Abstract
Purpose
Food stimulates changes to gastrointestinal secretion and motility patterns, however, the effect of smaller quantities of lipid, such as that contained in a lipid-based drug formulation, has not been detailed. This study aimed to examine the effects of small quantities of lipid on gastric emptying and biliary secretion.
Methods
The influence of oral administration of three lipid-based formulations and a negative control formulation on gastric emptying and biliary secretion was evaluated in 16 healthy male subjects using gamma scintigraphy, ultrasonography and duodenal aspiration.
Results
Low quantities (2 g) of long chain lipid stimulated gall bladder contraction and elevated intestinal bile salt, phospholipid and cholesterol levels. Changes in gastric emptying were also evident, although these did not reach statistical significance. Administration of a similar quantity of medium chain lipid, however, had little effect on gastric emptying and gallbladder contraction and did not stimulate appreciable increases in intestinal concentrations of biliary-derived lipids.
Conclusions
The quantities of long chain lipid that might be administered in a pharmaceutical formulation stimulate gallbladder contraction and elevate intestinal levels of bile salt and phospholipid. This effect is a likely contributor to the ability of lipid based formulations to enhance the absorption of poorly water-soluble drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The consumption of a meal, particularly one containing a large quantity of fat, stimulates a number of physiological responses, including a reduction in gastric transit, alterations in gastric pH, secretion of pancreatic enzymes, promotion of lymphatic transport, and stimulation of biliary lipid release from the gallbladder. These effects facilitate the appropriate rate of delivery of food from the stomach to the small intestine and promote the digestion and absorption of dietary nutrients (1). In addition, this ‘food effect’ may result in the enhanced absorption of co-administered drugs by providing a solubilising milieu of acid and homogenised fat. There are many examples of drugs that exhibit enhanced bioavailability when administered in combination with food, including griseofulvin (2), danazol (3), halofantrine (4), atovaquone and troglitazone (5,6), and the broader aspects of such food/drug interactions have been well reviewed (6–8).
The pronounced influence of food on the pattern of gastric motility is of particular interest and sustained drug release (and hence absorption) from the dosage form whilst within the stomach may provide a number of benefits. The mechanisms by which food promotes gastric retention are complex and interacting and factors such as meal osmolality, calorific volume, and pH have been suggested to mediate the observed post-prandial changes in gastric emptying (9–11). Biochemically, a number of secondary mediators, including cholecystokinin (CCK) (10,11), chylous lymph and apolipoprotein A-IV (Apo A-IV) (12–14), are implicated in mediating food-induced effects on satiety and thereafter gastric motility. The majority of studies suggest that the initial mediator of these events is the presence of dietary fat in the small intestine, and that long chain (rather than medium chain) fatty acids (FA) appear to be most effective in driving food-related inhibition of motility (11,15). In this regard, the implication of lymph-related species including apolipoprotein A-IV in the food effect/satiety cascade is consistent with the more efficient stimulation of lymphatic transport by long chain lipids (16).
Food also promotes the emptying of the gallbladder and maximal contraction has been demonstrated after ingestion of meals containing 10–25 g of lipid (17,18). The resultant release of biliary lipids, primarily bile salt (BS), phospholipid (PL), and cholesterol (CHOL) promotes the formation of a number of colloidal species within the small intestine, including mixed micelles and vesicles (19). The physiological purpose of these colloidal species is to aid in the solubilisation of the poorly water-soluble products of lipid digestion, i.e. FA and monoglycerides (MG) (20). However, the high affinity of poorly water-soluble drugs (PWSD) for these colloidal species (21–23) also dictates that this physiological response may be of great benefit in increasing the apparent intestinal solubility of drugs where low water-solubility is a barrier to efficient absorption (24).
Although a variety of food constituents (proteins, carbohydrates, and minerals) are likely to play a role in stimulating the physiological responses associated with a ‘food effect’, the lipid component of food is of particular importance for the absorption of lipophilic or poorly water-soluble drugs (PWSD). Several studies have examined the influence of lipid-containing meals on gastric emptying (9,10,25) and gallbladder contraction (17,18,26), however, the majority of these studies have examined the effects of administering large quantities of lipid (such as that contained within a meal) when compared with fasting conditions. In contrast, little evidence is available regarding the possible effects on drug absorption of quantities of lipid that might practically be included in a pharmaceutical formulation. A recent study has demonstrated that a liquid meal (containing 7.2 g of lipid) may at least partially stimulate the post-prandial intestinal secretory response (27), and Charman et al. have shown that administration of danazol (a poorly water-soluble steroid) in a lipid emulsion containing 10 g of glyceryl monooleate (GMO) stimulates the same increase in drug absorption in healthy volunteers as that observed following administration with a large meal (3). Similar effects have also been described pre-clinically where the oral bioavailability of danazol in beagle dogs was not significantly different after co-administration of drug with 3 g of soybean oil (in 3 × 1 g soft gelatin capsules) when compared with post prandial administration (28). However, the potential for lower lipid masses to induce similar bioavailability-enhancing effects as that seen post-prandially has not been widely reported, and to our knowledge, the impact of lower quantities of lipids on the likely physiological mediators of food effects (gastric emptying, biliary secretion and intestinal lipid concentrations) have to this point, not been described.
As a consequence, the current study was undertaken in healthy volunteers to explore the ability of lower quantities of lipid (2 g, i.e. the maximum quantity that might be contained in 2 × 1 g soft gelatin capsules) to activate the physiological indicators of a fed response. This quantity was chosen to represent the likely maximal amount of lipid that might practically be administered in a multiple capsule dose. The lipid formulation previously shown to provide equivalent drug absorption to that obtained in the fed state (10 g GMO) (3) was used as a positive control. In the low lipid dose (2 g) formulations, systems containing long (C18:1) and medium (C8–C10) chain lipids were also compared. The study was performed in two phases. In the first phase (Phase I), the lipid formulations described above, in addition to a placebo (water) formulation were administered to six healthy volunteers and the impact of formulation administration on gastric emptying (via γ scintigraphy) and the degree of gallbladder contraction (via ultrasonography) examined. In the second phase (Phase II), the associated changes in intestinal lipid and BS content were assessed (after administration of the same formulations to a further cohort of ten subjects) via duodenal sampling and biochemical analysis of the luminal content for BS, PL, CHOL, glyceride [G, comprised of TG, diglyceride (DG) and MG], and FA levels.
