Abstract
Purpose
The antitumor effect of paclitaxel-loaded PEGylated immunoliposome (PILs) was investigated in breast cancer cell lines and the xenograft model.
Methods
Herceptin was conjugated to paclitaxel-loaded PEGylated liposomes (PLs). In vitro cellular uptake and cytotoxicity of PILs were determined in breast cancer cell lines while in vivo antitumor efficacy was evaluated in the xenograft nude mouse model.
Results
The PILs formulation was able to significantly increase the HER2 mediated cellular uptake of paclitaxel compared to the PLs in cell lines overexpressing HER2 (BT-474 and SK-BR-3 cells). However, in the MDA-MB-231 cells, which express low levels of HER2, the difference between the PILs and PLs formulation was not significant. The biological activity of Herceptin was maintained throughout the conjugation process as exhibited by the antitumor dose–response curves determined for Herceptin itself, for the thiolated Herceptin alone and subsequently for the immunoliposome-coupled Herceptin. In BT-474 and SK-BR-3 cells, the cytotoxicity of the PILs was more potent than that of Taxol. Moreover, in in vivo studies, PILs showed significantly higher tumor tissue distribution of paclitaxel in the BT-474 xenograft model and more superior antitumor efficacy compared to Taxol and PLs. However, in the MDA-MB-231 xenograft model, PILs and PLs showed similar tumor tissue distribution as well as antitumor activity.
Conclusions
These results suggest that HER2-mediated endocytosis is involved in the PILs formulation. The ability of the PILs formulation to efficiently and specifically deliver paclitaxel to the HER2-overexpressing cancer cells implies that it is a promising strategy for tumor-specific therapy for HER2-overexpressing breast cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Human epidermal growth factor receptor-2 (HER2) is a 185-kDa transmembrane receptor tyrosine kinase that belongs to the epidermal growth factor receptor family (1, 2). HER2 overexpression in human breast cancer cells increases their intrinsic metastatic potential (3). HER2 is overexpressed in about 30% of tumors in patients with breast carcinoma, and HER2 overexpression correlates with reduced survival and a shorter time to disease progression in these patients (4,5). HER2-targeted therapy is promising because it targets a highly specific receptor protein for breast cancer growth. Moreover, HER2-overexpressing cancer cells express substantially more extracellular HER2 than do normal host cells, which allows selectivity for tumor cells (6).
Herceptin (Genentech, South San Francisco, CA), a recombinant humanized monoclonal antibody directed against the extracellular domain of the HER2 protein (7), is the only HER2-targeted therapeutic approved by the US Food and Drug Administration for the treatment of metastatic breast cancer. Herceptin has antitumor activity as a single agent (8,9), but it is most efficacious when combined with chemotherapy (10).
Paclitaxel, a taxane widely used in chemotherapy, is highly efficacious in the treatment of breast cancer, ovarian carcinoma, head and neck cancers, and nonsmall-cell lung cancer (11,12). By stabilizing the microtubule network and inhibiting microtubule dynamics in tumor cells (13,14), paclitaxel generally causes programmed cell death (apoptosis) after cell cycle arrest at the metaphase-anaphase transition (15). Pegram et al. have reported that Herceptin enhances the tumoricidal effects of paclitaxel against HER2-overexpressing tumors in athymic mice (16). This drug combination also elicits a better response rate and a longer time to disease progression in patients with HER2-overexpressing tumors than does paclitaxel alone (17).
Immunoliposomes are liposomes designed to actively target solid tumors by virtue of the monoclonal or polyclonal antibodies attached to their surface (18–20). Immunoliposomes have the potential to transfer large numbers of drug molecules to tumor cells, and drugs delivered via immunoliposomes have antitumor activities similar to or greater than those of the drug alone (21). The ability of immunoliposomes to target tumor cells overcomes many limitations of conventional liposomes and provides a novel strategy for tumor-targeted drug delivery (22). Anti-HER2 PEGylated immunoliposomes (PILs), which are made by coupling Herceptin to liposomes sterically stabilized with PEG, have yielded promising results for the treatment of HER2-overexpressing breast cancers (23). Specifically, the PEGylation can increase the circulation time of the encapsulated drug in the blood, and the Herceptin conjugation can increase the therapeutic index of an encapsulated drug by promoting selective delivery to HER2-overexpressing cells (24).
To combine the tumor-targeting properties of Herceptin with the drug delivery properties of PEGylated liposomes (PLs), we previously developed a paclitaxel-loaded PIL formulation that retained Herceptin integrity and exhibited a significantly longer half-life than that of PLs and Taxol (25). In the present study, we performed a systematic evaluation of the antitumor activity and mechanism of these paclitaxel-loaded PILs by performing an in vitro cellular uptake study in various breast cancer cells and an in vivo study in xenograft nude mouse models.
MATERIALS AND METHODS
Materials
Paclitaxel was purchased from Taihua Corporation (Xi’an, China). Soybean phosphatidylcholine (S100PC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine [methoxy(polyethyleneglycol)-2000] (MPEG2000-DSPE) were generous gifts of Lipoid GmbH (Ludwigshafen, Germany). Maleimide-derivatized PEG2000-DSPE (Mal-PEG2000-DSPE) and rhodamine-labeled phosphatidylethanolamine (Rh-PE) were purchased from Avanti Polar Lipids (Alabaster, AL), and cholesterol (CHOL) was purchased from Tokyo Kasei (Tokyo Kasei Kogyo Co., Ltd, Tokyo, Japan). The clinically marketed anti-HER2 monoclonal antibody (Herceptin) was a generous gift from Genentech (South San Francisco, CA). 3-(4,5-dimethyltiazol-2-ly)-2,5-diphenyl-tetrazolium bromide (MTT), 2-iminothiolane (Traut’s Reagent), and Sepharose CL-4B were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). Sephadex G-25 was purchased from Amersham Biosciences (Quebec, Canada), and Dulbecco’s Modified Eagle Medium (DMEM), Minimum Essential Medium (MEM), penicillin-streptomycin, fetal bovine serum (FBS), and TrypLE™ Express were obtained from Invitrogen (Burlington, Ontario, Canada). Hybri-Care culture medium was from the American Type Culture Collection (ATCC; Rockville, MD). All other chemicals were of analytical grade or higher.
