Abstract
Abstract
With application of molecular biology techniques, there has been rapid progress in understanding how many drugs and micronutrients (e.g., vitamins) are transferred across the choroid plexus (CP), the main transport locus of the blood–cerebrospinal fluid (CSF) barrier, and the renal tubular epithelial cells. In many cases, these molecules are transported by separate, specific carriers or receptors on the apical and/or basal side of the CP or renal epithelial cells. This commentary focuses on four micronutrient transport systems in CP (ascorbic acid, folate, inositol, and riboflavin), all of which have been recently cloned, expressed and for which knockout mice models were developed and transporter localization studies performed. Also reviewed is the recently cloned uric acid transport system in human kidney in which there exists a human “knockout” model. The implications of these transport systems for drug therapy of central nervous system and renal disorders are discussed, especially with regard to methods to circumvent the blood–brain and blood–CSF barriers to deliver drugs to the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last 7 years, there has been tremendous progress in understanding how molecules are transferred across the choroid plexus (CP) epithelial cells, the main transport locus of the blood–cerebrospinal fluid barrier (BCSFB). This knowledge has important pharmacological implications, especially in the central nervous system (CNS). There has also been comparable progress in understanding renal tubular transport with uric acid disposition being an edifying example. The purpose of this Commentary is to summarize this newer information, place it into perspective and provide specific examples of how it affects drug therapy.
Background
About 100 years ago, investigators began to define the blood-brain (BBB) and blood–CSF barriers (1). Over time, they established that the BBB is anatomically due to tight junctions between the brain capillary (endothelial) cells. The blood–CSF barrier is due to tight junctions between the CP epithelial cells, and the arachnoid membrane cells (1,2). The choroid plexus, which secretes the CSF, and the arachnoid membrane enclose the CSF compartment which surrounds the brain (1,2). This is minimal anatomical impediment to molecules moving between the subarachnoid or intraventricular CSF and the extracellular space of brain. The cerebral capillaries (1.0% of brain weight) and the fronded CP (0.5%) have comparable surface areas per unit weight (1–3). Unlike the brain capillaries, the choroid plexus epithelial cells resemble the renal tubular epithelial cells which are also joined by tight junctions (1,2). The CP and renal epithelial cells separate the blood compartment from the CSF and urine, respectively. The reader is referred to detailed up-to-date reviews and pictures of the anatomy (1–5).
In parallel with these anatomical observations, some proposed that the entry (and exit) of molecules into brain and CSF from blood was due to simple diffusion and thus depended predominantly on lipid solubility, charge and molecular size (1–5). Others thought that substances essential for brain, e.g., vitamins, would readily enter brain (6). They hoped to take advantage of this belief. For example, Linus Pauling and orthomolecular psychiatrists postulated lower vitamin cofactor affinity (single nucleotide and other polymorphisms) for certain brain apoenzymes in mental patients (6,7). They hoped to overcome this genetic abnormality by feeding large amounts of water soluble vitamins orally, to increase the concentrations of vitamin cofactors in brain. However, as described below [for vitamin C (AA), riboflavin, myo-inositol (inositol) and methyltetrahydrofolate (MeTHF)], there are very efficient, separate homeostatic systems for water-soluble vitamins in the body and CNS (6,7). Thus, it was not possible to increase the brain concentration of vitamin cofactors much if at all. Hence, at that time, it was not possible to test Pauling's hypothesis about apoenzyme polymorphisms in human brain (6).
It became progressively clear that a multitude of compounds (including many important drugs) do not distribute between brain, CSF and blood according to the postulated rules of simple diffusion based on charge, size and lipid solubility (1–5,7). Macronutrients, micronutrients including vitamins, “waste products” of metabolism, ions and exogenous substances seemed to depend on “carriers” to traverse the BBB and BCSFB (1–5,7). Over time, from the early studies of glucose and amino acid transport through the BBB by specific carriers, and Welch's documentation (1) of iodide efflux from CSF by a specific, active transport system in CP, several hundred different transport systems have been described or postulated in over 25 general classes (1,2,8). Moreover, the specificity of these systems overlaps considerably (8). Thus a single molecule might be transported on two or more systems (8).
Another complexity in the CNS is that there are two possible routes of entry into and exit from the CSF and brain: the BBB and the BCSFB, the CP (1–3). At first blush, most investigators thought that the BBB would be the most important locus of transport. But, in 1921, Stern and Gautier (1) presciently proposed that in both the developing mammalian fetus where the CP is disproportionately large (1,2) and adult, the CSF is “nourishing liquor” for the brain, an hypothesis we now know is partially correct (1,2,7). Although in most cases transport through the BBB predominates (e.g., influx of glucose and other macronutrients into brain, and efflux of certain xenobiotics and drugs into blood via P-glycoprotein); in other cases, the CP is more important (e.g., influx of AA into CSF, and efflux of penicillin G into blood) (1,2,9,10).
Finally, in recent years, two general attitudes arose that we believe are overly broad in scope or in error. First, there is excessive frustration over the difficulty in introducing some potentially useful chemicals into brain because of anatomical (penetration) or physiological reasons, e.g., efflux transport (11). Thus, the CNS is termed a “pharmacological sanctuary” implying there is little hope for working around the BBB and/or the blood–CSF barriers. Second, there have been incorrect generalizations about transport at the BBB or BCSFB (12). For example, it is stated that “nutrient transporters recognize only a small group of structurally related chemicals” whereas “most xenobiotic transporters are multi-specific, accepting a wide range of chemical structures” (12). Moreover, it is maintained that the reason for the existence of these “xenobiotic transporters” is “to rid the body of potential harm” (12). We discuss these issues below.
Focus
In past publications, we and others have reviewed the anatomy, defined the transport role of the CP and cerebral capillaries, discussed the types and physiology of the transport systems in the CNS, and outlined important methodological considerations and standards of proof for drawing firm conclusions about transport systems in the CNS (1–5). We refer the reader to those reviews. With the recent explosion of knowledge of these CNS transport systems, for the first time, we now understand several of these systems molecularly. Therefore, in this Commentary, we review four micronutrient systems in CP, all of which have been recently cloned, expressed and for which knockout (KO) mice models were developed and localization studies in CP performed. These new studies establish that each of these transport systems is unique. We also briefly review the new information about the recently cloned and expressed uric acid transport system in human kidney (URAT 1), in which there exists a human “knockout” model.