MATERIALS AND METHODS
Materials
Capmul GMO [minimum 60% glyceryl monooleate, (GMO)] was obtained from Abitec (Janesville, WI). Miglyol 810N, a medium chain TG (containing ∼70% C8 and ∼30% C10 TG) was supplied by Sasol (Witten, Germany). Oleic acid, as Crossential O94 (∼93% C18:1), was supplied by Croda (Staffordshire, UK). Potassium hydroxide was obtained from Merck Chemicals (Darmstadt, Germany). Sodium carboxymethylcellulose and ethylcellulose were purchased from Aqualon (Wilmington, DE). Lactose was supplied by Borculo Domo Ingredients (Zwolle, Netherlands). Orlistat (Roche, Basel, Switzerland) was purchased as commercial capsules containing 120 mg of active component. Olive oil was obtained from Thornton and Ross (Huddersfield, UK). Sodium chloride, ferric (III) chloride anhydrous, ammonium thiocyanate, ascorbic acid, sodium dihydrogen orthophosphate (dihyrate), hydrochloric acid, and acetonitrile were obtained from BDH Chemicals (Dorset, UK), and chloramphenicol, sodium azide and ethanol were purchased from Sigma Chemical Co. (St. Louis, MO). 99mTc-diethylenetriamine-pentaacetic acid (DTPA), 99mTc-pertechnetate and 111In-DTPA radiolabels were supplied by the Radiopharmaceutical Department at the Western Infirmary (Glasgow, UK). Purified water was obtained from Baxter Healthcare (Deerfield, IL).
Experimental Design Summary
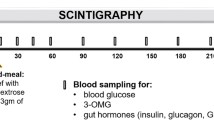
Clinical aspects of the study were undertaken at the Bio-Imaging Centre (Glasgow, UK). The study was undertaken as a single centre, analyst blinded, randomised crossover study in 16 healthy male subjects. The influence of four formulations (Table I) on measurable physiological ‘fed’ response indicators was assessed—a positive control comparator formulation [10 g GMO in 30 ml emulsion as studied by Charman et al. (3)], a negative control/placebo (10 ml water) and two formulations containing low volumes of either long chain lipids (2 g GMO in 10 ml emulsion) or medium chain lipids (2 g medium chain TG in 10 ml emulsion). Six subjects were entered into phase I, which was conducted as a four-way crossover study, where each subject received each formulation. Ten subjects were entered into phase II (see Fig. 1). Phase II was conducted as a three-way crossover study ie. each subject received three formulations. The protocol planned for data from eight subjects for the positive control (GMO) formulation and the low dose long chain lipid formulation and from seven subjects for the placebo and low dose medium chain lipid formulation. Due to non-treatment related subject withdrawal, data was actually obtained from six subjects for the positive control (GMO) formulation, eight subjects for the low dose long chain lipid formulation, six subjects for the placebo formulation and seven subjects from the low dose medium chain lipid formulation.
Study Subjects
Sixteen healthy male volunteers (aged 35–60 years) provided written informed consent to participate in the study, with pre-study medical screening taking place approximately 3 weeks prior to study commencement. Volunteers were recruited into two cohorts, the first cohort containing six subjects entered into phase I of the study and the second cohort containing ten subjects entered into phase II of the study. In both phases of the study subjects were fasted overnight prior to dosing. Water was allowed ad libitum during the fasting period but was withheld for 2 h pre-dose. No evidence of significant (including gastrointestinal-related) pre-existing pathological conditions was found in any subject. The study was conducted in accordance with the declaration of Helsinki, was approved by the North Glasgow Universities NHS Trust Ethics Committee and the Administration of Radioactive Substances Advisory Committee, and was conducted to Good Clinical Practice (GCP).
Preparation of Investigational Formulations
Four separate formulations were prepared as detailed in Table I. The positive control formulation contained 10 g of Capmul GMO emulsified in 30 ml of distilled water and was stabilised with potassium oleate (2% w/w). This was identical to the formulation previously used by Charman et al. (3). The negative control formulation consisted of 10 ml of distilled water.
Two further lipidic formulations were assessed which contained relatively low (2 g) quantities of lipid. The long chain lipid formulations contained 2 g glyceryl monooleate (Capmul GMO), chosen to match the long chain lipid used in the positive control formulation, and was formulated as an emulsion in 10 ml distilled water. The medium chain formulation contained 2 g medium chain triglyceride (Miglyol 810N) also formulated as an emulsion in 10 ml distilled water. For the medium chain lipid formulation, triglyceride (Miglyol 810N) rather than monoglyceride (as used in the long chain formulation) was employed since the equivalent medium chain monoglycerides formulation was found to be extremely unpalatable. Both of the low volume lipid emulsions were stabilised with potassium oleate (1% w/w for the Capmul GMO formulation and 2% w/w for the Migylol 810N formulation). All the lipid emulsions were produced using a Branson model 450 digital sonifier (Branson Ultrasonics, Danbury, CT). The mean particle size of each formulation was measured by dynamic light scattering on a Zetasizer 3000 (Malvern, UK), and was 300 ± 4 nm (mean±SD) for the positive control, and 302 ± 7 and 193 ± 2 nm for the long and medium chain low volume lipid formulations, respectively. The physical stability of the formulations was evaluated by monitoring changes in particle size. Changes in mean particle size did not exceed 15% over a 5-day period (at room temperature) for all formulations.
Lipidic formulations were freshly prepared on the evening prior to each study day. In phase I of the study, the lipid-soluble radiolabel 99mTc-thiocyanate was incorporated into the lipid phase of all the lipidic formulations during the emulsification process. The water-soluble label 99mTc-DTPA was added to the placebo/negative control (10 ml water) formulation. Incorporation of radiolabelled makers was not required for phase II of the study.
Gamma (γ) Scintigraphy
External radioactive markers were taped to the anterior and posterior chest of subjects to allow accurate alignment of sequential images. Scintigraphic imaging was performed using a Siemens E-Cam γ camera (Siemens AG, Erlangen, Germany) and all subjects were imaged in a standing position. For each subject, a standard region of interest (ROI) was drawn around the stomach and a small area of background on the 99mTc-channel image from the γ camera (after 99mTc-label administration). Anterior and posterior static acquisitions of 30-s duration were collected immediately after dosing and then every 5 min up to 1 h post-dose, followed by images every 10 min up to 4 h post-dose.
Scintigraphic Protocol and Image Analysis
The lipophilic radiolabel 99mTc-thiocyanate was produced from 99mTc-pertechnetate in an olive oil vehicle, according to the method described by Cunningham et al. (9). The 111In radiolabelled, non-disintegrating tablet was prepared as previously described by Kelly et al. (29) and was used to evaluate the impact of the test formulations on the gastric emptying of a tablet. Labelled test formulations were dosed to subjects and the percentage of formulation-associated radiolabel remaining in the stomach ROI at 10 min was recorded. At 15 min post-dose, the 111In-labelled non-disintegrating tablet and 100 ml of 99mTc (as 99mTc-DTPA) labelled water were administered in order to determine the effects of the test formulations on tablet and liquid emptying, respectively. The time required for removal of the tablet from the stomach ROI was determined qualitatively from the scintigraphic images by two independent, trained personnel. Liquid emptying was determined by quantifying the amount of activity remaining in the stomach at each time point. Scintigraphic images were analysed using the WebLink™ image analysis program, and were corrected for background and decay. The geometric means of the corrected counts were calculated using data from anterior and posterior images. For images post-15 min (i.e. after tablet dosing), an additional correction was applied to account for the contribution of 111In counts from the tablet in the 99mTc channel.