Cell Lines
The human breast cancer cell lines SK-BR-3 and BT-474, which express high levels of HER2, and the human breast cancer cell line MDA-MB-231, which expresses low levels of HER2, were purchased from ATCC. BT-474 cells were cultured in Hybri-Care medium supplemented with 10% FBS and antibiotics (100 U/ml penicillin G and 0.1 mg/ml streptomycin), and SK-BR-3 cells were cultured in MEM supplemented with 10% FBS and antibiotics. MDA-MB-231 cells were cultured in DMEM with 10% FBS and antibiotics. All of the cells were cultured in incubators maintained at 37°C with 5% CO2 in a humidified atmosphere.
Preparation of PEGylated Liposomes (PLs) and PEGylated Immunoliposomes (PILs)
PEGylated liposomes (PLs) composed of S100PC:CHOL:mPEG2000-DSPE (90:10:5 molar % ratio) were prepared using the thin-film hydration method, as described previously (26), and were used as controls for comparison with PEGylated immunoliposomes (PILs). Briefly, paclitaxel, CHOL, and lipids (S100PC and mPEG2000-DSPE) were dissolved in chloroform and dried in a rotary evaporator to form a thin film. This film was resuspended in phosphate-buffered saline (PBS, pH 4.0) with 3% (v/v) Tween 80 until completely hydrated. The resulting liposomal dispersion was serially passed through an extruder (Northern Lipids, Inc, Canada). Free paclitaxel was removed from the liposome suspensions by centrifugation, and the liposome pellet was washed twice with PBS (pH 7.4). The pellet was then resuspended in distilled water containing sucrose to produce a final sugar:lipid molar ratio of 2.3:1 and freeze-dried. The final PL particles were stored in tight containers at 4°C.
PILs composed of S100PC:CHOL:MPEG2000-DSPE:Mal-PEG2000-DSPE (molar ratio 90:10:4:1) were prepared from PLs as previously described (27). To immobilize antibody on the PLs, Herceptin was treated with 2-iminothiolane at a 1:20 molar ratio in degassed HEPES-buffered saline (25 mM HEPES, 140 mM NaCl, pH 8.0). The mixture was allowed to react for 1 h at room temperature in the dark under an inert N2 atmosphere. After purification by gel chromatography on a Sephadex G-25 column (Amersham Biosciences, Quebec, Canada), the reduced Herceptin was incubated overnight at 4°C with Mal-PEG2000-DSPE at a protein:linker lipid molar ratio of 1:4. Finally, the PEG-DSPE-conjugated Herceptin was incubated overnight with PLs at 4°C to form PILs, and any extraliposomal molecules were removed using a Sepharose CL-4B column. The amounts of conjugated and free Herceptin were indirectly measured using a protein assay (BCA Protein Assay Reagent; Pierce, Rockford, IL) (28).
Cellular Uptake Study
Cellular uptake studies were performed as described previously, with slight modification (29). Briefly, the human breast cancer cell lines were seeded in 24-well flat-bottom tissue-culture plates at 1 × 106 cells/well and cultured for 24 h to permit the attachment of cells. Paclitaxel-loaded liposomes were diluted with culture media to produce various final concentrations of paclitaxel, added in triplicate to the wells, and incubated with the cells for 2 h at either 4 or 37°C. The cells were then washed twice with ice-cold PBS to remove unbound liposomes and collected using 0.25% TrypLE™ Express. The cell pellet was destroyed by addition of 0.1 ml of 10% sodium dodecyl sulfate after centrifugation. An equal volume of acetonitrile was added to the cell solution to precipitate the protein, and 20 μl of supernatant was removed for HPLC analysis (26) after centrifugation at 13,000 rpm for 5 min.
In Vitro Cytotoxicity of Liposomal Paclitaxel
The cytotoxicity of the PILs formulation was determined in three breast cancer cell lines (BT-474, SK-BR-3, and MDA-MB-231) using the MTT assay (30). For comparison, Taxol and PLs were also used. Briefly, cells were plated in 96-well flat-bottom tissue-culture plates at 2.0 × 104 cells/well and incubated at 37°C in a 5% CO2 incubator for 24 h, which provided sufficient time for cells to attach and resume growth. Liposomes were diluted with culture media to make various concentrations of paclitaxel and then 200 μl were added to triplicate wells. Control wells were treated with equivalent volumes of paclitaxel-free media. After 2 h, the supernatant was removed. The cells were washed twice with PBS, fresh medium was added, and the cells were incubated for another 24 h. MTT dye was added to each well at a final concentration of 0.5 mg/ml. After 4 h, the nonreduced MTT and medium were discarded. Each well was washed with 200 μl of PBS, and 200 μl of dimethylsulfoxide were added to dissolve the MTT formazan crystals. Plates were shaken for 20 min, and absorbance was read at 560 nm using a microplate reader (Molecular Devices Corporation, Sunnyvale, CA). The concentration of paclitaxel resulting in 50% growth inhibition (IC50) was determined graphically from the concentration-effect curve, in which the optical density of the control well was taken as 100% (31). To evaluate the effect of Herceptin on the cytotoxicity of PILs, Herceptin was added at a final concentration of 5 μg/ml following the protocol described above.
