90% RH, in which particle size reduction was observed. Figure 5 shows no significant moisture uptake or loss up to 60% RH followed by a sharp weight loss of approximately 1.6% from 60 to 90% RH. Even though the experimental run was carried out at relatively high humidity of 90% RH, the particle surface did not turn deliquescent because adipic acid is only slightly-hygroscopic (Class 2) and has critical relative humidity (CRH) of 85% RH at 27 °C (20). No surface wetting was seen using in-line microscopy during the DVS run. With such a high CRH, the influence of a wide range of storage humidity on the particle size reduction process can be studied without the need to consider the effect of surface wetting and agglomeration. Though there were clear differences in moisture uptake and loss behaviour at different relative humidity from 25 to 90% as shown in the DVS profiles, this moisture sorption or loss did not seem to affect the size reduction process. It is difficult to account for the sharp weight loss observed in the DVS isotherm even though such results usually imply plasticisation and re-crystallisation of amorphous regions. DVS isotherm of crystallised adipic acid also showed a similar weight loss at a smaller magnitude even though the crystallised sample was not expected to have significant amount of amorphous content. PXRD and DSC analyses were performed on crystallised and milled adipic acid as shown in Figs. 6 and 7. The absence of peak broadening in the PXRD diffractograms and glass transition in DSC profiles suggested that adipic acid retained significant crystallinity even after milling (21). Karl Fischer titration of crystallised and milled samples showed water contents of 0.5 wt.% and 0.1 wt.%, respectively. It may be possible that part of the weight loss during the DVS runs could have been contributed by the expulsion of such internal water.
Results so far showed that as long as milled adipic acid was stored below CRH or the hygroscopic point, moisture uptake or loss did not seem to be the underlying cause behind particle size reduction. This supports the hypothesis of stress relaxation proposed by Joshi et al. (6), who have attributed the particle size reduction of micronised budesonide to the self-relaxation of residual stresses stored in the form of cracks and other defects created during the milling process (22). SEM images showed that micronised budesonide were composed of agglomerates just like milled adipic acid (23) and may have undergone a similar breakage pattern. The hypothesis is further supported by Kendall’s theory on agglomerate breakage (24), which suggested that defects present in form of small cracks within the agglomerate structure increase the ease of particle breakage and size reduction. During milling process, as particle fracture occurs at high velocities in the order of 102 m/s (25), freshly cleaved and highly active surfaces of new primary particles are created and these particles agglomerate very quickly. The occurrence of particle breakage and fusion over such short durations could have left behind residual defects inside the inter-particle bridges.
Influence of Storage Conditions on Bulk Flow and Surface Adhesion Forces
The differences in bulk flow properties between milled and crystallised adipic acid prior and after storage at different humidity conditions were analysed using the annular shear test and plotted in the form of yield loci in Fig. 8. The yield loci of crystallised adipic acid particles were straight lines passing close to the origin with shear index of 1, which is typical of free-flowing powders. The yield loci of milled particles approached parabolic curves with shear indices between 1 and 2, typical of cohesive powders (17). Bulk flow property is interpreted in terms of bulk tensile strength, which is the interception of the extrapolated yield loci with the axis of normal stress and tabulated in Table I (26). Those for milled adipic acid before storage were in agreement with previously reported values (27). The significant difference in bulk tensile strength between crystallised and milled particles is expected because the rougher surfaces of milled particles (as shown by SEM) make them more cohesive and have a greater tendency to interlock than smooth surfaces (28). The higher tensile strength of milled particles can also be explained by an increase in cohesive forces due to more contact points or the average co-ordination number according to Rumpf theoretical model (17).
After storage at two humidity conditions of 25 and 75% RH and at 22 °C, there was no obvious change in yield loci or bulk tensile strength of crystallised adipic acid as shown in Fig. 8 and Table I. The flow properties of post-storage milled particles also remained fairly unchanged at 25% RH. However, at 75% RH, a sharp decrease in bulk tensile strength and the shifting of the yield locus towards the normal stress axis indicated a significant improvement in the flowability. This was unexpected because the PSD of post-storage milled adipic acid at 25 and 75% RH were both similarly shifted to finer fractions after storage. Moreover, finer particles should have larger contact surface areas, which lead to higher bulk tensile strengths and poorer bulk flow properties. Recent literature has shown that bulk tensile strength can be correlated with surface adhesion force at single particle level measured using AFM. Therefore, AFM measurements were taken to evaluate if changes in surface adhesion forces could explain the observed decrease in tensile strength after storage at 75% RH. Pull-off forces of crystallised and milled particles prior and after storage at 25 and 75% RH were measured and the results were tabulated in Table I. The low AFM pull-off force values for crystallised particles at ≤10.4 nN appeared to be associated with single asperity contacts. A four-ten fold increase in pull-off force after milling could be attributed to an increase in number or area of contacts due to the much rougher surfaces typical of cohesive powders (29). Though the pull-off forces of milled adipic acid decreased after storage at 75% RH, the value is still several times higher than that of crystallised adipic acid. Therefore, it is unlikely that the decrease in surface adhesion forces alone can explain the large decrease in bulk tensile strength. It is noted that the AFM results of milled adipic acid can only be interpreted qualitatively as the contact formed between AFM tip and particle surface is far from the idealised smooth contact in theory. Varying contact geometry and surface roughness between measurements may have rendered the results less consistent.