Ultrasonography
Gallbladder volume was assessed using the ultrasound methods described by Rasyid and Lelo and employed a real time ultrasound system (ACUSON Sequoia Ultrasound System, Siemens AG, Erlangen, Germany) (30). Baseline gallbladder volume was determined at 5 min prior to dosing of each formulation. Gallbladder volume was subsequently determined every 15 min for up to 2 h post-dose. Gallbladder volume was calculated in ml, and then expressed as a percentage of the “pre-dose” volume (which was taken as the baseline fasting volume).
Duodenal Fluid Analysis and Sampling Strategy
Based on the available information in the literature, it was anticipated that intestinal sample volumes of up to 5 ml could be obtained during this study. Previous studies have described samples (3–15 ml) being taken from the duodenum of overnight fasted subjects after the administration of both placebo formulations (26,31,32) and standard meals (11,19,31,33). The methodologies employed in these studies, however, varied from the current methodology, particularly for the studies conducted in fasted subjects, where duodenal samples were either collected using a modified cannula (Loc-I-Gut®) which occludes the intestine downstream of the collection point (27,32), or where samples were collected continually over a 30 min period (26). In reality, duodenal sampling volumes were considerably lower, and variable.
Following overnight fasting, the ten subjects involved in Phase II of the trial were intubated with an oral–duodenal feeding tube. The tube was positioned with the aid of an endoscope, and the distal region of the tube was placed just past the ampulla of Vater within the duodenum. The procedure was performed in the endoscopy unit at the Glasgow Royal Infirmary (Glasgow, UK) by a consultant gastroenterologist.
Sampling was performed after removing the dead volume of the feed tube. At time points of −0.5, −0.25, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, and 4 h, duodenal fluid samples of up to 5 ml were collected using a syringe and placed into polypropylene centrifuge tubes containing 100 μl of an ethanolic solution of both an antimicrobial cocktail (2 mg sodium azide and 2.5 mg chloramphenicol) (19) and the lipase inhibitor orlistat (600 μg). This concentration of orlistat was in excess of that previously shown to completely inhibit the in vitro digestion of the initial formulations, using a standard model of lipid digestion (34) and similar to that employed recently to inhibit the ex-vivo lipolysis of intestinal samples (27).
Volumes of duodenal samples up to 0.5 ml (following the addition of lipolysis inhibitor, antimicrobial, and centrifugation) were added to a tube containing 1 ml of acetonitrile prior to assay for BS, PL, CHOL, G, and FA content. Commercially available enzymatic kits were used to quantify BS (Biostat Diagnostics Systems, San Diego, CA), PL (Roche, Mannheim, Germany), CHOL (Roche, Mannheim, Germany) and G (which essentially represents total glycerides, including TG, DG and MG; Roche, Mannheim, Germany) and non-esterified FA (NEFA, or free FA; Wako, Richmond, VA). Where sample volumes were not attainable or resulted in data below LOQ, data were excluded. Each reported data point represents the mean data obtained from n = 3–7 subjects.
Data Analysis
Results are expressed as mean±SEM. Formulation group responses were compared using a one-way analysis of variance. Differences across all formulations were initially assessed using analysis of variance (ANOVA) and subsequent differences between the test formulations and the control (placebo) formulation were assessed post-hoc using Dunnett’s test comparison (SigmaStat 2.03, Systat Software, San Jose, CA). Differences were considered significant where p < 0.05.
RESULTS
Phase I
Gastric Emptying
The rate of gastric emptying of the lipid/control formulations following oral administration was determined by calculating the percentage distribution of total dosed activity within the stomach ROI using quantitative analysis of the scintigraphic images. The proportion of the formulation remaining in the stomach could only be determined at 10 min post-dose (since at 15 min post-dose, the 111In labelled non-disintegrating tablet and 99mTc labelled water were administered) and these data are presented in Fig. 2a. Approximately 66 to 81% of each of the test and positive control (10 g GMO) formulations remained in the stomach after 10 min. In contrast, only 28% of the placebo (water) ‘formulation’ was retained in the stomach at this time, and these differences were statistically significant (p < 0.05). Variability between subjects was relatively high, with subject 006 showing unusually slow emptying of the placebo formulation. Scintigraphic data from subject 005 after taking the long chain formulation was excluded from all analyses due to an abnormally low fasting gallbladder volume (see ‘gallbladder volume’ below).
Gastric emptying data showing: a the percentage of each formulation remaining in the stomach 10 min post-dose as determined by γ scintigraphy. b The time taken for 50% and c 90% of the 99mTc label (comprising a mixture of the 99mTc-thiocyanate label contained in the lipid formulations administered at t = 0 and the 99mTc-DTPA labelled water that was co-administered with the non-disintegrating tablet at time = 15 min) to empty from the stomach. d The time taken for the 111In radiolabelled tablet to empty from the stomach after administration (15 min after the lipid formulations). For all panels, asterisk indicates a statistical difference (p < 0.05) from placebo. Data are presented as mean±SEM; n = 6 in all cases except for data for the low dose long chain lipid formulation where n = 5.
Fifteen minutes after administration of the lipid and control formulations, 100 ml of 99mTc-DTPA labelled water was co-administered with a 111In labelled non-disintegrating tablet. The principle aim of this aspect of the study was to examine the impact of the lipid formulations on the patterns of gastric emptying of a tablet formulation. Administration of the tablet therefore required co-administration of a quantity of water to assist with swallowing. A decision was therefore taken to add a 99mTc-DTPA label to the water to facilitate an examination of the effects of the formulations on both solid (tablet) and liquid (water) gastric emptying. Distinguishing between the 99mTc-thiocyanate lipid label and the 99mTc-DTPA water label, however, was not possible, and consequently, only an overall indication of total liquid emptying could be generated from t = 15 min onwards. Nonetheless, the times taken for 50% (t 50) and 90% (t 90) of the 99mTc-labelled liquid (formulation plus water) to be removed from the stomach are presented in Fig. 2b and c. Relatively small differences between liquid emptying patterns were evident from the t 50 data, and whilst the mean t 50 emptying time for the long chain formulation appeared to be greater, this was almost entirely a result of the data from a single subject (subject 003). The lack of difference between formulations most likely indicates that the aqueous label was removed rapidly and emptied independently of the test formulations. The t 90 data suggest that the last proportion of liquid took longer to empty from the stomach in the lipid containing formulation groups, however, a significant difference to placebo (p < 0.05) was only observed for the positive control (GMO) group.