Antitumor Activity of Herceptin, Thiolated Herceptin, and Liposome-Conjugated Herceptin
The antitumor activity of Herceptin, thiolated Herceptin, and liposome-conjugated Herceptin was assessed in three different human breast cancer cell lines (BT-474, SK-BR-3, and MDA-MB-231) using MTT assays. Briefly, cells were plated in 96-well flat-bottom tissue-culture plates at 2.0 × 104 cells/well and incubated at 37°C for 24 h in a 5% CO2 incubator. Herceptin, thiolated Herceptin, and liposome-conjugated Herceptin were diluted with culture media, and added to the wells at final Herceptin concentrations of 1, 5, 10, 20, and 40 μg/ml. Control wells were treated with equivalent volumes of Herceptin-free media. After a 24 h incubation, cell viability was evaluated using the MTT assay.
Combined Effect of Herceptin and Liposomal Paclitaxel on Breast Cancer Cells
The combined effect of Herceptin and paclitaxel on MDA-MB-231, SK-BR-3, and BT-474 breast cancer cells was determined using the MTT assay following two different procedures. In the first procedure, the cells were treated with Herceptin (5 μg/ml) with or without paclitaxel (500 ng/ml) delivered either as Taxol or in liposomes and incubated for 2 h at 37°C. Then, the cells were washed twice with PBS, fresh medium was added, and the cells were incubated for another 24 h. The second procedure was identical to the first except that paclitaxel, when present, was at 200 ng/ml (as Taxol or in liposomes), and the incubation time was 24 h. Control cells were not treated with Herceptin or paclitaxel. After a predetermined incubation period, the cell viability associated with each treatment was evaluated using MTT assays, and the resulting values were compared with those of the control.
Tumor Tissue Distribution Study
The human breast cancer cell lines, BT-474 and MDA-MB-231, in their exponential growth were inoculated into 4-week-old (18–20 g) female BALB/C nu/nu athymic (nude) mice (Charles River, Korea). In brief, for the xenograft model expressing low levels of HER2, 100 μl of MDA-MB-231 cells (5 × 106 cells) were injected subcutaneously into the breast tissue of nude mice. For the HER2-overexpressing xenograft model, a 17-β-estradiol pellet (0.72 mg, 60-day release; Innovative Research of America, Sarasota, FL) was implanted subcutaneously on the back of each mouse 1 day before tumor inoculation (32). The following day, 100 μl of BT-474 cells (2 × 107 cells) were mixed with an equal volume of Matrigel (BD Biosciences, Bedford, MA) and injected subcutaneously into the breast tissue of each mouse. Animals were kept in a SPF facility and had free access to food and water. When the tumor volumes became 100–200 mm3 after 2–3 weeks of inoculation, PLs and PILs (7.5 mg/kg as a paclitaxel) were administered via tail vein. After 24 h of injection, blood samples were collected from the eyes of three mice in each group, after which the mice were sacrificed by cervical dislocation in order to obtain tumor tissue samples. The samples were washed twice with physiological solution (0.9% NaCl), weighed and stored at −20°C until analyzed by HPLC, as described previously (26).
In Vivo Antitumor Efficacy
Two human breast cancer xenograft models were created using BT-474 and MDA-MB-231 cells, as described above. PLs or PILs were administered intravenously three times to each xenograft model over a period of 8 days; they were injected into tail veins at days 0, 4, and 8 after implantation when the tumor volume was about 50 mm3. Injections of Taxol (diluted to 1.5 mg/ml with PBS, pH 7.4) or saline solution served as the positive and negative controls, respectively. The dose of paclitaxel in each administration was 7.5 mg/kg body weight for a total treatment dose of 22.5 mg/kg. The tumor volumes of the xenograft models were monitored twice a week for up to 60 days. The tumor volume was calculated as 0.4ab 2, where a and b were the largest and smallest diameters, respectively, that are known to correlate well with actual tumor weight (r = 0.980) (33). At the end of the study, all mice in each group were weighed and then sacrificed by cervical dislocation. The tumors were removed, washed twice with saline solution, wiped, and weighed. The tumor weight percent was calculated from the equation:
Statistical Analysis
Statistical comparisons were performed using a two-tailed Student’s t test. All experiments were performed at least three times. The data are shown as the mean ± standard deviation (S.D.).
RESULTS
Characterization of PEGylated Immunoliposomes (PILs)
Our previous study showed that the PILs were not significantly different in size from the PLs, despite the incorporation of Herceptin conjugate (25). The mean diameters of both types of liposome were <200 nm, and they also exhibited similar zeta potentials (−18.50 and −16.88 mV, respectively). The paclitaxel percentage content of the PILs powder (0.34 ± 0.01%, n = 3) was lower than that of PLs (1.08 ± 0.02%, n = 3) because the addition of Herceptin increased the total weight of the liposomes.