In addition, the differences between the average particle size distributions of milled adipic acid after storage at 75% RH and 25% RH were insignificant, and could not account for the better flowability at the higher humidity. Closer analysis of Fig. 1(a) shows a larger deviation in PSD measurements after storage at 75% RH than that at 25% RH. This showed that in some samples, the agglomerates stored at 75% RH have remained intact (unbroken). However, due to the relaxation of residual stress, the inter-particle bonds may have become weaker even though the agglomerates are intact. It is speculated that these weakened agglomerates could have broken up easily during the shear test leading to improved flowability as compared to the agglomerates stored at 25% RH, which have already broken up prior shear testing.
CONCLUSIONS
An experimental study was conducted to investigate the particle size shift phenomenon during storage of post-milled adipic acid. To the best of the authors’ knowledge, it was observed for the first time that the particle size of the same milled material can shift to finer fractions, remain unchanged or even to coarser fractions depending on the storage humidity and temperature conditions. In the case of particle size reduction, SEM analysis showed that the milled agglomerates exhibited a breakage pattern termed as localised disintegration whereby clusters of primary particles broke off from the outer surface of milled agglomerates. Empirical analysis showed that the inter-particle bonds, which were broken up, were not electrostatic or liquid bridging in nature but were likely contributed by Van-der-Waals attraction, inter-locking or solid bridges, or a combination of these bonds. The breakage process occurred regardless whether there was moisture uptake or loss as long as the storage humidity was kept below 90% RH at 22 °C (below the critical relative humidity). These findings support the “stress relaxation” mechanism first proposed by Joshi et al. (6), who suggested that particle breakage resulted from self-relaxation of residual stresses stored in the form of cracks and other defects created during the milling process. When storage conditions were kept just above CRH, the particle size remained relatively unchanged while storage at an elevated temperature of 110 °C resulted in re-crystallisation, agglomeration and particle size enlargement. Improvement in powder flow properties was also measured using annular shear test for milled adipic acid stored at 75% RH but not at 25% RH. The unexpected change in powder flow properties cannot be attributed to the decrease in AFM-measured surface adhesion forces or to the average particle size distribution alone. This gives rise to the possibility that weakened inter-particle bonds at 75% RH may account for the differences in flowability. The results suggested that appropriate storage conditions for milled materials is not only important for controlling the particle size distribution, but also for bulk powder flow, which are both crucial to good formulability and bioavailability of pharmaceutical products. However, the complex behaviour of the milled powders during storage is still not fully understood and this highlights the need for further research.
References
V. Chikhalia, R. T. Forbes, R. A. Storey, and M. Ticehurst. The effect of crystal morphology and mill type on milling induced crystal disorder. Eur. J. Pharm. Sci. 27:19–26 (2006).
M. D. Ticehurst, P. A. Basford, C. I. Dallman, T. M. Lukas, P. V. Marshall, G. Nichols, and D. Smith. Characterisation of the influence of micronisation on the crystallinity and physical stability of revatropate hydrobromide. Int. J. Pharm. 193:247–259 (2000).
G. H. Ward, and R. K. Shultz. Process-induced crystallinity changes in albuterol sulfate and its effect on powder physical stability. Pharm. Res. 12:773–779 (1995).
K. B. Pfeiffer, H. Haeusler, P. Grass, and P. Langguth. Influence on particle growth of salbutamol sulfate. Drug Dev. Ind. Pharm. 29:1077–1084 (2003).
K. B. Pfeiffer, P. Langguth, P. Grass, and H. Haeusler. Influence of mechanical activation on the physical stability of salbutamol sulphate. Eur. J. Pharm. Biopharm. 56:393–400 (2003).
V. Joshi, D. Sarvajna, and G. H. Ward. Increase in the specific surface area of budesonide during storage postmicronization. Pharm. Res. 19:7–12 (2002).
L. Mackin, S. Sartnurak, I. Thomas, and S. Moore. The impact of low levels of amorphous material (<5%) on the blending characteristics of a direct compression formulation. Int. J. Pharm. 231:213–226 (2002).