The influence of each formulation on the gastric emptying of a co-administered non-disintegrating tablet is presented in Fig. 2d. A significant increase in the gastric emptying time of the tablet was evident in the positive control (GMO) group (p < 0.05). However, the increases in mean gastric emptying time noted after administration of the low volume lipid formulations were not statistically significant when compared to the control (placebo) formulation.
Gallbladder Volume
Similar fasting gallbladder volumes were calculated for each subject (18.7 ± 1.4 ml, mean±SEM) with the exception of subject 005 prior to administration of the long chain formulation. For subject 005, the gallbladder volume was significantly lower (4.4 ml). It is known that pre-meal gall bladder emptying can occur as a result of thought or the smell of food, or through sham eating (35). As this data set would skew the calculation of the mean, it was concluded that a resting volume of this size would be too small to discern a lipid induced contraction. Accordingly, the results from subject 005 following administration of the long chain formulation were excluded from the mean calculations for all aspects of Phase I of the study (gastric emptying and gallbladder volume). Data are displayed in Fig. 3 for each formulation group as a percentage of individual fasting gallbladder volume. Maximal contraction of the gallbladder was achieved with the positive control (10 g GMO) formulation, with a plateau (maximal contraction) being reached 45 min post-dose and gallbladder refilling starting approximately 2 h post-dose. Gallbladder contraction was also observed after administration of the long and medium chain test formulation groups, and contraction to ∼50% (45 min) and ∼70% (30 min) of initial volume was observed for the long and medium chain formulations, respectively, followed by a return (refilling) to baseline volume in less than 1 h. Statistically significant (p < 0.05) differences in gallbladder volume relative to control were observed for the positive control formulations at all time points after 15 min, and for the long chain formulation at 45 min post−dose. A series of ultrasound images illustrating the contraction of the gall bladder in a subject receiving the GMO formulation is illustrated in Fig. 4.
Changes in gallbladder volume (% relative to fasted volume) with time (h) after dosing the test formulations; (filled circle) placebo, (empty circle) 10 g GMO (positive control), (filled triangle) low dose long chain and (empty triangle) low dose medium chain formulations. Asterisk indicates statistical difference (p < 0.05) from placebo. Data are presented as mean±SEM; n = 6 in all cases except for data for the low dose long chain lipid formulation where n = 5.
Phase II
Influence of Formulations on Intestinal Fluid Composition
Changes in the luminal concentration of the biliary-derived lipids (BS, PL, CHOL), and lipids derived primarily from exogenous (i.e. formulation) sources (G, NEFA) are shown in Figs. 5 and 6. Subject to subject variation, coupled with the difficulties associated with effective duodenal sampling (and therefore relatively sparse samples in some groups, especially the positive control group and placebo group at later time points) resulted in considerable variability in the data. Nonetheless, consistent trends in the data were evident. Thus pooled fasted (pre-dose) levels of BS, PL and CHOL were 1.93 ± 0.25, 0.34 ± 0.07 and 0.16 ± 0.01 mM, respectively, values in line with those in the literature ((23,32,36) and as expected these levels did not alter substantially after administration of the negative control (10 ml water) formulation. Similarly, administration of the low dose medium chain formulation led to relatively minor alterations in BS, PL and CHOL levels, consistent with the limited effect on gallbladder emptying observed in Phase I. In contrast, administration of the positive control (10 ml of GMO) formulation resulted in large (albeit variable) increases in maximum luminal concentration of BS (up to 20 mM), PL (>1 mM) and CHOL (>0.5 mM; Fig. 5). Increases to BS, PL and CHOL were also apparent after administration of the long chain low dose formulation, although, at least in the case of BS, these increases were limited to mean maximum concentrations of approximately 10 mM (Fig. 5). The trends observed in the relative changes in luminal concentrations of the individual biliary lipids were similar, consistent with the fact that BS, PL and CHOL are likely to be co-secreted in bile, and therefore, that changes to the concentration of one biliary-derived lipid will most likely reflect changes to the others.
Changes to concentrations (mM) of biliary-derived lipids in the intestinal lumen. Data are expressed as mean±SEM. a Bile salt (BS) levels for (filled circle) placebo (n = 3–6), (empty circle) 10 g GMO (positive control; n = 3–4), (filled triangle) low dose long chain (n = 4–7) and (empty triangle) low dose medium chain (n = 4–7) formulations. b Phospholipid (PL) levels for (filled circle) placebo (n = 3–6), (empty circle) 10 g GMO (positive control; n = 3–4), (filled triangle) low dose long chain (n = 3–7) and (empty triangle) low dose medium chain (n = 4–7) formulations. c Cholesterol (CHOL) levels for (filled circle) placebo (n = 3–4), (empty circle) 10 g GMO (positive control; n = 3–4), (filled triangle) low dose long chain (n = 3–6) and (empty triangle) low dose medium chain (n = 3–7) formulations.
Changes to concentrations (mM) of formulation derived lipids in the intestinal lumen. Data are expressed as mean±SEM. a Glyceride (G) concentrations for (filled circle) placebo (n = 4–6), (empty circle) 10 g GMO (positive control; n = 3–4), (filled triangle) low dose long chain (n = 3–6) and (empty triangle) low dose medium chain formulations (n = 5–6). b Non-esterified fatty acid (NEFA) concentrations for (filled circle) placebo (n = 3–5), (empty circle) 10 g GMO (positive control; n = 3–4), (filled triangle) low dose long chain (n = 3–7) and (empty triangle) low dose medium chain formulations (n = 4–6). c Shows the intestinal G concentrations normalised to the moles of glyceride lipid (in mM) administered. d Shows the intestinal NEFA concentrations normalised to the administered dose of lipid (in mM FA equivalents). For long chain lipids 1 mM of administered GMO was assumed to provide 1 mM of FA equivalent, whereas for the medium chain lipid 1 mM of administered triglyceride was assumed to provide 3 mM of FA equivalents.
Luminal glyceride and FA concentrations are presented in Fig. 6a and b. Total luminal glyceride concentration (Fig. 6a) and the concentration of luminal FA (Fig. 6b) was elevated most significantly after administration of the high lipid dose (10 g) positive control GMO formulation. In this group lipid concentrations were also sustained over the 4 h sampling period, likely reflecting reduced gastric emptying (Fig. 2) and providing a driver of sustained gallbladder contraction (Fig. 3) and luminal biliary lipid elevation (Fig. 5). Luminal FA concentrations were also high in the low dose medium chain formulation group (Fig. 6b), in spite of the limited effects of this formulation on gallbladder contraction and intestinal biliary lipid levels.