Cellular Uptake of Liposomal Paclitaxel
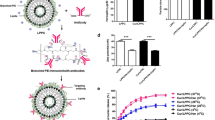
We previously reported that HER2 on the cell surface plays an important role in the cellular uptake of PILs by measuring the uptake of fluorescence-labeled empty PILs into BT-474, SK-BR-3, and MDA-MB-231 cells using confocal laser-scanning microscopy (25). We also observed the uptake of paclitaxel loaded PILs into the cells that express HER2 in a temperature dependent manner (25), suggesting HER2-mediated endocytosis as a possible mechanism. To further verify this mechanism, the concentration dependence of PILs on the HER2-mediated endocytosis was observed. The cellular uptake of paclitaxel was measured after the cells were incubated with liposomes containing various concentrations of paclitaxel for 2 h at 4 or 37°C. HER2-mediated endocytosis was calculated by subtracting the amount of uptake of paclitaxel at 4°C from that of paclitaxel at 37°C (Fig. 1) in order to exclude values corresponding to nonspecific adsorption to cells. In BT-474 and SK-BR-3 cells, HER2-mediated uptake of paclitaxel from PILs was significantly higher than that from PLs (Fig. 1a, b) and the HER2 mediated endocytosis was saturated when the concentration of paclitaxel in PILs exceeded 500 ng/ml (as paclitaxel). On the other hand, in MDA-MB-231 cells, PLs and PILs did not show significant differences in paclitaxel uptake (Fig. 1c).
HER2 mediated cellular uptake of paclitaxel in PEGylated liposomal (PLs; closed circle) and PEGylated immunoliposomal (PILs; open circle) formulations in BT-474 cells (a), SK-BR-3 cells (b), and MDA-MB-231 cells (c). Each point shown represents an average of three replicates ±S.D. of three independent experiments.
In Vitro Cytotoxicity of Liposomal Paclitaxel
In our previous studies, we found that the biological half-life of paclitaxel after intravenous injection was 1.65 h (26), and the maximum uptake of PILs into BT-474 and SK-BR-3 cells occurred after 2 h exposure (25). In the present study, we determined the in vitro IC50 values of the liposomes after 2 h exposure. As shown in Table I, the IC50 value of PILs in BT-474 cells (451.3 nM) was significantly lower than that of PLs (1645.9 nM), and even lower than that of Taxol (675.8 nM). A similar phenomenon was observed in SK-BR-3 cells (Table I). In MDA-MB-231 cells, on the other hand, the IC50 value of PILs (1743.8 nM) did not differ significantly from the IC50 value of PLs (1715.0 nM), and it was significantly higher than that of Taxol (507.1 nM; Table I).
Notably, the IC50 values of PILs in the HER2-overexpressing cancer cell lines (BT-474 and SK-BR-3) were significantly increased by the addition of 5 μg/ml Herceptin. This competitive inhibition implies that PILs and Herceptin share the same target (i.e., HER2); this conclusion is also supported by the fact that Herceptin did not exhibit an inhibitory effect in cancer cells expressing low levels of HER2 (MDA-MB-231).
Antitumor Activity of Herceptin, Thiolated Herceptin, and Liposome-Conjugated Herceptin
The PILs used in the present study were prepared by conjugating PEG to thiolated Herceptin. Since Herceptin itself is a well-known curative agent for breast cancer (8,9), it is possible that, despite its chemical modification, the PL-conjugated Herceptin has antitumor activity in addition to the ability to recognize HER2. Therefore, we examined the cytotoxicity of Herceptin, thiolated Herceptin, and non-paclitaxel-loaded PILs in BT-474, SK-BR-3, and MDA-MB-231 cells. The cytotoxic effect of Herceptin against BT-474 and SK-BR-3 cells was greatest at concentrations above 5 μg/ml. Similar patterns were found for thiolated Herceptin and for non-paclitaxel-loaded PILs (Fig. 2a, b). However, none of the three Herceptin formulations exhibited cytotoxicity toward MDA-MB-231 cells (Fig. 2c).
Antitumor activity of Herceptin (closed circle), thiolated Herceptin (open circle), liposome-conjugated Herceptin (closed triangle) against breast cancer cells after incubation for 24 h. a BT-474, b SK-BR-3, and c MDA-MB-231 cells were treated with varying concentrations of Herceptin. Cell survival was measured using an MTT assay and is expressed as a percentage of cell survival of control cells. Each experiment was performed at least in triplicate. The points shown represent the means of three experiments.
Combined Effect of Herceptin and Liposomal Paclitaxel on Breast Cancer Cells
To evaluate the potential of PILs for use in cancer chemotherapy, we examined the cytotoxicity of combination treatment with Herceptin and paclitaxel. Based on the IC50 values of Taxol after 2 h (Table I) and 24 h (26) exposures, the paclitaxel concentrations for the 2 and 24 h exposure experiments were set at 500 and 200 ng/ml, respectively. The Herceptin concentration in both experiments was fixed at 5 μg/ml based on the results shown in Fig. 2. As expected, Herceptin had no effect on MDA-MB-231 cells, regardless of the incubation time, and it was more cytotoxic in SK-BR-3 and BT-474 cells after 24 h than after 2 h (Fig. 3a, b). For 24 h exposure, the addition of Herceptin increased the cytotoxic effect of Taxol or PLs in cell lines overexpressing HER2 (Fig. 3b). In BT-474 cells, for example, the addition of Herceptin in Taxol changed cell viability from 52 to 36%, and that in PLs decreased it from 61 to 45%. These results confirm the rationale for combination treatment with Herceptin and chemotherapeutic agents in breast cancer chemotherapy. However, when the exposure was limited to 2 h, no significant effect of Herceptin on the cytotoxicity of Taxol or PLs was observed (Fig. 3a).