L. Mackin, R. Zanon, J. M. Park, K. Foster, H. Opalenik, and M. Demonte. Quantification of low levels (<10%) of amorphous content in micronised active batches using dynamic vapour sorption and isothermal microcalorimetry. Int. J. Pharm. 231:227–236 (2002).
P. Begat, P. M. Young, S. Edge, J. S. Kaerger, and R. Price. The effect of mechanical processing on surface stability of pharmaceutical powders: visualization by atomic force microscopy. J. Pharm. Sci. 92:611–619 (2003).
J. Subero, and M. Ghadiri. Breakage patterns of agglomerates. Powder Technol. 120:232–243 (2001).
K. Y. Chow, J. Go, M. Mehdizadeh, and D. J. W. Grant. Modification of adipic acid crystals: influence of growth in the presence of fatty acid additives on crystal properties. Int. J. Pharm. 20:3–24 (1984).
G. Steele. Preformulation as an aid to product design in early drug development. In M. Gibson (ed.), Pharmaceutical Preformulation and Formulation: A Practical Guide from Candidate Drug Selection to Commercial Dosage Form, Interpharm/CRC, Boca Raton, 2004, pp. 175–289.
T. R. Keel, C. Thompson, M. C. Davies, S. J. B. Tendler, and C. J. Roberts. AFM studies of the crystallization and habit modification of an excipient material, adipic acid. Int. J. Pharm 280:185–198 (2004).
Organisation Internationale de Métrologie Légale (OIML). The scale of relative humidity of air certified against saturated salt solutions. OIML: R121 (1996).
M. Mathlouthi, and B. Roge. Water vapour sorption isotherms and the caking of food powders. Food Chem. 82:61–71 (2003).
W. Pietsch. Agglomeration Process: Phenomena, Technologies, Equipment. Wiley-VCH, Weinheim Germany, 2002, pp. 35–59.
M. Suzuki. Powder mechanics. In K. Gotoh, H. Masuda, and K. Higashitani (eds.), Powder Technology Handbook, Marcel Dekker, New York, 1997, pp. 361–369.
R. Boerefin, Z. Ning, and M. Ghadiri. Disintegration of weak lactose agglomerates for inhalation applications. Int. J. Pharm. 172:199–209 (1998).
A. Samimi, R. Ghadiri, R. Boerefin, A. Groot, and R. Kohlus. Effect of structural characteristics on impact breakage of agglomerates. Powder Technol. 130:428–435 (2003).
K. Umprayn, and R. W. Mendes. Hygroscopicity and moisture adsorption-kinetics of pharmaceutical solids: a review. Drug Dev. Ind. Pharm. 13:653–693 (1987).
A. S. Gerhardt, C. Ahlneck, and G. Zografi. Assessment of disorder in crystalline solids. Int. J. Pharm. 101:237–247 (1994).
R. Huettenrauch, S. Fricke, and P. Zielke. Mechanical activation of pharmaceutical systems. Pharm. Res. 2:302–306 (1985).
T. E. Tarara, M. S. Hartman, H. Gill, A. A. Kennedy, and J. G. Weers. Characterization of suspension-based metered dose inhaler formulations composed of spray dried budesonide microcrystals dispersed in HFA-134a. Pharm. Res. 21:1607–1614 (2004).
J. R. Coury, and M. L. Aguiar. Rupture of dry agglomerates. Powder Technol 85:37–43 (1995).
R. Weichert. Anwendung von Fehlstellenstatistik und Bruchmechanik zur Bescreibung von Zerkleinerungsvorgaengen. Zement-Kalk-Gips 45:1–8 (1992).
F. M. Thomson. Storage of particulate solids. In M. E. Fayed, and L. Otten (eds.), Handbook of Powder Science, Chapman & Hall, New York, 1997, pp. 416–427.
H. Pasley, P. Haloulos, and S. Ledig. Stickiness—a comparison of test methods and characterization parameters. Dry. Technol. 13:1587–1601 (1995).
J. Staniforth. Powder flow. In A. E. Aulton (ed.), Pharmaceutics: The Science of Dosage Form Design, Churchhill Livingstone, Spain, 2001, pp. 197–210.
R. Jones. From single particle AFM studies of adhesion and friction to bulk flow: forging the links. Granul. Matter 4:191–204 (2003).
Acknowledgements
The authors would like to thank Junwei, Chin Lee, Ron Lim, Ai Tee, Prashant, Lay Yong, Gary Liu and Wenyi for their contribution in particle characterisation and Aaron Yuen for assisting in theoretical calculations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ng, W.K., Kwek, J.W. & Tan, R.B.H. Anomalous Particle Size Shift During Post-milling Storage. Pharm Res 25, 1175–1185 (2008). https://doi.org/10.1007/s11095-007-9497-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9497-8