In the current study two different masses of formulation lipid were administered (2 g in the low dose lipid groups and 10 g in the positive control group). The molecular weight of the long and medium chain lipids also differ (the molecular weight of GMO was assumed to be 356 and for Miglyol 810 an average molecular weight of 496 was assumed based on a proportional content of 70% w/w C8 triglyceride and 30% w/w C10 triglyceride). As such different molar quantities of lipid were administered. Furthermore, the medium chain formulation was administered as TG and the long chain formulation as MG, and therefore the equivalent moles of FA administered (and therefore expected to be produced post digestion) were threefold higher for the TG based formulation when compared to the MG based formulation (at equivalent molar glyceride doses). Fig. 6c therefore shows the luminal glyceride concentration, dose normalised to the mM glyceride dose, to facilitate comparison of luminal concentrations of glyceride lipid relative to the molar lipid dose. Fig. 6d describes the luminal FA concentration similarly dose normalised to the mM FA equivalent dose. The data suggest that whilst the absolute luminal glyceride levels were higher in the high dose GMO group and luminal FA levels were higher in the medium chain lipid group this largely reflected the administration of larger quantities of lipid in the GMO group and larger quantities of FA equivalents in the medium chain lipid group.
It is generally accepted that infusion of lipids into the duodenum exerts marked changes on gastric motility via enteric neuronal inhibition (37) and the secretion of hormones such as CCK. One issue described previously (38) and illustrated by the present study is that following the administration of small volumes of lipid, gastric contents do not pool until they reach the jejunum as shown in Fig. 7. Similar observations have been made by other colleagues utilising magnetic moment imaging, in which onward movement of the labelled matrix proceeds rapidly though the duodenum and then may stop for considerable periods of time in the jejunum (Weitschies, personal communication).
DISCUSSION
In recent years increasing numbers of studies have described the utility of lipid based formulations as a means to enhance the absorption of poorly water-soluble drugs (39). The performance of these formulations has, at least in part, been attributed to their capacity to stimulate changes to the intestinal environment (such as slowing of gastric emptying and stimulation of gallbladder contraction) that are similar to those observed after ingestion of the much larger quantities of lipid in food. It is apparent, however, that little, if any, data is available in the literature to support this contention and this realisation has driven the genesis of the studies described in the current publication.
The current study has therefore examined whether oral administration of relatively small quantities of lipid (2 g of either a long or medium chain lipid) are capable of stimulating a fed (or post-prandial) response in the gastrointestinal tract. The study was conducted in two separate phases. In phase I, the effects of these small quantities of lipid on gastric emptying and gallbladder contraction were assessed. In phase II the duodenal fluid was sampled after lipid administration to assess for changes to biliary-derived (BS, PL, CHOL) lipid secretion as a function of changes in exogenous (TG/DG/MG and FA) lipid levels.
Phase I
Gastric Emptying
Gamma scintigraphy was successfully utilised as a means to monitor the gastric emptying of formulation-derived radiolabelled lipid. Assessment of the percentage of each formulation remaining in the stomach 10 min post-dose dosing showed that significant quantities of lipid remained (Fig. 2a) relative to placebo. This suggests that the initial gastric emptying of the low volume lipid formulations (medium and long chain) and the positive control (10 g GMO) may be reduced relative to a simple aqueous solution. These results are in agreement with those reported by Cunningham et al. (9), where the transit of an oily meal was significantly delayed relative to that of an aqueous liquid.
To examine the potential impact of the formulation lipids on the gastric transit of a solid dose form, a radio-labelled (non-disintegrating) tablet was administered 15 min after dosing the lipid formulation. The positive control formulation (10 g GMO) significantly (p < 0.05) slowed the gastric emptying of the tablet relative to placebo (86 min versus 25 min, respectively, Fig. 2d), however the increases in gastric emptying time for the tablet after administration of the low volume lipid formulations were not statistically significant. The data from the positive control formulation are in agreement with Edelbroek et al. (40), where the influence of lipid containing meals on the emptying of solid material (i.e. solid food) was investigated. These studies found that the solid portions of a meal were more effectively retained in the stomach in the presence of the lipidic material. The positive control data are also in accord with many studies that have described the ability of food to slow the gastric emptying of tablet dosage forms (2,7,41). Unfortunately an inherent limitation of any study of the gastric emptying patterns of non-disintegrating solids in fasted subjects is that the emptying profiles are highly dependent on the timing of dose-form administration relative to passage of the interdigestive migrating myoelectric complex (IMMC, commonly referred to as the ‘housekeeper wave’), since passage of the IMMC will stimulate rapid emptying of all gastric contents (42). The ability of the current experimental protocol to show changes to emptying (after administration of the lipidic formulation) is therefore potentially complicated by the time of dosing relative to the IMMC. Unfortunately, this can only be synchronised in a practical sense by inducing fed state intestinal motility patterns, which in itself would preclude useful interpretation of the data in the current investigation.
From analysis of the liquid emptying data, it appears that the initial 50% of fluid in the stomach (Fig. 2b) is emptied at an approximately equal rate, regardless of formulation constituents and likely represents aqueous liquid emptying. However, consistent with the data describing the impact of the lipid formulations on the gastric emptying of the non-disintegrating tablet, the time taken for 90% emptying of the liquid (Fig. 2c) was significantly longer for the GMO (positive control) formulation (p < 0.05). Whilst increases in the mean t 90 data were evident after administration of the low volume lipid formulations these were not statistically significant when compared with the control (placebo) data.
It is well established that a reduction in GI transit can be brought about by the presence of lipids (and particularly lipids of long chain origin, such as oleic acid) in the terminal ileum (43) and this ‘ileal brake’ has been proposed to have the potential to prolong the transit of co-administered tablets (43,44). Several secondary mediators of this event have also been suggested, including CCK (10,11) and more recently, Apo A-IV (12–14). Tso and Liu (14) have further suggested that Apo A-IV is secreted by the intestine in response to chylomicron formation via the lymphatics, and may also act centrally (within the hypothalamus) to control satiety (45). Further investigation of the physiological functions of these biochemical mediators and their relationship to, for example, gastric emptying, may provide greater insight into the effects of lipids on gastric motility, although in the current investigations, it appears unlikely that the relatively small quantities of lipids employed would be sufficient to provide an ileal stimulus.
Gallbladder Volume
Ingestion of a lipidic meal has been well documented to lead to gallbladder contraction (18) however, few studies have examined the influence of small quantities of lipid on this response. Ultrasound imaging was therefore utilised in the current study to compare the magnitude of gallbladder emptying after administration of the low volume (2 g) lipidic formulations, placebo, and the high lipid volume (10 g GMO) positive control. A reduction in gallbladder volume was evident following administration of all lipidic formulations (Fig. 3), however, a statistical difference (p < 0.05) from placebo only occurred after administration of the positive control formulation (between 0.5 to 1.5 h post-dose) and the low volume (2 g) long chain formulation (45 min post-dose). The low volume medium chain formulation appeared to have less impact on gallbladder contraction than the long chain equivalent, which may reflect the fact that the digestion of medium chain lipid is less dependent on the presence of intestinal biliary lipid (34) than that of long chain lipid.