Effects of cotreatment with Herceptin and different paclitaxel formulations on BT-474 (shaded bar), SK-BR-3 (open bar) and MDA-MB-231 (striped bar) cell viability at 2 h (a) and 24 h (b). Her Herceptin; PL PEGylated liposome; PIL PEGylated immunoliposome. The data shown represent the mean ± S.D. for four independent experiments.
Notably, PILs were the most potent cytotoxic agents of those tested in BT-474 and SK-BR-3 cells, even after only a 2 h incubation (Fig. 3a). Moreover, after a 24 h incubation, cytotoxicity of PIL was significantly higher than that of Taxol, PLs, and PLs plus Herceptin, whereas it was comparable to that of Taxol plus Herceptin in both HER2-overexpressing cell lines (Fig. 3b).
Tissue Distribution Study
In vivo tumor tissue distribution of paclitaxel was evaluated after intravenous injection of each formulation, PLs and PILs, in breast carcinoma xenograft mouse model. In BT-474 cells, the PILs formulation showed a significantly greater paclitaxel concentration ratio of tumor to plasma (T/P ratio) compared to the PLs formulation. However, this HER2 selective tumor distribution was not observed in MDA-MB-231 cells (Fig. 4), which suggests that PILs were selectively delivered into the HER2 expressing tumor tissues via a HER2-mediated endocytosis.
T/P ratio (concentration ratio of tumor/plasma) of paclitaxel after 24 h of intravenous injection of PEGylated liposome (PLs; 7.5 mg/kg as paclitaxel) and PEGylated immunoliposome (PILs; 7.5 mg/kg as paclitaxel) in nude mice bearing BT-474 and MDA-MB-231 human breast cancer xenografts. Each data represents the mean ± standard deviation (n = 3). Asterisk p < 0.05 compared with MDA-MB-231. Pound sign p < 0.05 compared with PL.
In Vivo Antitumor Efficacy
The antitumor efficacy of PILs was evaluated in breast cancer xenograft models expressing high or low levels of HER2 (BT-474 and MDA-MB-231, respectively; Fig. 5). Because tumor volumes in the saline treatment group (control) increased rapidly, the experiments for this treatment group ended at 43 and 35 days, respectively, whereas those for the other treatment groups ended at 60 days. In the BT-474 xenograft model, the antitumor efficacy of PILs was superior to that of all other treatments (Fig. 5a, c). For example, tumor growth in the PILs group was inhibited to such an extent that, at 60 days, tumor volume in this group was only 25 and 42% that of the Taxol and PLs groups, respectively (Fig. 5a). Similar results for antitumor efficacy were obtained in terms of tumor weight percent (Fig. 5c). The pharmacodynamic data for saline, Taxol, and PLs treatment in the MDA-MB-231 xenograft model, which were obtained previously (25) are shown for comparison. As shown in Fig. 5b and d, PILs and PLs exhibited equivalent antitumor efficacies and were much more effective than saline or Taxol. These results might be explained by the increased local concentration of paclitaxel in the tumor tissue, since accumulation of PLs in tumors is favored by their long circulation time and by their enhanced permeability and retention (EPR) effect. Thus, the PILs appeared to retain the desirable effects of “PEGylation,” including long biological half-life and passive targeting, whereas also exhibiting the active targeting property conferred by the antibody. Taken together, our results show that the antitumor efficacy of PILs treatment is greatly superior to that of Taxol treatment in both the BT-474 and MDA-MB-231 xenograft models.
a, b Antitumor efficacy of different paclitaxel formulations in BT-474 (a) and MDA-MB-231 (b) human breast cancer xenograft models. c, d Ratio of tumor weight to total body weight in BT-474 (c) and MDA-MB-231 (d) human breast cancer xenograft models. In the saline group, monitoring was ended at 43 and 35 days for the BT-474 and MDA-MB-231 xenograft models, respectively, because of the great increase in tumor volumes; other groups were monitored for 60 days. Xenograft models (n = 5 or 6 for each group) were injected intravenously with Saline (closed circle), Taxol (open circle), PLs (closed triangle) or PILs (open triangle) at 0, 4, and 8 days (arrow; three injections total), when the tumor volume was about 50 mm3. The injected solutions of Taxol and liposomal paclitaxel were adjusted to 7.5 mg paclitaxel/kg for each administration. The zero time point indicates the initiation of therapy. Asterisk p < 0.05 compared with Taxol. Pound sign p < 0.05 compared with PL.
DISCUSSION
An ideal dosage form for therapeutic agent specifically delivers an appropriate dose of medication to the target action site over the required period of time. Liposomes have gained considerable attention as carriers for a wide range of drugs (34), largely because their biodegradability and structural flexibility allow easy manipulation of the in vivo fate of drugs by changing the composition of liposomes (35). Steric stabilization by PEGylation, a recent advance in liposome technology, greatly increases the circulation time of liposomes by increasing their resistance to clearance by the mononuclear phagocyte system, thereby facilitating selective extravasation in solid tumors (36). However, PLs do not directly enter tumor cells; rather, they accumulate within the tumor interstitium, from which the drug then passively diffuses into the tumor cells (37). To enhance the therapeutic efficacy of liposome-encapsulated drugs, we need to increase their uptake into, and intracellular retention by, tumor cells. In this context, anti-HER2 PILs constitute a promising strategy for tumor-targeted drug delivery, in that this formulation combines the tumor-targeting properties of Herceptin with the pharmacokinetic and drug-delivery properties of long-circulating liposomes.
