Purpose
The aim of this study was to develop a rheological method to evaluate the stability of highly viscous pharmaceutical emulsions. Thereby, the time devoted to the storage tests could be reduced and manufacturers could save time in optimizing their formulations and manufacturing techniques for topical pharmaceutical forms. The influence of the type of oil and the type of emulsifier on the microstructure of the emulsions was also studied.
Materials and Methods
The samples were stored at 25 and at 50°C for 6 months and analysed every month using rheological as well as microscopic techniques. The size and the organization of the droplets within the emulsion were determined by freeze-fracture electron microscopy and optical contrast phase microscopy.
Results
A decrease in the rheological parameters was observed for the OC emulsions (“Tween/Span” emulsions made with olive oil) and the “Montanov” emulsions. The rheological measurements showed that the structure of the OC emulsions and that of the emulsions made with the Montanov 82 emulsifier become more brittle when submitted to a shear force. The micrographs obtained by freeze-fracture electron microscopy showed that for OC only the oil droplets arrange in a network structure. Storage tests at 50°C confirmed the rheological assumptions on the stability of emulsions.
Conclusion
The study of the effect of shearing on the emulsions allowed quick discrimination between the emulsions according to their stabilities. The rheological tool gave information on the structure of the emulsions and on the aging process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Oil in water” (O/W) or “water in oil” (W/O) emulsions are among the most frequently used colloidal systems, largely present in various fields including cosmetics, pharmaceuticals, foods, paints, petrochemicals, etc... All these emulsions evolve with time. They are thermodynamically unstable, usually splitting into two distinct phases. This instability could become manifest through a variety of physicochemical destabilizing processes, such as creaming (or sedimentation), flocculation, coalescence or phase inversion (1–4).
Depending on the field of application of these emulsions, their stability and rheology vary widely. Thus industries need a better understanding of the factors that determine the rheological properties and the stability of their products in order to obtain the necessary physical characteristics. Emulsions are complex fluids whose rheological parameters should always be considered in relation with the properties and interactions of the droplets constituting the colloid. The understanding of the emulsion microstructure is a main issue when it comes to optimizing the formulation and manufacturing techniques of topical pharmaceutical forms.
For the last few years, a large amount of experimental and theoretical research has been carried out to study and determine the factors which influence the rheology and stability of emulsions (5–7). The most significant factors are the rheological behaviour of the continuous phase, the nature of the particles, their concentration, their size distribution and the particle–particle interactions (8–11). Moreover, one of the main concerns of the scientists and manufacturers is to reduce as much as possible the time they spend studying the stability of their new formulations. The influence of the temperature and of the storage time on the rheological properties of the emulsions has been dealt with in several papers (7,12–16). According to these studies, the aging of the emulsions seems to affect their rheological properties. However no study has yet defined a method to quickly determine the stability of pharmaceutical or cosmetic creams. The purpose of this work was to develop a simple and fast rheological method which would initially make it possible to compare the stability of the formulated emulsions without having to carry out storage tests that could last several months. This would save manufacturers considerable time when choosing the emulsifiers and additives and optimizing their formulations.
Seven highly viscous O/W emulsions were formulated with three different oils, three different emulsifiers and a polymer. Four batches of each emulsion type were made, of which two were stored for 6 months at 25°C (room temperature) and two for 6 months at 50°C. They were characterized over time by rheological measurements and microscopic analyses. The effect of aging on the rheological parameters of the emulsions was studied. In this work, we assessed the effect of the interactions between the emulsifier, the oil and the polymer on the emulsion stability with time. The viscoelastic behaviour of creams can be determined by dynamic and static rheological methods. These methods give detailed information about the structural changes. Thus, steady-state flow and oscillatory measurements were used to characterize the rheological properties of the emulsions. These analyses were completed with macroscopic observations and microscopic measurements (at 25 and 50°C).
The aim of this study was to follow the evolution of the viscoelastic properties of the different emulsions during aging using a rheological method, and then to classify these emulsions according to their stability.