In contrast to the graded effect of lipids on gallbladder emptying described here, previous studies have suggested that a ‘threshold’ level of lipid is required to stimulate an ‘all or none’ gallbladder contraction (18). The previously proposed threshold quantity of lipid (10–25 g), however, is likely to be the quantity required to obtain a maximal reduction in gallbladder volume, as others have shown that lower quantities (4 g) may still induce gallbladder contraction, albeit to a lesser extent (∼50% maximal) (17). The current study, where up to 50% gallbladder contraction was obtained after administration of only 2 g of long chain lipid (at 45 min post-dose), suggests that even smaller quantities of lipid may stimulate gallbladder contraction. It is important to note that the observed changes in gallbladder volume via ultrasonography comprised a composite of both gallbladder emptying and refilling (46), however, it was expected that the refilling rate was similar in each subject.
Phase II
Duodenal Fluid–Lipid Analysis
Phase II of the current study was designed to assess whether oral administration of relatively small (and pharmaceutically relevant) volumes of lipid were capable of increasing the intestinal concentration of biliary-derived lipids (BS, PL, CHOL) above the levels typically observed in the fasted state. Changes to luminal lipid concentrations were assessed after administration of the positive control (10 g GMO) and long and medium chain low volume lipid formulations relative to placebo. As suggested by the gallbladder studies, maximum release of biliary-derived lipids (BS, PL, CHOL) was observed for the positive control (10 g GMO) formulation, followed by the low volume (2 g) long chain lipid formulation.
In contrast to the long chain formulations, the ability of the medium chain lipid formulation to stimulate biliary release was less clear. The relatively limited response following ingestion of medium chain lipid may reflect the reduced dependence of medium chain lipids on solubilisation within biliary-derived lipid (BS/PL/CHOL) micelles in order to facilitate absorption, as the medium chain digestion products (FA/MG) are considerably more water-soluble than their long chain equivalents (34,47,48).
When the mean BS profiles for each formulation were compared to the commonly published ranges for accepted fed (post-prandial) and fasted BS levels (see Fig. 8), it is evident that the positive control formulation (10 g GMO) resulted in increases in luminal biliary lipid concentrations to levels similar to those commonly observed post prandially. In contrast, levels obtained in the placebo group fell, as expected, within the typical fasted BS range. The medium chain group also fell similarly within the fasted range, highlighting the low dependence of medium chain lipid digestion on BS release. Interestingly, administration of the low lipid load (2 g) long chain lipid formulation resulted in luminal BS levels above typical fasted levels (see Fig. 8), although these were well below peak post-prandial levels and therefore might be most accurately described as a ‘partially fed’ or as having stimulated a ‘partial-prandial’ response.
Figure showing the data describing changes to luminal concentrations (mM) of bile salt in the intestinal lumen after administration of (filled circle) placebo, (empty circle) 10 g GMO (positive control), (filled triangle) low dose long chain and (empty triangle) low dose medium chain formulations (from Fig. 5) overlayed with the typically observed ranges of luminal BS concentrations under fasted and fed (post-prandial) conditions [from refs (19,23,26,32,52,53)].
Analysis of the lipid chemistry of the intestinal fluid samples (for all lipid formulation groups) showed that the release of biliary lipid (BS/PL/CHOL) coincided with the presence of elevated concentrations of G (TG/DG/MG) and NEFA (free FA), suggesting that biliary lipid release occurred in response to the presence of exogenous (formulation-derived) lipid. These biliary-derived lipid (BS/PL/CHOL) concentration patterns (within the duodenal fluid) also broadly mirrored the gallbladder contraction data arising from Phase I, confirming that decreased gallbladder volume resulted in increased intestinal biliary lipid levels. In agreement with the data described here, recent studies by Persson et al. (27) using human intestinal fluid samples have highlighted the importance of formulation (or dietary) lipids in the solubilisation (and dissolution) of poorly water-soluble drugs. In the first of these studies, human intestinal aspirates were taken under fasted and post-prandial conditions and BS levels were found to be ∼2 mM in the fasted state (in close agreement with the data obtained here where fasted BS levels were 1.93 ± 0.25 mM), and ∼8 mM in the fed state (27). The current study therefore suggests that both the positive control and long chain low volume formulations stimulate secretion of BS to levels consistent with the fed state conditions described by Persson et al. (27) whereas BS levels after administration of the placebo and medium chain lipid formulations reflect basal (fasted) levels. Follow-up studies by Persson et al. also analysed the changes observed in the intestinal lipid environment after administration of a nutritional drink (NuTRIflex®) containing 7.2 g of lipid (3.6 g of long chain triglyceride and 3.6 g of medium chain triglyceride). These studies were performed via single pass perfusion of the model liquid meal into the proximal human jejunum using the Loc-I-Gut® perfusion system, and collection of intestinal samples over the 90 min perfusion period (36). The lipid quantities infused by Persson were larger than the low dose lipid formulations employed here (and lower than the 10 g GMO positive control formulation), however, the data obtained were in general agreement with the current studies and a rapid increase in luminal BS, PL and CHOL concentrations was observed in response to perfusion of the NuTRIflex®. Control infusions in the absence of lipid confirmed that this was not simply a response to the volume of perfusate (H. Lennernas, personal communication). In terms of time dependency, Persson et al. (27) described an increase in BS concentrations to give peak BS concentrations approximately 40 min after initiation of the infusion. This is again consistent with the current data where peak BS levels occurred at approximately 30 min post-dose for the low volume long chain lipid formulation. A similar profile was also evident after administration of the positive control (10 g GMO) formulation, although in this case the effect was sustained for a longer period, possibly reflecting prolonged gastric emptying. Interestingly, the ratio of the biliary lipid components in the current study did not vary significantly across the different formulation groups or with time after administration, with ratios of BS:PL:CHOL (±SEM) being 19(3):2(0):1(0) for placebo, 25(3):3(0):1(0) for long, 18(1):2(0):1(0) for medium and 24(3):3(0):1(0) for the positive control. Although a slight enrichment in BS was evident after administration of the long chain groups, the change was not statistically significant suggesting that the influence of formulation on biliary lipid concentration (output) was not specific to a single component (ie BS, PL or CHOL) but rather led to secretion of BS:PL:CHOL mixed micelles of similar composition. In contrast, Person et al. (27) noted slightly lower BS:PL ratios (6:1–2:1) and also described a time dependency where BS:PL ratios were higher (6:1) at early sampling points and lower (2:1) at later time points.