In each micronutrient example, we will discuss the transported moiety, the direction of transport through the CP, the location of the entry and exit carriers (see Fig. 1), the specificity of the transporter, and its molecular biology. We will also review the role of the BBB, the mechanism of entry of the transported moiety into brain cells and finally the implications for drug therapy. The overall objective is to show that, with detailed anatomical, physiological, biochemical and now molecular knowledge, it is possible, at least in principle, to use or manipulate these systems to improve drug therapy.
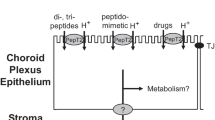
The established carrier mechanisms for vectorially transporting several micronutrients across the epithelial cells of choroid plexus are displayed. Ascorbate (AA) from the blood is actively transported across the basolateral membrane by SVCT 2 (Na-ascorbate cotransporter) into the epithelium and then released into CSF. Folate (in the reduced form as MeTHF) is actively taken up from the blood by the folate receptor (FRα) and then subsequently extruded across the apical membrane into the CSF, likely by the reduced folate carrier (RFC1). Myo-inositol (inositol) is actively transported from the blood into the choroidal epithelium by a sodium-dependent transporter (SMIT1); its release into CSF is by a mechanism not yet identified. In contrast to the case for AA, MeTHF and inositol, the net active transport of riboflavin is in the reverse direction, i.e., from CSF to plasma. Riboflavin is actively removed from CSF by the organic acid transporter 3 (OAT 3) carrier in the apical membrane of CP. The basolateral efflux of riboflavin from the epithelial cells is by a concentrative mechanism that awaits clarification.
Ascorbic Acid Transport Through Choroid Plexus
AA concentrations in mammalian plasma, CSF and brain are homeostatically maintained at approximately 0.05, 0.2, and 1.2 mM, respectively (2,3,7). AA is not synthesized in mammalian brain where it turns over about 40% per day (7). The concentration of AA in some neurons approaches 10 mM (13). AA in the CNS serves as an essential cofactor for norepineprine production and as an antioxidant. In severe depletion experiments in species that do not synthesize AA, e.g., guinea pigs, the concentration of AA in brain is better maintained than in all other organs, thus explaining the CNS resistance to AA deficiency in scurvy (7).
To understand AA transport in the CNS, in elegant autoradiographic studies, Hammarstrom showed that 14C-AA, after intravenous injection, first entered the CNS via the CP and then slowly entered the brain substance from the CSF surfaces (14). We confirmed this work and showed that AA can penetrate into deep brain structures when injected directly into the CSF (7,15).
In a series of subsequent studies, we and others showed that AA is transported from blood into CSF by an active, saturable transport system in CP (Fig. 1) (7,15,16). The K T (0.05 mM) for this system (i.e., the concentration which half saturates the carrier) is the normal plasma concentration (Table I) (7). The AA CP system has also been studied in vitro and in porcine monolayers (16). In these studies, CP pumps AA from the basolateral to the apical side (Fig. 1), where the AA is released into the CSF (16,17). In vivo, at the normal plasma concentration, the concentration of AA in the secreted CSF is four times the plasma concentration (1,7,15–17). This highly specific CP active transport system (and the fact that AA can only leave the CNS by simple diffusion and bulk blow of CSF) explains in part the remarkable homeostasis of AA in brain (7). When the plasma levels of AA fall, relatively more AA is pumped into the CSF from blood; when plasma levels are raised, relatively less. Moreover, the CSF contains 0.2 mM AA which is about four times the half-saturation concentration of AA entry into brain cells (0.05 mM). Thus, the brain is constantly surrounded by “nourishing liquor” as far as AA goes (Table I) (1,7). There are also saturable absorption and reabsorption systems in gut and kidney, respectively, that tend to keep the plasma levels constant (1,7). These transport systems in: 1) kidney/gut, 2) CP and 3) brain cells, all of which are half-saturated at the normal plasma concentration (0.05 mM), work in series and make for an extremely powerful mechanism to control brain AA (1,7).
The AA system in vivo and in CP is very specific; isoascorbic acid has one-tenth the affinity of AA for the CP system; carboxylic acids, e.g., penicillin, salicylate and probenecid have less or no affinity (Table I) (18).
There are two experiments that suggest the above-described data and interpretation are incomplete. First, Lam and Daniel suggested there was a saturable transport system for AA at the BBB (K T ∼ 0.1 mM) (19). However, they did not include a passive marker for intravascular space (e.g., mannitol or sucrose) and did not chromatograph the (14C)-AA. Moreover, others have not been able to reproduce this observation (20). Almost certainly, this work is inconsistent with Hammarstrom's findings (14) and the cloning and knockout (KO) mice experiments described below.
Second, several groups have suggested that dehydroAA (DHAA), the oxidized form of AA, is the moiety normally transported through the BBB. DHAA, once in brain, can readily be reduced back to AA (20). In 1966, Hammarstrom showed that, unlike AA, DHAA readily traverses the BBB on what we now know is the glucose (GLUT 1) transporter (14,20). However, there is no or very little DHAA in plasma making this possibility unlikely (3). Moreover, the molecular biology and KO mice experiments make the notion that transport of DHAA at the BBB can supply the brain with AA extremely unlikely (see below).
Recently, two sodium-dependent vitamin C transporters (SVCT 1 and 2) have been cloned, expressed and localized (Table I) (21). Only SVCT 2 is expressed in the CNS, specifically in the CP (very strongly), some neurons, tanycytes, and the arachnoid membrane. SVCT 1 is expressed in the kidney and other organs (21). The specificity and K T of expressed SVCT 2 (0.05 mM) is similar to that of the intact AA transport system in choroid plexus (Table I) (7,21). DHAA has minimal affinity for SVCT 2. The finding of SVCT 2 in neurons and not glia in vivo is consistent with the much higher concentration of AA in neurons (13,21). Three groups of investigators have looked for and not found the SVCT transporters at the BBB, i.e., in brain capillaries (21–23).
In homozygous SVCT 2 KO mice, although there is ∼ 50% as much AA in blood, there is virtually no AA in the brain of the nonviable newborn mice (24). The concentration of AA in CSF was not measured. [In normal newborn mammals, the CP AA system is fully developed and functional (7).] These results strongly suggest there is no system (other than SVCT 2) in the CNS to provide AA to brain. Trace amounts of DHAA in blood are not enough to provide AA to brain via GLUT 1 in the SVCT 2 KO mice (24). Moreover, what is absolutely clear is that, without the SVCT 2 system in series in CP and neurons, simple diffusion of AA from blood is grossly insufficient to provide AA to brain (7,13,15,24).