We previously described the basic properties (conjugation, stability, in vitro release, and pharmacokinetics) of our paclitaxel-loaded anti-HER2 PILs formulation (25). We also have suggested that HER2 mediated endocytosis is involved in the cellular uptake of PILs in cells that express HER2 receptor, but not in cells without HER2 (25). In the present study, we observed that the HER2 mediated endocytosis of PILs was saturated at the paclitaxel concentration of over 500 ng/ml (Fig. 1) and was inhibited by free Herceptin in a competitive manner (Table I). These observations strongly suggest that HER2-mediated endocytosis is involved in the cellular uptake process of PILs. Moreover, the cytotoxicity of PILs was compared with that of Taxol and PLs in breast cancer cell lines with various HER2 expression levels. Drug-loaded PLs are generally less cytotoxic than free drug solutions because drug internalization is retarded by the steric effect of the PEG chains (38,39). When the exposure time was short (2 h), Taxol was the most cytotoxic of the formulations toward MDA-MB-231 cells (Table I). In the case with Taxol, it would be likely that paclitaxel would easily penetrate the cells, due to its lipophilicity. Although the PILs also exhibited a steric effect, caused by PEGylation, they were more cytotoxic than Taxol in HER2-overexpressing cell lines (BT-474 and SK-BR-3). These results might be explained by quick internalization of PILs by tumor cells through HER2-mediated endocytosis, which is consistent with the inhibition results of Herceptin cotreatment (Table I).
Herceptin is used in combination with Taxol to treat patients with metastatic breast cancer whose tumors overexpress HER2 and who have not received previous chemotherapy for their metastatic disease (40). The use of Herceptin in conjunction with chemotherapeutics is strongly supported by preclinical tumor xenograft studies showing improved treatment efficacy of chemotherapeutics such as cisplatin, doxorubicin, and paclitaxel, when used in combination with Herceptin (41,42). If Herceptin retains its tumoricidal activity in the PILs formulation, the rationale for use of PILs is improved. Thiolated Herceptin, liposome-conjugated Herceptin, and Herceptin exhibit similar cell viability profiles in all three cell lines (Fig. 2), suggesting that the PILs preparation process, namely thiolation of Herceptin and conjugation of thiolated Herceptin to PLs, does not greatly affect the tumoricidal activity of Herceptin. Therefore, we further evaluated the cytotoxicity of PILs compared with that of Herceptin combined with Taxol or PLs. Valeriote’s method was used to evaluate the combined effect (43, 44). As shown in Fig. 3b, we observed an additive effect for Herceptin treatment in combination with Taxol or PLs over a 24 h incubation period, which is consistent with results of a previous study by Baselga et al. (42), who reported that the effect of Herceptin was additive when used in conjunction with doxorubicin or Taxol. PILs proved to be more cytotoxic than Taxol, PLs, or Herceptin plus PLs, and were comparable in cytotoxicity to Taxol plus Herceptin after 24 h incubation (Fig. 3b).
Chemotherapeutic treatment of most tumors is hindered by several pharmacokinetic problems (45). Several chemotherapy drugs, including paclitaxel, docetaxel, doxorubicin, and daunorubicin, are rapidly eliminated from the systemic circulation, are not specific for tumor cells, and accumulate not only in tumors but also in healthy tissues. As a result, very high doses are required for sufficient antitumor efficacy, which are also related with side effects. Considering these facts, our present results suggested a promising rationale for using paclitaxel-loaded PEGylated immunoliposomes in breast cancer chemotherapy, in that this formulation retains both the desirable pharmacokinetic properties of long-circulating liposomes and the cytotoxic and tumor-targeting activities of Herceptin.
We attempted to confirm the in vivo antitumor efficacy of this approach using xenograft models. In a HER2-overexpressing (BT-474) xenograft model, PILs were significantly more effective against tumors than were any of the other treatments tested (Fig. 5a, c). In a xenograft model expressing low levels of HER2 (MDA-MB-231), the antitumor efficacy of PILs was equivalent to that of PLs and better than that of saline or Taxol (Fig. 5b, d). These results are consistent with the observation that the T/P ratio of paclitaxel was higher when administered as a PILs formulation compared to the PL formulation in BT474 cells (Fig. 4), suggesting a HER2 selective tumor tissue targeting of PILs. PLs were more effective than Taxol against tumors, irrespective of HER2 expression levels, possibly because of passive targeting via the EPR effect. It is important to note that in the BT-474 xenograft model, PILs were more potently tumoricidal than PLs, which was probably at least partially due to differences in their HER2 mediated intratumoral distribution and uptake mechanism.
Regarding the tumor targeting mechanism of immunoliposomes, it was reported that they are selectively transferred to tumor cells via a targeted receptor, are internalized by constitutive endocytosis after target cell binding, and then reach the endosome (46). This mechanism is also supported by the results in this study that PILs were uptaken into tumor cells via HER2 in a temperature and concentration dependent manner competitively in vitro. Thus, the PILs formulation is expected to deliver paclitaxel into tumor cells more effectively than the PLs, thereby resulting in more efficacious antitumor therapy. A more detailed mechanism study of the HER2 mediated endocytosis of PILs is under way.
CONCLUSIONS
The in vitro and in vivo drug delivery characteristics of a PEGylated immunoliposomal formulation of paclitaxel (PILs) have been investigated. The PILs were transferred to tumor cells via a HER2-mediated endocytosis in a HER2-specific manner and showed potent cytotoxicity against HER2 overexpressing cells (BT-474 and SK-BR-3) compared to that against HER2 low-expressing cells (MDA-MB-231). Studies in a BT-474 xenograft model showed that PILs exhibited a significantly greater antitumor effect than that of PLs or Taxol. Therefore, the efficient and specific delivery of paclitaxel by PILs to HER2-overexpressing cancer cells implies that this strategy is a promising approach for tumor-specific treatment in HER2-overexpressing breast cancers.