CONCLUSION
The current studies suggest that even relatively low quantities (2 g) of long chain lipid, (and importantly quantities that might realistically be administered on a single occasion in, for example, 2 × 1 g soft gelatin capsules), are capable of stimulating gall bladder contraction and therefore elevating intestinal BS/PL/CHOL levels. In contrast administration of a similar quantity of medium chain lipid had relatively limited effects on gallbladder contraction and did not stimulate appreciable increases in intestinal concentrations of biliary-derived lipids. It is possible that larger quantities of medium chain lipids may more effectively stimulate changes to gastric emptying or biliary secretion, but this was not examined in the current study. In the case of the low dose long chain lipid formulation, the response obtained appears to be somewhat less than that obtained by co-administration with larger quantities of lipid such as that contained in the positive control formulation (which has previously been shown to lead to increases in drug (danazol) absorption equivalent to that observed on co-administration with food (3)). As such, the response obtained with the smaller quantity of lipid might more correctly be described as a ‘partial-prandial’ response. Nonetheless, this partial-prandial response may play a role in enhancing drug absorption after the administration of lipid-based delivery systems, via an enhancement of intestinal drug solubility through an increase in biliary lipid secretion. As a caveat, however, is important to note that the enhancement in absorption of poorly water-soluble drugs commonly observed after administration of a lipid-based formulation is likely to reflect events in addition to the partial-prandial effect described above Thus, increases in intestinal solubilisation capacity are likely to be driven more avidly by the incorporation of the products of digestion of lipidic formulation into BS/PL/CHOL mixed micelles, rather than simply by raising the concentrations of luminal endogenous BS/PL/CHOL levels (48–50). The choice of formulation lipids should therefore be informed not only by potential stimulation of the partial-prandial effect described here—but also by an understanding of the solubilisation capacity of the species that form in the intestinal lumen on digestion of the formulation and intercalation of the digestion products into endogenous solubilising species. Finally, some lipid-based formulations, most notably the Neoral® formulation of Sandimmune have been shown to effectively support drug absorption even in the absence of bile (51). Under these circumstances the potential solubilisation advantages that stimulation of changes to luminal BS/PL/CHOL may provide are not required.
References
P. Borel, B. Pasquier, M. Armand, V. Tyssandier, P. Grolier, M. Alexandre-Gouabau, M. Andre, M. Senft, J. Peyrot, V. Jaussan, D. Lairon, and V. Azais-Braesco. Processing of vitamin A and E in the human gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G95–G103 (2001).
N. Aoyagi, H. Ogata, N. Kaniwa, and A. Ejima. Effect of food on the bioavailability of griseofulvin from microsize and PEG ultramicrosize (GRIS-PEG) plain tablets. J. Pharmacobio-Dyn. 5:120–124 (1982).
W. N. Charman, M. C. Rogge, A. W. Boddy, and B. M. Berger. Effect of food and a monoglyceride emulsion formulation on danazol bioavailability. J. Clin. Pharmacol. 33:381–386 (1993).
A. J. Humberstone, C. J. H. Porter, and W. N. Charman. A physicochemical basis for the effect of food on the absolute oral bioavailability of halofantrine. J. Pharm. Sci. 85:525–529 (1996).
E. Nicolaides, M. Symillides, J. B. Dressman, and C. Reppas. Biorelevant dissolution testing to predict the plasma profile of lipophilic drugs after oral administration. Pharm. Res. 18:380–388 (2001).
L. E. Schmidt and K. Dalhoff. Food–drug interactions. Drugs 62:1481–1502 (2002).
W. N. Charman, C. J. H. Porter, S. Mithani, and J. B. Dressman. Physicochemical and physiological mechanisms for the effects of food on drug absorption: the effects of lipids and pH. J. Pharm. Sci. 86:269–282 (1997).
D. Fleisher, C. Li, Y. Zhou, L. Pao, and A. Karim. Drug, meal and formulation interactions influencing drug absorption after oral administration. Clin. Pharmacokinet. 36:233–254 (1999).
K. M. Cunningham, R. J. Baker, M. Horowitz, A. F. Maddox, M. A. Edelbroek, and B. E. Chatterton. Use of technetium-99m(V)thiocyanate to measure gastric emptying of fat. J. Nucl. Med. 32:878–881 (1991).
C. Feinle, T. Rades, B. Otto, and M. Fried. Fat digestion modulates gastrointestinal sensations induced by gastric distension and duodenal lipid in humans. Gastroenterology 120:1100–1107 (2001).
M. Fried, E. A. Mayer, J. B. Jansen, C. B. Lamers, I. L. Taylor, S. R. Bloom, and J. H. Meyer. Temporal relationships of cholecystokinin release, pancreatobiliary secretion, and gastric emptying of a mixed meal. Gastroenterology 95:1344–1350 (1988).
T. Okumura, K. Fukagawa, P. Tso, I. L. Taylor, and T. N. Pappas. Apolioprotein A-IV acts in the brain to inhibit gastric emptying in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 33:G49–G53 (1996).
J. Glatzle, T. J. Kalogeris, T. T. Zittel, S. Guerrini, P. Tso, and H. E. Raybould. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G86–G91 (2002).
P. Tso and M. Liu. Apolipoprotein A-IV, food intake, and obesity. Physiol. Behav. 83:631–643 (2004).
H. E. Raybould, J. H. Meyer, Y. Tabrizi, R. A. Liddle, and P. Tso. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am. J. Physiol. 274:R1834–R1838 (1998).
S. M. Caliph, W. N. Charman, and C. J. H. Porter. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J. Pharm. Sci. 89:1073–1084 (2000).
B. G. Stone, H. J. Ansel, F. J. Peterson, and R. L. Gebhard. Gallbladder emptying stimuli in obese and normal-weight subjects. Hepatology 15:795–798 (1992).
F. Froehlich, J. J. Gonvers, and M. Fried. Role of nutrient fat and cholecystokinin in regulation of gallbladder emptying in man. Dig. Dis. Sci. 40:529–533 (1995).
O. Hernell, J. E. Staggers, and M. C. Carey. Physical–chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry 29:2041–2056 (1990).
M. C. Carey, D. M. Small, and C. M. Bliss. Lipid digestion and absorption. Annu. Rev. Physiol. 45:651–677 (1983).
L. J. Naylor, V. Bakatselou, and J. B. Dressman. Comparison of the mechanism of dissolution of hydrocortisone in simple and mixed micelle systems. Pharm. Res. 10:865–870 (1993).
B. L. Pedersen, H. Brondsted, H. Lennernas, F. N. Christensen, A. Mullertz, and H. G. Kristensen. Dissolution of hydrocortisone in human and simulated intestinal fluids. Pharm. Res. 17:183–189 (2000).
B. L. Pedersen, A. Mullertz, H. Brondsted, and H. G. Kristensen. A comparison of the solubility of danazol in human and simulated gastrointestinal fluids. Pharm. Res. 17:891–894 (2000).
G. L. Amidon, H. Lennernas, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
J. N. Hunt and M. T. Knox. A relation between the chain length of fatty acids and the slowing of gastric emptying. J. Physiol. 194:327–336 (1968).
S. D. Ladas, P. E. T. Isaacs, G. M. Murphy, and G. E. Sladen. Comparison of the effects of medium and long chain triglyceride containing liquid meals on gall bladder and small intestinal function in normal man. Gut 25:405–411 (1984).