A comparison between the handling of AA by CP and renal tubule cells is instructive. The tubule cells normally reabsorb (presumably by SVCT 1) essentially all the filtered AA. In a human adult on a normal diet, about 2 g of AA are filtered a day, and less than 30 mg is lost in the urine (21). In CP, greater than 50% of the AA in the blood flowing through the CP (400 ml/100g/min) is cleared of AA via SVCT 2; then, the cleared AA is secreted into the CSF at a concentration four times that of plasma (7,16,17,21). Thus, SVCT 1 in kidney and SVCT 2 in choroid plexus are powerful, specific saturable pumps (7,16,17,21–24). How AA exits the tubule cells or CP cells into blood or CSF, respectively, awaits elucidation.
These studies have important implications for therapy. For example, in murine stroke models, there is up-regulation of SVCT 2 in the penumbra around the necrotic center of the infarcted brain, interpreted as a protective (antioxidant) response (25). With this information, several investigators hypothesized AA might be neuroprotective in acute stroke (25). As pointed out above, because of the barrier to AA and the powerful homeostatic systems for AA in the CNS, even massive doses of intravenous AA did not increase the brain AA concentration significantly (7,20,25). But as noted above, intravenous DHAA, unlike AA can enter brain via Glut 1 at the BBB acutely, and increase brain AA concentrations by a factor of two or more (14,20,25). When intravenous DHAA was given to mice after induction of experimental strokes, as expected, there was rapid penetration of DHAA into brain, in situ reduction of DHAA to AA, and unequivocal neuroprotection i.e., a significant decrease in infarct volume (25). In short, there is a way to increase AA in brain. Linus Pauling's hypothesis for AA, at least acutely, is now testable. One could test whether a doubling of AA in brain makes a clinical difference.
Folate Transport through Choroid Plexus
Like AA, MeTHF, the principal folate in plasma and CSF, is present in CSF at approximately four times the plasma concentration (26,27). Like AA, it is difficult to deplete adult brain folates (which exist predominantly in polyglutamate forms). This is not because of lack of turnover of folates in brain but due to powerful homeostatic systems in the body and CNS which normally maintain total brain folate concentrations fairly constant (26,27). In vivo, the half-saturation concentration of MeTHF for entry into CSF and brain is approximately equal to the plasma concentration (0.02 μM) (27). In vitro, in the isolated CP, there is an active transport system for MeTHF that is also half saturated at 0.02 μM (Table II) (26,27).
There is now substantial evidence that this system in CP is a principal locus of MeTHF transport into the CNS (26–29). On the basal (blood) side of the CP, there exists the Folate Receptorα (FRα), which is attached to the membrane by a phosphatidylinositol anchor (Fig. 1) (29). The FRα in CP efficiently accumulates MeTHF from blood, transfers it by an endosomal mechanism inside the CP epithelial cell and then releases it (26–31). The MeTHF is then transported from the cell interior into the CSF by a saturable mechanism at the apical surface, probably the Reduced Folate Carrier 1 (RFC 1) (29). This system, like the CP AA system, is fully operational at birth (31).
The specificity of the FRα is much greater for reduced and oxidized folates than methotrexate or other weak acids, e.g., AA or probenecid. Unlike FRα, the RFC1 has much higher affinity for reduced than oxidized folate, e.g., folic acid (FA) (26). So although FRα can transport both MeTHF and FA into the CP cell, the released FA tends to be reduced by intracellular CP dihydrofolate reductase (26,27,32). Moreover, FA is not readily transported out of the CP epithelial cell into CSF by RFC1 (32). Thus, the mechanism in the intact CP is specific for transferring reduced, not oxidized, folates, particularly MeTHF, from blood into CSF (26–32). Like AA, the concentration of MeTHF in newly formed CSF is approximately four times the plasma concentration.
At the BBB, there is some evidence for a very low capacity transport system for folates (33). However, this system could not be detected in bovine capillaries in vitro (33). Whether this system is, in fact, a low level expression of RFC1 remains to be established. However, quantitatively, this carrier is much less important than the vigorous system in the CP, which continuously transfers MeTHF into the newly formed CSF (34). The consequence is that brain cells are surrounded by a steady, high concentration of MeTHF (27). In immunolocation studies, the RFC 1 protein is present not only on the apical surface of CP cells but also on adult neuronal dendrites and axons (Fig. 1) (29). In adults, RFC 1 is almost certainly the mechanism for MeTHF uptake by neurons since FRα is not present in adult neuronal membranes (29).
In FRα homozygous KO mice, there was an arrest of neural tube development and the fetuses were reabsorbed (35). Massive doses of reduced folates given to the dams throughout pregnancy overcame the lack of FRα receptor in CP and other locations, and led to normal neural tube development (35). Knowing the CSF level of MeTHF, and the CSF/plasma ratio, after the megadosing of reduced folates might provide insight on the mechanism(s) of MeTHF distribution in the CNS of FRα KO animals. Whether the MeTHF enters fetal brain by simple diffusion or by another non-FRα carrier is unknown. Whether such KO mice will survive postnatally with massive doses of reduced folates is also uncertain. The abnormal neural tube development in FRα homozygous mice does not prove the exclusive need for MeTHF transport via CP (in brain development) since FRα is temporarily upregulated in the brain of developing fetuses (35). Thus, the situation is complex. We hypothesize that the normal development of the FRα KO homozygous mice in utero in dams given massive doses of reduced folates is due at least in part to low level transport by residual RFC1 at the BBB and other locations in the fetuses.
In summary, in CP, there is a complex FRα endosomal mechanism that is an integral part of the transfer and homeostatic system for folates into adult CSF and brain (Fig. 1) (26–30,34,35). Without FRα, the CNS cannot develop in utero as described above, or function properly in children or adults as described below. It is worth noting that the renal tubule reabsorbs almost all the filtered MeTHF in the glomerular filtrate by a similar, saturable FR-dependent mechanism.