Abbreviations
- CHOL:
-
cholesterol
- DMEM:
-
Dulbecco’s modified eagle medium
- EPR:
-
enhanced permeability and retention
- FBS:
-
fetal bovine serum
- HER2:
-
human epidermal growth factor receptor-2
- MEM:
-
minimum essential medium
- MPEG2000-DSPE:
-
1,2-distearoyl-sn-glycero-3-phosphoethanolamine [methoxy(polyethyleneglycol)-2000]
- MTT:
-
3-(4,5-dimethylthiazol-2-ly)– 2,5-diphenyl-tetrazolium bromide
- PBS:
-
phosphate-buffered saline
- PEG:
-
polyethylene glycol
- PILs:
-
PEGylated immunoliposomes
- PLs:
-
PEGylated liposomes
- S100PC:
-
soybean phosphatidylcholine
- Rh-PE:
-
rhodamine labeled-phosphatidylethanolamine
References
T. Yamamoto, S. Ikawa, T. Akiyama, K. Semba, N. Nomura, N. Miyajima, T. Saito, and K. Toyoshima. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature 319:230–240 (1986).
C. I. Bargmann, M. C. Hung, and R. A. Weinberg. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 319:226–230 (1986).
M. Tan, J. Yao, and D. Yu. Overexpression of the c-erbB-2 gene enhanced intrinsic metastasispotential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 57:1199–1205 (1997).
G. N. Hortobagyi. Treatment of breast cancer. N. Engl. J. Med. 339:974–984 (1998).
D. J. Slamon, G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182 (1987).
M. Toi, K. Horiguchi, H. Bando, S. Saji, and L. W. C. Chow. Trastuzumab: updates and future issues. Cancer Chemother. Pharmacol. 56:s94–s99 (2005).
P. Carter, L. Presta, C. M. Gorman, J. B. Ridgway, D. Henner, and W. L. Wong. Humanization of an anti-p185her2 antibody for human cancer therapy. Proc. Natl. Acad. Sci. USA 89:4285–4289 (1992).
J. Baselga, D. Tripathy, J. Mendelsohn, S. Baughman, C. C. Benz, L. Dantis, N. T. Sklarin, A. D. Seidman, C. A. Hudis, J. Moore, P. P. Rosen, T. Twaddell, I. C. Henderson, and L. Norton. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 14:737–744 (1996).
M. A. Cobleigh, C. L. Vogel, D. Tripathy, N. J. Robert, S. Scholl, L. Fehrenbacher, J. M. Wolter, V. Paton, S. Shak, G. Lieberman, and D. J. Slamon. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in woman who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 17:2639–2648 (1999).
D. J. Slamon, B. Leyland-Jones, S. Shak, H. Fuchs, V. Paton, A. Bajamonde, T. Fleming, W. Eiermann, J. Wolter, M. Pegram, J. Baselga, and L. Norton. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cnacer that overexpresses HER2. N. Engl. J. Med. 344:783–792 (2001).
E. K. Rowinsky, and R. C. Donehower. Paclitaxel (Taxol). N. Engl. J. Med. 332:1004–1014 (1995).
T. M. Mekhail, and M. Markman. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 3:755–766 (2002).
A. M. Yvon, P. Wadsworth, and M. A. Jordan. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol. Bio. Cell 10:947–959 (1999).
A. Goncalves, D. Braguer, K. Kamath, L. Martello, C. Briand, S. Horwitz, L. Wilson, and M. A. Jordan. Resistance to Taxol in lung cancer cells associated with increased microtubule dynamics. Proc. Natl. Acad. Sci. USA 98:11737–11742 (2001).
M. A. Jordan, and L. Wilson. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 4:253–265 (2004).
M. D. Pegram, A. Lopez, G. Konecny, and D. J. Slamon. Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin. Oncol. 27(6 Suppl 11):21–25 (2000).
V. Dieras, P. Beuzeboc, V. Laurence, J. Y. Pierga, and P. Pouillart. Interaction between Herceptin and taxanes. Oncology 61(Suppl 2):43–49 (2001).
S. Suzuki, S. Watanabe, T. Masuko, and Y. Hashimoto. Preparation of long-circulating immunoliposomes containing adriamycin by a novel method to coat immunoliposomes with polyethylene glycol. Biochem. Biophys. Acta 1245:22–29 (1995).
S. M. Nam, H. S. Kim, W. S. Ahn, and Y. S. Park. Sterically stabilized anti-G(M3), anti-Le(x) immunoliposomes: targeting to B16BL6, HRT-18 cancer cells. Oncol. Res. 11:9–16 (1999).
G. Pagnan, P. G. Montaldo, F. Pastorino, L. Raffaghello, M. Kirchmeier, and T. M. Allen. GD2-mediated melanoma cell targeting and cytotoxicity of liposome-entrapped fenretinide. Int. J. Cancer 81:268–274 (1999).
K. R. Reddy. Controlled-release, pegylation, liposomal formulation: new mechanisms in the delivery of injectable drugs. Drug Deliv. 34:915–923 (2000).
J. W. Park, K. Hong, D. B. Kirpotin, D. Papahadjopoulos, and C. C. Benz. Immunoliposomes for cancer treatment. Adv. Pharmacol. 40:399–435 (1997).