E. M. Persson, A.-S. Gustafsson, A. S. Carlsson, R. G. Nilsson, L. Knutson, P. Forsell, G. Hanisch, H. Lennernas, and B. Abrahamsson. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm. Res. 22:2141–2151 (2005).
C. J. H. Porter, A. M. Kaukonen, B. J. Boyd, G. A. Edwards, and W. N. Charman. Susceptibility to lipase-mediated digestion reduces the oral bioavailability of danazol after administration as a medium-chain lipid-based microemulsion formulation. Pharm. Res. 21:1405–1412 (2004).
K. Kelly, B. O’Mahony, B. Lindsay, T. Jones, M. McDonagh, L. Martini, H. N. E. Stevens, and C. G. Wilson. The effect of long chain fatty acids on the gastric emptying of a co-administered tablet in fasted human volunteers. AAPS PharmSci. 4:T3072 (2002).
A. Rasyid and A. Lelo. The effect of curcumin and placebo on human gall-bladder function: an ultrasound study. Aliment. Pharmacol. Ther. 13:245–249 (1999).
L. Kalantzi, T. Fuerst, B. Abrahamsson, K. Goumas, V. Kalioras, J. B. Dressman, and C. Reppas. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability studies in the fasting and in the fed state AAPS PharmSci. 5: R6068 (2003).
A. Lindahl, A. Ungell, L. Knutson, and H. Lennernas. Characterisation of fluids from the stomach and proximal jejunum in men and women. Pharm. Res. 14:497–502 (1997).
H. P. Porter and D. R. Saunders. Isolation of the aqueous phase of human intestinal contents during the digestion of a fatty meal. Gastroenterology 60:997–1007 (1971).
L. Sek, C. J. H. Porter, A. M. Kaukonen, and W. N. Charman. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J. Pharm. Pharmacol. 54:29–41 (2002).
S. Ellenbogen, S. A. Jenkins, J. S. Grime, M. Critchley, C. R. Mackie, and J. N. Baxter. Preduodenal mechanisms in initiating gallbladder emptying in man. Br. J. Surg. 75:940–945 (1988).
E. M. Persson, R. G. Nilsson, G. I. Hansson, L. J. Lofgren, F. Liback, L. Knutson, B. Abrahamsson, and H. Lennernas. A clinical single-pass perfusion investigation of the dynamic in vivo secretory response to a dietary meal in human proximal small intestine. Pharm. Res. 23:742–751 (2006).
P. Holzer. Gastrointestinal afferents as targets of novel drugs for the treatment of functional bowel disorders and visceral pain. Eur. J. Pharmacol. 429:177–193 (2001).
C. G. Wilson and K. Kelly. Gastrointestinal transit and drug absorption. In J. B. Dressman and J. Kramer (eds.), Pharmaceutical Dissolution Testing, Taylor and Francis, London, 2006, pp. 97–125.
C. J. H. Porter, N. L. Trevaskis, and W. N. Charman. Lipids and lipid based formulations: optimising the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 6:231–248 (2007).
M. A. Edelbroek, M. Horowitz, A. F. Maddox, and J. Bellen. Gastric emptying and intragastric distribution of oil in the presence of a liquid or a solid meal. J. Nucl. Med. 33:1283–1290 (1992).
N. Kaniwa, N. Aoyagi, H. Ogata, H. Motoyama, and H. Yasumi. Gastric emptying rates of drug preparations. II. Effects of size and density of enteric-coated drug preparations and food on gastric emptying rates in humans. J. Pharmacobio-Dyn. 11:571–575 (1988).
R. L. Oberle, T. S. Chen, C. Lloyd, J. L. Barnett, C. Owyang, J. Meyer, and G. L. Amidon. The influence of the interdigestive migrating myoelectric complex on the gastric emptying of liquids. Gastroenterology 99:1275–1282 (1990).
C. L. Dobson, S. S. Davis, S. Chauhan, R. A. Sparrow, and I. R. Wilding. The effect of oleic acid on the human ileal brake and its implications for small intestinal transit of tablet formulations. Pharm. Res. 16:92–96 (1999).
C. L. Dobson, S. S. Davis, S. Chauhan, R. A. Sparrow, and I. R. Wilding. The effect of ileal brake activators on the oral bioavailability of atenolol in man. Int. J. Pharm. 248:61–70 (2002).
K. Fujimoto, J. A. Cardelli, and P. Tso. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am. J. Physiol. 262:G1002–G1006 (1992).
R. P. Jazrawi, P. Pazzi, M. L. Petrioni, N. Prandini, C. Paul, J. A. Adam, S. Gullini, and T. C. Northfield. Postprandial gallbladder motor function: refilling and turnover of bile in health and in cholelithiasis. Gastroenterology 109:582–591 (1995).
G. A. Kossena, B. J. Boyd, C. J. H. Porter, and W. N. Charman. Separation and characterization of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water-soluble drugs. J. Pharm. Sci. 92:634–648 (2003).
G. A. Kossena, W. N. Charman, B. J. Boyd, D. E. Dunstan, and C. J. H. Porter. Probing drug solubilisation patterns in the gastrointestinal tract after administration of lipid based delivery systems: a phase diagram approach. J. Pharm. Sci. 93:332–348 (2004).
C. J. H. Porter, A. M. Kaukonen, A. Taillardat-Bertschinger, B. J. Boyd, J. M. O’Connor, G. A. Edwards, and W. N. Charman. Use of in vitro lipid digestion data to explain the in vivo performance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J. Pharm. Sci. 93:1110–1121 (2004).
G. A. Kossena, W. N. Charman, B. J. Boyd, and C. J. H. Porter. Influence of the intermediate digestion phases of common formulation lipids on the absorption of a poorly water-soluble drug. J. Pharm. Sci. 94:481–492 (2005).
A. K. Trull, K. K. C. Tan, L. Tan, G. J. M. Alexander, and N. V. Jamieson. Enhanced absorption of new oral cyclosporin microemulsion formulation, Neoral, in liver transplant recipients with external biliary diversion. Transplant Proceedings 29:2977–2978 (1994).
A. Tangerman, A. van Schaik, and E. W. van der Hock. Analysis of conjugated and unconjugated bile acids in serum and jejunal fluid of normal subjects. Clin. Chim. Acta 159:123–132 (1986).
O. Fausa. Duodenal bile acids after a test meal. Scand. J. Gastroenterol. 9:567–570 (1974).
Acknowledgments
We are grateful to GSK for financial support and to Dr. Paul Ingram and Kirsteen Wilson (Bio-Images Research Ltd) for analytical assistance. The co-ordination roles of Eilis O’Driscoll and Paul Linacre at GSK are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kossena, G.A., Charman, W.N., Wilson, C.G. et al. Low Dose Lipid Formulations: Effects on Gastric Emptying and Biliary Secretion. Pharm Res 24, 2084–2096 (2007). https://doi.org/10.1007/s11095-007-9363-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9363-8