This FRα system in CP has many implications for drug therapy. For example, in recent years several devastating pediatric CNS syndromes including developmental delays, autism, peculiar behavior patterns (e.g., compulsive hand-washing) and mental retardation are associated with low CSF MeTHF levels, although plasma concentrations are normal (30,34). In these cases, there is substantial evidence the CP FRα is dysfunctional, and thus cannot transfer reduced folates from blood into CSF, and secondarily, the brain adequately (30,34). This is not due to a genetic abnormality; the FRα gene was sequenced and normal (30,34). In some cases, this appears to be due to an autoantibody to the FRα (36). Since the FRα is on the basal (blood) side of the CP, the antibodies can attach to it (36). Fortunately, as in the FRα homozygous KO mice, megadoses of oral reduced folates can normalize the CSF MeTHF concentration, possibly by simple diffusion, with improvement in these devastating illnesses (30,34,36). This syndrome is now termed “cerebral folate deficiency” a potentially treatable condition especially if discovered before irreversible CNS damage occurs (34,36).
Also, these studies provide indirect support for fortification of food with folates but that discussion is beyond the scope of this Commentary (37).
Inositol Transport Through Choroid Plexus
Myo-inositol (inositol), one of nine isomers and the principal cyclitol in mammals, has at least three roles in brain: osmolyte (see below), precursor for inositol-containing second messengers and part of anchor-structures for membrane-bound proteins (38). The concentration of inositol in plasma, CSF and brain is 0.05, 0.2–0.3, and 4–5 mM, respectively (39). About 8% of rabbit brain inositol is replaced by inositol from blood each day (39–41). Although inositol can be synthesized from glucose in brain capillaries, the large majority of inositol is transported into CSF and brain from blood, especially in developing mammals (see below) (38,40). Like folate and AA, inositol is transported from blood into CSF by a separate, specific, active transport system in CP which is half-saturated at 0.05 mM, the plasma concentration (Fig. 1; Table III) (39–41). Unlike AA and folates, there is also an easily demonstrable, saturable, inward myo-inositol transport system (KT ∼ 0.05 mM) at the BBB (42). Brain slices accumulate inositol by a saturable system with a KT ∼ 0.05 mM (43).
Clarifying the mechanism of inositol transport into the CNS, the sodium-dependent myo-inositol transporter (SMIT l) was cloned, expressed and found to be present in large amounts in CP and in much lesser amounts in some neurons (44). The transporter, as expected, is located on the basal side of the CP and both, in vivo, and in monolayers of porcine CP in vitro is part of the mechanism that pumps inositol from the basal surface into the CP cell (16,17,38,39,44). How inositol exits the cell into the CSF needs to be determined (Fig. 1).
In vitro, in isolated choroid plexus, in choroid plexus monolayers, in cells with cloned and expressed SMIT1, and in vivo, the specificity for cyclitols is the same: myo-inositol ∼ scyllitol, one of the nine cyclitol isomers (KT 0.05 mM) >> other cyclitols and glucose (16,17,38,41).
However, in brain slices and in vivo entry into the brain substance, unlike in isolated CP, (+) chiro-inositol (another cyclitol isomer) has substantial affinity for inhibiting inositol transport (43). This anomaly was finally explained when SMIT2 (K T ∼ 0.12 mM) was cloned, expressed and found to exist in neurons but not CP (45). Unlike SMIT1, the expressed SMIT2 transporter has similar affinity for myo-inositol, scyllitol and (+) chiro-inositol (45). Thus, neurons possess two different systems (SMIT 1 and 2) to accumulate myo-inositol to levels approximately 10–20 times higher than in CSF (38,44,45). The exact role(s) of SMIT2 in brain is not well understood (Table III).
Under normal circumstances, brain cells are surrounded by CSF (and presumably the extracellular space of brain) at a concentration of inositol approximately four times higher than the SMIT 1 and 2 K T values (29,44,45). This is due, in large part, to the active inositol (SMIT 1) transport system in CP (2,9,38–45). Thus, increasing CSF inositol concentrations further will not appreciably alter brain concentrations, since inositol passes minimally through membranes by simple diffusion (39–43). Moreover, raising plasma concentrations will saturate the carriers at both the CP and BBB, which are half saturated at the normal plasma concentration (39–43). Consequently, these systems will transport relatively less inositol into CSF and the extracellular space brain. On the other hand, with low plasma concentrations, relatively more inositol is pumped into CSF and brain at the BBB and CP, thus completing an elegant and powerful homeostatic system for inositol under normal circumstances (39–43).
An unexpected discovery was that the inositol homeostatic system in the CNS is flexible as discovered in its osmolyte role (38,46). When the plasma concentration of sodium or other osmolytes was increased, the concentration of SMIT1 in rat brain increased (38,46). This was associated with increased SMIT1 activity and the accumulation of more inositol in brain cells, which partially compensated for the increased plasma osmolarity (38,46). Strong hybridization signals for SMIT mRNA were also observed in choroid plexus (46). The brain cannot tolerate osmotic imbalances and swelling, since it resides in the rigid skull.
In homozygous SMIT1 KO mice, although the brain is anatomically normal at birth, the brain concentration of inositol is only 7% of normal (47). The concentration of inositol in CSF was not measured but almost certainly would be low. At birth, the newborn mice did not breathe, and immediately died, secondary to functional abnormalities in the brain respiratory control system and abnormal peripheral nerve development, especially in the phrenic nerve (47). These findings in the SMIT1 KO mice are expected in view of the data showing the crucial in vivo role of SMIT1 in the CP and in the neuronal uptake of inositol (47). However, when massive oral doses of inositol were given to the dams and post-weanling homozygous KO SMIT1 mice, many of the KO mice survived, but had only 10 and 39% as much inositol as normal mice in sciatic nerve and brain, respectively (48). Functionally, these KO mice had only minimal abnormalities as long as they received 1% inositol in their drinking water (48).
These studies have important therapeutic implications. They reaffirm that it is not possible to increase brain levels of inositol appreciably in normal mammals including humans (49) by oral intake and explain why. Thus, oral inositol for therapy of psychiatric disease e.g., depression, is not rational. Moreover, these studies not only provide deep insight into inositol homeostasis in brain under normal circumstances, but also under osmotic challenges, i.e., with up-regulation of SMIT1 and increased transport of inositol (and other osmolytes via other systems) into brain (38).
Finally, these studies with SMIT1 KO mice provide compelling evidence that the “inositol depletion hypothesis” for the mechanism of action (and toxicity) of lithium, carbamazapine and valproate as mood stabilizers is highly unlikely (50). For decades investigators have postulated that lithium, and more recently carbamazapine and valproate cause depletion of inositol in brain by various mechanisms, that this depletion leads to an impairment of inositol signaling, and therefore mood stabilization (50). In homozygous KO mice, with markedly decreased inositol brain levels, there is no decrement in the concentration of phophatidylinositol or the potential for inositol signaling (50). A large amount of literature and extensive, expensive drug discovery efforts have erroneously been based on the “inositol depletion hypothesis” (51).