J. S. Chen, K. L. Lan, and M. C. Hung. Strategies to target HER2/neu overexpression for cancer therapy. Drug Resist. Updat. 6:129–136 (2003).
J. W. Park, K. Hong, P. Carter, H. Asgari, L. Y. Guo, G. A. Keller, C. Wirth, R. Shalaby, C. Kotts, W. I. Wood, D. Papahadjopoulos, and C. C. Benz. Development of anti-p185HER2 immunoliposomes for cancer therapy. Proc. Natl. Acad. Sci. USA 92:1327–1331 (1995).
T. Yang, M. K. Choi, F. D. Cui, J. S. Kim, S. J. Chung, C. K. Shim, and D. D. Kim. Preparation and evaluation of paclitaxel-loaded PEGylated immunoliposome. J. Control. Release 120:169–177 (2007).
T. Yang, F. D. Cui, M. K. Choi, J. W. Cho, S. J. Chung, C. K. Shim, and D. D. Kim. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int. J. Pharm. 338:317–326 (2007).
U. B. Nielsen, D. B. Kirpotin, E. M. Pickering, K. Hong, J. W. Park, M. R. Shalaby, Y. Shao, C. C. Benz, and J. D. Marks. Therapeutic efficacy of anti-ErbB2 immnoliposomes targeted by a phage antibody selected for cellular endocytosis. Biochim. Biophys. Acta 1591:109–118 (2002).
C. Fonseca, J. N. Moreira, C. J. Ciudad, M. C. Pedroso de Lima, and S. Simões. Targeting of sterically stabilised pH-sensitive liposomes to human T-leukaemia cells. Eur. J. Pharm. Biopharm. 59:359–366 (2005).
K. Laginha, D. Mumbengegwi, and T. Allen. Liposomes targeted via two different antibodies: assay, B-cell binding and cytotoxicity. Biochim. Biophys. Acta 1711:25–32 (2005).
P. R. Twentyman, and M. Luscombe. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 56:279–285 (1987).
A. Sharma, U. S. Sharma, and R. M. Straubinger. Paclitaxel-liposomes for intracavity therapy of intraperitoneal P388 leukemia. Cancer Lett. 107:265–272 (1996).
C. X. Wang, D. C. Koay, A. Edwards, Z. Lu, G. Mor, I. T. Ocal, and M. P. DiGiovanna. In vitro and in vivo effects of combination of trastuzumab (Herceptin) and tamoxifen in breast cancer. Breast Cancer Res. Treat. 92:251–263 (2005).
N. Maeda, Y. Takeuchi, M. Takada, Y. Sadzuka, Y. Namba, and N. Oku. Anti-neovascular therapy by use of tumor neovasculature-targeted long-circulating liposome. J. Control. Release 100:41–52 (2004).
M. Owais, and C. M. Gupta. Liposome-mediated cytosolic delivery of macromolecules and its possible use in vaccine development. Eur. J. Biochem. 267:3946–3956 (2000).
S. C. Semple, A. Chonn, and P. R. Cullis. Influence of cholesterol on the association of plasma proteins with liposomes. Biochemistry 35:2521–2525 (1996).
M. C. Woodle. Controlling liposomal blood clearance by surface-grafted polymers. Adv. Drug Deliv. Rev. 32:139–152 (1998).
Y. P. Hu, N. Henry-Toulme, and J. Robert. Failure of liposomal encapsulation of doxorubicin to circumvent multidrug resistance in an in vitro model of rat glioblastroma cells. Eur. J. Cancer 31:389–394 (1995).
M. L. Immordino, P. Brusa, S. Arpicco, B. Stella, F. Dosio, and L. Cattel. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing decetaxel. J. Control. Release 91:417–429 (2003).
P. Crosasso, M. Ceruti, P. Brusa, S. Arpicco, and L. Cattel. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J. Control. Release 63:19–30 (2000).
P. Carter. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer Nov1:118–129 (2001).
R. J. Pietras, B. M. Fendly, V. R. Chazin, M. D. Pegram, S. B. Howell, and D. J. Slamon. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 9:1829–1838 (1994).
J. Baselga, L. Norton, J. Albanell, Y. M. Kim, and J. Mendelsohn. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 58:2825–2831 (1998).
A. Ito, Y. Kuga, H. Honda, H. Kikkawa, A. Horiuchi, Y. Watanabe, and T. Kobayashi. Magnetic nanoparticle-loaded anti-immunoliposomes for combination of antibody therapy with hyperthermia. Cancer Lett. 212:167–175 (2004).
F. Valeriote, and H. Lin. Synergistic interaction of anticancer agents: a cellular perspective. Cancer Chemother. Rep. 59:895–900 (1975).
U. Massing, and S. Fuxius. Liposomal formulations of anticancer drugs: selectivity and effectiveness. Drug Resist. Updat. 3:171–177 (2003).
E. Mastrobattista, G. A. Koning, and G. Storm. Immunoliposomes for the targeted delivery of anticancer drugs. Adv. Drug Deliv. Rev. 40:103–127 (1999).
Acknowledgements
This research was financially supported by the Ministry of Science and Technology (M10528010004-06N2801-00410) in Korea. The authors would like to thank the Lipoid GmbH (Germany) and Genentech companies for providing phosphatidylcholine and Herceptin, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, T., Choi, MK., Cui, FD. et al. Antitumor Effect of Paclitaxel-Loaded PEGylated Immunoliposomes Against Human Breast Cancer Cells. Pharm Res 24, 2402–2411 (2007). https://doi.org/10.1007/s11095-007-9425-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9425-y