Riboflavin Transport Through Choroid Plexus
In 1979 and 1980, Bressler et al. working with fluorescein (52) and our group with riboflavin (53) studied the transport of these molecules in the isolated choroid plexus in vitro using fluorescence microscopy and other techniques. Both fluorescein and riboflavin were transported from the apical side into the choroid plexus epithelial cells and then pumped out of the basolateral side into the subepithelial/vascular space (Fig. 1) (52,53). On both sides, there was a large step-up increase in fluorescence. This was especially striking with riboflavin as it was pumped out of the CP cells across the basolateral membrane into the subepithelial/vascular space (∼ tenfold increase) (53). We postulated that there must be two specific active transport systems for riboflavin, one at the apical membrane (influx) and another at the basolateral surface (efflux into blood in vivo) (Fig. 1). We immediately realized that riboflavin transport by CP was completely different from the transport of AA, inositol and MeTHF discussed above; the directionality was opposite (Fig. 1).
When we initiated our studies with riboflavin, we knew it was difficult to deplete riboflavin in brain by dietary means (54,55). To understand this profound total riboflavin homeostasis in brain, we first studied riboflavin transport in the CNS (55). [By total riboflavin, we mean riboflavin plus the active phosphorylated forms FMN and FAD (55)] The concentrations of total riboflavin in plasma, CSF and brain were 0.2, 0.1 and 8.8 μM, respectively, in adult rabbits; the riboflavin concentrations, the moiety actually transported, were 0.05, 0.02, and 0.1–0.2 μM, respectively (Table IV) (55).
As part of our studies we found a saturable riboflavin transport system in vivo at the BBB with a K T ∼ 0.1 μM, similar to the plasma concentration. We also found a facilitated diffusion system in brain slices in vitro with a K T ∼ 0.1 μM (56). Riboflavin transport by these systems was inhibited by other flavins with or without sugars (e.g., lyxoflavin, lumiflavin and lumichrome) but not by probenecid or penicillin (55–57). Thus, we understood how and where riboflavin entered brain, i.e., by two gates (transport systems) in series; first through the BBB and then into brain cells by facilitated diffusion.
However, we were surprised at how rapidly intraventricularly injected riboflavin was cleared from the CSF by the saturable system in CP (K T ∼ 80 μM) (54,55). In vivo in rabbits, 2 h after the intraventricular injections of tracer quantities of riboflavin and the much smaller passive marker mannitol, less than 1% of the injected riboflavin was in the CSF versus 35% of the mannitol; this was not due to riboflavin uptake by brain (55). Concurrently injected probenecid decreased the clearance of riboflavin from the CSF (55). We were also surprised to find (both in vitro and in vivo) that the active, sodium-dependent riboflavin uptake system in CP had a very broad specificity; penicillin G was a competitive inhibitor of riboflavin transport in CP and vice versa; probenecid, quinine, quinidine, aminohippurate (PAH), fluorescein and various flavins inhibited riboflavin and/or penicillin transport (K I) at concentrations between 0.01 and 0.1 mM (Table IV) (53–55,57). These riboflavin studies confirmed and extended our prior studies of penicillin transport in the CNS (58).
Subsequently, other investigators have corroborated and expanded these studies. Galla and associates showed; 1) the ability of porcine CP monolayers to transport riboflavin from the apical to basal side, and then release riboflavin into the CSF side, 2) the K T values for riboflavin and penicillin in the porcine system were almost identical to the K T values in vitro in the isolated rabbit CP, and 3) that riboflavin and penicillin were competitive inhibitors of each others transport (16). However, a small amount of riboflavin transport into CP appeared to be by a separate undefined system (16).
Since the initial descriptions of penicillin, fluorescein and riboflavin transport in CP, there have been several hundred transporters described and classified (8). Until recently it was unclear which transporters transported which substances (8). With the recent cloning and expression of the organic acid transporter 3 (OAT 3), we are now on firmer ground for understanding CP riboflavin and weak acid transport (59). Moreover, when homozygous OAT 3 KO mice were studied, they were: 1) apparently normal, and 2) their CP, as expected, could only transport about 25% as much fluorescein or PAH as normal, thus confirming the role of OAT 3 in transporting these substances into CP epithelial cells (59,60). As expected, taurocholate is not transported by OAT 3 (59,60). Recently OAT 3 has also been described on the abluminal side of the brain capillaries, where it transports OAT 3 ligands (e.g., penicillin) from the extracellular space of brain into blood (61).
However, it still remains unclear how the putative OAT 3 ligands including riboflavin, penicillin and fluorescein exit CP on the basolateral (blood) side, or by analogy exit brain capillaries on the luminal side. We suspect that in CP there may be different efflux carriers for various molecules transported into CP from the apical side by OAT 3. One candidate is the ATP-requiring Multidrug Resistance Protein 1 (MRP1), located on the basal side of the CP; another is the organic acid transport protein 2 (OATP 2), for equilibrative efflux transport (62).
It is worth noting that the CP apical membrane contains many other transport systems, some of which have been cloned e.g., peptide transporter 2 (PEPT 2) that transports aminopenicillins out of CSF via the CP, and organic acid transport protein 3 (OATP 3) that probably transports substantial amounts of estrone sulfate out of CSF (63,64). How these substances leave the basolateral surface of CP is unclear. Another separate system transports the methotrexate–fluorescein conjugate into CP and is visualizable with confocal fluorescence microscopy (64). Unlike riboflavin, the entry of the conjugate through the apical membrane of the CP epithelial cell is equilibrative, but the conjugate is then vigorously pumped out of the cell into the subepithelial/vascular space by a powerful, clearly concentrative mechanism (64).
In summary, riboflavin which slowly turns over in brain (∼1% per hour) enters brain through the BBB and brain cells by two systems in series, both approximately one-half saturated at the plasma concentration (0.2 μM). These systems have no affinity for penicillin or probenecid. These serial systems prevent excess transfer of riboflavin from blood into brain. At low concentrations of riboflavin in blood, they pump relatively more riboflavin into the CNS. Most surprising is the powerful system (presumably OAT 3) in CP (K T for riboflavin ∼ 80 μM) that rapidly clears riboflavin and many other ligands and drugs from CSF in a two-step process as noted above.
A comparison with the kidney is revealing. The kidney contains a saturable uptake system in the tubules that reabsorbs almost all the filtered riboflavin (65). However, when the plasma concentration goes above 0.5 μM, the system becomes saturated and riboflavin spills into the urine. Moreover, above 0.5 μM, a riboflavin secretory system in the tubules becomes apparent, and the renal clearance of riboflavin goes well above the filtration rate. The one-half saturation concentration of the renal secretory system for riboflavin is ∼80 μM (65); in comparison, the same value of 80 μM was determined for CP reabsorptive transport (54,55). Gerhard Levy suggested and provided evidence 40 years ago that riboflavin was secreted on the same system that secreted penicillin and PAH into the urine (65). We provided confirmatory evidence for Levy's hypothesis in rabbit kidney slices (66). Thus, both in the CNS and kidney, there are bidirectional systems for riboflavin to provide exquisite homeostasis. By chance, many drugs apparently “hitchhike” on the riboflavin secretory systems in kidney, CP, and brain capillaries. In our view, the name OAT 3 is a misnomer. From a teleological viewpoint, the OAT 3 system is a riboflavin transporter that provides riboflavin homeostasis in kidney and CP.
As described above, the OAT 3 system in CP and the abluminal side of the BBB, transports many important drugs out of the brain and CSF. More recently, cimetidine, certain statins, and AZT have also been shown to be substrates for transport by OAT 3 (3,8). Not only does OAT 3 obviously interfere with therapy (e.g., by transporting AZT out of the CNS in the therapy of AIDS or penicillin in the treatment of meningitis) but also there are other drug efflux transporters like p-glycoprotein at the BBB, and MRP 1 in CP which also transport drugs out of CSF and brain (3,8). Now that OAT 3 has been sequenced, cloned and expressed it seems eminently possible to make a high affinity inhibitor of OAT 3 which will block efflux transport (59,60). In null OAT 3 KO mice, the mice seem normal so this approach is probably safe (59,60). Also, as noted above, not all riboflavin transport through the CP is via OAT 3 so there is a “safety valve” for riboflavin transport from CSF. Years ago, we tried this approach by pretreating normal and meningitic rabbits with probenecid to increase penicillin levels in CSF (58). Unfortunately, although we achieved a small effect, probenecid does not have a high enough affinity for the carrier (OAT 3) in CP at a safe, achievable CSF concentration (58). A better (submicromolar) inhibitor is needed.
Urate Transport Through Renal Tubules
In the past, the explanation of urate handling by kidney was complex. Of the filtered urate at the glomerulus, about 90% is reabsorbed. This was explained by presecretory reabsorption, secretion, and postsecretory absorption (67). This theory, based on physiological and indirect pharmacological experiments, we now believe is incorrect (67). A better understanding of urate handling in the human kidney was proposed after the principal urate transporter was cloned, expressed and termed URAT 1 (67). Patients who are homozygous (or compound heterozygotes) for abnormalities in URAT 1 have very low serum uric acid concentrations and do not respond to probenecid or pyrizinamide, unlike normal subjects (68). With this recent information, the newly cloned urate-anion exchanger (URAT 1) alone can explain urate handling by the kidney; there is no need to invoke urate secretion and postsecretory reabsorption (68). Specifically it is now clear that probenecid inhibits urate reabsorption by URAT 1 and that pyrazine carboxylic acid, the active metabolite of pyrazinamide, enhances urate reabsorption via URAT 1 by enhancing urate–pyrazine carboxylic acid exchange, thus increasing urate reabsoption (68). In short, understanding urate handling by the kidney has simplified and corrected our understanding of the pharmacology of drugs that interact with URAT 1 (67,68). But why humans reabsorb 90% of filtered urate, an apparent waste product of purine metabolism, remains unclear. Soluble uric acid can stimulate vascular smooth muscle cell proliferation by activating mitogen-activated protein kinases (69). Whether this is an important physiological role for uric acid remains to be determined.
Conclusions
As described in the five examples in this commentary, the transfer of many drugs and most nutrients through the CP and urate through the renal tubules cannot be explained by lipid solubility, charge or molecular size. Simple diffusion accounts for little of the throughput transfer. Instead, there are multiple different systems with varying specificities. Furthermore, in most cases there are (or must be) carriers (or receptors) on both sides of the epithelial cells, each with different specificities. There have been many surprises. For example, the riboflavin transport systems in choroid plexus and the renal tubule exemplify endogenous nutrient systems with very broad specificities, notwithstanding the widespread belief that nutrient systems tend to specific.
With our recent expanded knowledge of these systems, we can now usefully employ that information and manipulate these and/or related systems. AA can be delivered to brain not as AA but as DHAA with reduction to AA in brain. Massive doses of reduced folate can be employed to treat FRα transport failure. We now have strong evidence that the inositol depletion hypothesis for lithium action is extremely unlikely. Finally, it is formally possible to increase the concentration of many important drugs in CSF and brain, e.g., penicillin and AZT. The previous notion of the brain and CSF as a pharmacological sanctuary is not absolute. Moreover, there are many other systems in CP and the BBB that can be employed, manipulated or altered to improve the delivery of small molecules into brain (70) without having to resort to direct injection into the brain substance or CSF.
However, there are still many unknowns. For example, we do not have a clear idea why URAT 1 exists. What is the purpose of the dynamic, two-step riboflavin transport system in CP that so vigorously removes riboflavin from the CSF? Is it just there for total riboflavin homeostasis in brain? What is the nature of the CP transporters for AA, folate, inositol and riboflavin that extrude these micronutrients from the epithelium? We understand reasonably well the uptake transporters, i.e., SVCT 2, FRα, SMIT1 and OAT 3, respectively, but that is only half of the story. Why does OAT 3 have such broad specificity? From a medicinal chemistry point of view, how does OAT 3 manage such a broad specificity? It is like P-glycoprotein and the MRP systems, which also have very broad but different specificities (8). Finally, in our view, although complex, all the above questions are now solvable with time, dedication and resources. The pharmacological future is bright. As documented above, as molecular knowledge advances, drug therapy in general and in the CNS in particular will steadily improve.
References
H. Davson and M. B. Segal. Physiology of the CSF and Blood–Brain Barriers, CRC, Boca Raton, 1996.
R. Spector and C. E. Johanson. The mammalian choroid plexus: structure, development and function. Sci.Am. 261:68–74 (1989).
R. Spector. Drug transport in the mammalian central nervous system: multiple complex systems. Pharmacology60:58–73 (2000).
D. E. Smith, C. E. Johanson, and R. F. Keep. Peptide and peptide analog transport systems at the blood–CSF barrier. In Y. Sugiyama and J-F. Ghersi-Egea (eds.), Drug Transfer in the Choroid Plexus. Multiplicities and Substrate Specificities of Transporters, Adv. Drug Deliv. Rev., 56:1765–1791 (2004).
C. E. Johanson. The choroid plexus–CSF nexus: gateway to the brain. In P. M. Conn (ed.), Neuroscience in Medicine, 2nd ed., Humana, Totowa, New Jersey, 2003, pp. 165–195.
R. Spector. Megavitamin therapy and the central nervous system. In M. H. Briggs (ed.), Vitamins in Human Biology andMedicine, CRC, Boca Raton, Florida, 1981, pp. 138–156.
R. Spector. Vitamin homeostasis in the central nervous system. N. Engl. J. Med. 296:1393–1398 (1977) (Seminar in Medicine).
K. M. Giacomini and Y. Sugiyama. Membrane transporters and drug response. In L. L. Brunton, J. S. Lazo, and K. L. Parker (eds.), Goodman and Gilman's The Pharmacological Basis of Therapeutics, McGraw Hill, New York, 2005, pp. 41–70.
R. Spector. Micronutrient homeostasis in mammalian brain and cerebrospinal fluid. J. Neurochem. 53:1667–1674 (1989).
Y. Nagata, H. Kusuhara, H. Endou, and Y. Sugiyama. Expression and functional characterization of rat organic anion transporter 3 (rOAT 3) in the choroid plexus. Mol. Pharm. 61:982–988 (2002).
W. M. Pardridge, Introduction to the Blood–Brain Barrier. Cambridge University Press, UK, 1998.
J. B. Pritchard and D. S. Miller. Expression systems for cloned xenobiotic transporters. Toxicol. Appl. Pharmacol. 204:256–262 (2005).
M. E. Rice. Ascorbate regulation and its neuroprotective role in brain. TrendsNeurosci. 23:209–216 (2000).
L. Hammarstrom. Autoradiographic studies on the distribution of 14C-labelled ascorbic acid and dehydroascorbic acid. Acta Physiol. Scand.70(suppl. 289):1–79 (1966).
R. Spector. Penetration of ascorbic acid from cerebrospinal fluid into brain. Exp. Neurol. 72:645–653 (1981).
A. Hakvoort, M. Haselbach, and H. J. Galla. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 795:247–256 (1998).
S. Angelow, P. Zeni, and H. J. Galla. Usefulness and limitation of primary cultured porcine choroid plexus epithelial cells as an in vitro model to study drug transport at the blood–CSF barrier. Adv. Drug Del. Rev. 56:1859–1873 (2004).
R. Spector and A. V. Lorenzo. The specificity of ascorbic acid transport system of the central nervous system. Am. J. Physiol. 226:1468–1473 (1974).
D. K. C. Lam and P. M. Daniel. The influx of ascorbic acid into the rat's brain. Quart. J. Exp. Physiol. 71:483–489 (1986).
D. B. Agus, S. S. Bambhir, W. M. Pardridge, et al. Vitamin C crosses the blood–brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 100:2842–2848 (1997).
H. Tsubaguchi, T. Tokui, B. Mackenzie, et al. A family of mammalian Na+-dependent l-ascorbic acid transporters. Nature399:70–75 (1999).
A. Astuya, T. Caprile, M. Castro, et al. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J. Neurosci. Res. 79:146–156 (2005).
M. D. L. A. Garcia, K. Salazar, C. Millan, et al. Sodium vitamin C cotransporter SVCT 2 is expressed in hypothalamic glial cells. Glia50:32–47 (2005).
S. Sotiriou, S. Gispert, J. Cheung, et al. Ascorbic acid slc 23 a1 is essential for vitamin C transport into brain and for perinatal survival. Nat. Med. 8:514–517 (2002).
J. Hung, D. B. Agus, C. J. Winfree, et al. Dehydorascorbic acid, a blood–brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. PNAS98:11720–11724 (2001).
R. Spector and A. V. Lorenzo. Folate transport by the choroid plexus in vitro. Science187:540–542 (1975).
R. Spector and A. V. Lorenzo. Folate transport in the central nervous system. Am. J. Physiol.229:777–782 (1975).
S. A. Suleiman and R. Spector. Purification and characterization of a folate binding protein from porcine choroid plexus. Arch. Biochem. Biophys.208:87–94 (1981).
Y. Wang, R. Zhao, R. G. Russel, and I. D. Goldman. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochim. Biophys. Acta1513:49–54 (2001).
V. T. Ramaekers, S. I. Hansen, J. Holm, et al. Reduced folate transport to the CNS in female Rett patients. Neurology61:506–515 (2003).
R. Spector. Development of the vitamin transport system in choroid plexus and brain. Neurochemistry33:1317–1319 (1979).
B. A. Kamen and A. K. Smith. A review of folate receptor alpha and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv. Drug Del. Rev.56:1085–1097 (2004).
D. Wa and W. M. Pardridge. Blood–brain transport of reduced folic acid. Pharm. Res.16:415–419 (1999).
V. T. Ramaekers and N. Blau. Cerebral folate deficiency. Dev. Med. Child Neurol.46:843–851 (2004).
J. A. Piedrahita, B. Oetama, G. D. Bennett, et al. Mice lacking the folic-acid binding protein Folbp1 are defective in early embryonic development. Nat. Genet.23:228–232 (1999).
V. T. Ramaekers, S. P. Rothenberg, J. M. Sequeira, et al. Auto antibodies to folate receptors in the cerebral folate deficiency syndrome. N. Engl. J. Med.352:1985–1991 (2005).
S. Xiao, D. K. Hansen, E. T. M. Horsley, et al. Maternal folate deficiency results in selective upregulation of folate receptors and heterogenous nuclear ribonucleoprotein-E1 associated with multiple subtle aberrations in fetal tissues. Birth Defects Res. (Part A): Clin. and Mol Teratol.73:6–28 (2005).
S. K. Fischer, J. E. Novak, and B. W. Agranoff. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J. Neurochem.82:736–754 (2002).
R. Spector and A. V. Lorenzo. Myo-inosital transport in central nervous system. Am. J. Physiol.228:1510–1518 (1975).
R. Spector and A. V. Lorenzo. The origin of myo-inositol in brain, cerebrospinal fluid and choroid plexus. J. Neurochem.25:353–354 (1975).
R. Spector. The specificity and sulfhydryl sensitivity of the inositol transport system of the central nervous system. J. Neurochem.27:229–236 (1976).
R. Spector. Myo-inositol transport through the blood–brain barrier. Neurochem. Res.13:785–787 (1988).
R. Spector. Inositol accumulation by brain slices in vitro. J. Neurochem.27:1273–1276 (1976).
I. Inoue, S. Shimoda, Y. Minami, et al. Cellular localization of Na+/myo-inositol 1 co-transporter in RNA in the rat brain. NeuroReport7:1195–1198 (1996).
M. J. Coady, B Wallendorff, D. G. Gagnon, and J. Y. Lapointe. Identification of a novel Na+/myo-inositol cotransporter. J. Biol. Chem.277:35219–35224 (2002).
Y. Minami, K. Inoue, S. Shimada, et al. Rapid and transient upregulation of Na+/myo-inositol cotransporter transcription in the brain of acute hypernatremic rats. Mol. Brain Res.40:64–70 (1996).
G. T. Berry, S. Wu, R. Buccafusca, et al. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. J. Biol. Chem.278:18297–18302 (2003).
J. F. L. Chau, M. K. Lee, J. W. Law, et al. Sodium myo-inositol cotransporter-1 is essential for the development and function of the peripheral nerves. FASEB J. 19:1887–1889 (2005).
C. M. Moore, J. L. Breeze, T. J. Kukes, et al. Effects of myo-inositol ingestion on human brain myo-inositol levels: a proton magnetic resonance spectroscopic study. Biol. Psychiatry45:1197–1202 (1999).
G. T. Barry, R. Buccafusca, J. J. Greer, and E. Eccleston. Phosphoinositide deficiency due to inositol depletion is not a mechanism of lithium action in brain. Mol. Genet. Metab. 82:87–92 (2004).
J. R. Alack. Inositol monophosphate inhibitors—lithium mimetics? Med. Res. Rev. 17:215–224 (1997).
S. E. Bresler, V. M. Bresler, E. N. Kazbekov, A. A. Nikitorov, and N. N. Vasilieva. On the active transport of organic acids fluorescein in the choroid plexus of the rabbit. Biochim. Biophys. Acta. 550:110–119 (1979).
R. Spector. Riboflavin transport in the central nervous system: characterization and effects of drugs. J. Clin. Invest. 66:821–831 (1980).
R. Spector and B. Boose. Active transport of riboflavin by the isolated choroid plexus in vitro. J. Biol. Chem. 254:10286–10289 (1979).
R. Spector. Riboflavin homeostasis in the central nervous system. J. Neurochem. 35:202–209 (1980).
R. Spector. Riboflavin accumulation by rabbit brain slices in vitro. J. Neurochem. 34:1768–1771 (1980).
R. Spector. Lumiflavin and lumichrome transport in the central nervous system. J. Neurochem. 36:1186–1191 (1981).
R. Spector and A. V. Lorenzo. Inhibition of penicillin transport from the cerebrospinal fluid after intracranial inoculation of bacteria. J. Clin. Invest. 54:316–325 (1974).
D. H. Sweet, D. S. Miller, J. B. Pritchard, Y Fiyuvare, D. R. Brier, and S. K. Nigam. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat 3 slc22a8) knockout mice. J. Biol. Chem. 277:26934–26943 (2002).
D. Sykes, D. H. Sweet, S. Lowes, S. K. Nigam, J. B. Pritchard, and D. S. Miller. Organic anion transport in choroid plexus from wild-type and organic anion transporter 3 (slc22a8)-null mice. Am. J. Physiol. 286:F972–F978 (2004).
R. Kikuchi, H. Kusuhara, D. Sugiyama, and Y. Sugiyama. Contribution of organic anion transporter 3 (slc22a8) to the elimination of p-aminohippuric acid and benzylpenicillin across the blood–brain barrier. J. Pharmacol. Exp. Ther. 306:51–58 (2003).
C. M. Breen, D. B. Sykes, G. Fricker, and D. S. Miller. Confocal imaging of organic anion transport in intact rat choroid plexus. Am. J. Physiol. 282:F877–F885 (2002).
S. M. Ocheltree, H. Shen, Y. Hu, J. Xiang, R. F. Keep, and D. E. Smith. Mechanisms of cefadroxil uptake in choroid plexus: studies in wild-type and PEPT 2 knock-out mice. J. Pharmacol. Exp. Ther. 308:462–467 (2004).
D. S. Miller. Confocal imaging of xenobiotic transport across the choroid plexus. Adv. Drug Del. Rev. 56:1811–1824 (2004).
W. J. Jusko and G. Levy. Absorption, protein binding and elimination of riboflavin. In Rivlin (ed.), Riboflavin, Plenum, New York, 1975, pp. 100–152.
R. Spector. Riboflavin transport by rabbit kidney slices: characterization and relation of cyclic organic acid transport. J. Pharmacol. Exp. Ther. 221:394–398 (1982).
A. Enomoto, H. Kimura, A. Chairougdue, et al. Molecular identification of a renal-urate exchanger that regulates blood urate levels. Nature417:447–451 (2002).
K. Ichida, M. Hosoyamada, I. Hisatome, et al. Clinical and molecular analysis of patients with renal hypouricemia in Japan—influence of URAT 1 gene on urinary urate excretion. J. Am. Soc. Nephrol. 15:164–173 (2004).
D. Kang, L. Han, X. Ouyang, A. Kahn, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am. J. Nephrol. 25:425–433 (2005).
C. Johanson, J. Duncan, E. Stopa, and A. Baird. Enhanced prospects for drug delivery and brain targeting by the choroid plexus–CSF route. Pharm. Res. 22:1011–1037 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spector, R., Johanson, C. Micronutrient and Urate Transport in Choroid Plexus and Kidney: Implications for Drug Therapy. Pharm Res 23, 2515–2524 (2006). https://doi.org/10.1007/s11095-006-9091-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9091-5
Key words
- ascorbic acid
- blood–brain barrier
- blood–cerebrospinal fluid (CSF) barrier
- choroid plexus epithelium
- folate
- folate receptor (FRα)
- inositol
- myo-inositol cotransporter (SMIT 1)
- organic acid transporter 3 (OAT 3)
- penicillin
- riboflavin
- sodium ascorbate cotransporter (SVCT 2)
- uric acid transporter (URAT 1)
- vitamin homeostasis