Materials and Methods
Raw Materials and Emulsion Preparations
The following substances were used for the preparation of the emulsions. Oils: sweet almond oil (COOPER, France), apricot core oil (COOPER, France), olive oil (COOPER, France); humectant: glycerine (glycerol, COOPER, France); emulsifiers: Montanov 68 (cetearyl alcohol and cetearyl glucoside, SEPPIC, France), Montanov 82 (cetearyl alcohol and coco-glucoside, SEPPIC, France), Tween 20 (polyoxyethylene (20) sorbitan monolaurate, PROLABO, France), Span 60 (sorbitan monostearate, SEPPIC, France); thickener: Carbopol® 980 (carboxyvinylic polymer C980, BF GOODRICH); preservatives: Sepicide CI (imidazolidinyl urea, SEPPIC, France), Methylparaben (methyl-parahydroxybenzoate, COOPER, France), Propylparaben (propyl-parahydroxybenzoate, COOPER, France). Sodium hydroxide (10% (w/w), Merck) was added to neutralize the polymer. Demineralized water was used to prepare the emulsions.
Seven highly viscous O/W emulsions (OC, NC, ADC, OM68, NM68, OM82, NM82) were prepared according to a direct emulsification process (17,18) with different oils (olive oil, apricot core oil and sweet almond oil) and different emulsifiers (“Tween20/Span60” and “Montanov 68/82”). The compositions of the emulsions (Table I) were chosen so that it would be possible to determine the influence of the oil and of the emulsifier on their stability and the interaction between the emulsifier and the polymer in the case of the “Tween/Span” emulsions (OC, NC, ADC). For each formulation, two batches were made for each temperature to ensure reproducibility of the results (OC-1 and OC-2 for 25°C storage, OC-3 and OC-4 for 50°C storage).
The oil phase (oil with emulsifier) and the aqueous phase were heated at 75°C and mixed together using a Polytron® (homogenizer rotor-stator) at 20,000 rpm for 3 min. They were then stirred at 300 rpm for 20 min. Preservatives and glycerine were added at 30°C. Then, each emulsion was stirred for 5 min at room temperature to be homogenized. The polymer (Carbopol®) was dispersed gradually into the aqueous phase and neutralized (pH = 7) by a 10% sodium hydroxide solution for the “Tween/Span” emulsions. Carbopol® was solubilized in the aqueous phase 24 h before the preparation in order to obtain a better homogenization of the polymer in the preparation.
The emulsions were stored for 6 months at 25 and 50°C to visualize a possible destabilization and analysed every month. Three measurements were performed for each emulsion to validate the repeatability of the results.
Rheology
The rheological properties were determined using a strain-controlled AR 1000 rheometer (TA Instrument) with cone-plate geometry (diameter 60 mm, angle 1°59, truncation 68 μm). The measuring device was equipped with a temperature unit (Peltier plate) that provided very good temperature control over an extended period of time. For all experiments, the temperature was maintained at 25°C ± 0.01. Each sample was characterized 24 h after its preparation in order to ensure a complete equilibration of the emulsions, as commonly performed in many studies (19–21) and then measurements were run every month.
Steady-state flow
To characterize the system the steady-state flow mode was used. Each emulsion was submitted to a shear stress (τ) which increased from 0.2 to 120 Pa. This protocol allowed the measurement of two parameters: the apparent viscosity (η 0) and the yield stress (τ 0), represented on Fig. 1a. A log–log plot of viscosity versus shear stress made the spotting of the yield stress easier, as opposed to stress–strain curves (22). Two successive steady-state flow measurements were carried out with a two-minute interval to test if the shearing has an influence on the rheological parameters.
Oscillatory rheology
Oscillatory rheological measurements (dynamic rheology) were performed to complete the information obtained on the viscoelastic properties of the emulsions. This oscillatory mode makes it possible to avoid the breakdown of the emulsion structure, i.e., keeping the material within its linear viscoelastic region, within a determined frequency range. Above a certain value of the applied strain, the deformation is no longer sinusoidal and the material starts flowing (end of the linear viscoelastic region). In the oscillation stress sweep test, τ was increased from 0.01 to 200 Pa and the frequency was kept constant (1 Hz). The linear viscoelastic region of each emulsion was determined thanks to the oscillation stress sweep test (Fig. 1b). In the linear viscoelastic region, the stress to strain ratio was only a function of time. In the oscillation frequency sweep test, the frequency was increased from 0.001 to 30 Hz and the stress was kept constant (τ = 1 Pa).
Four rheological parameters were measured using the oscillatory mode: the storage modulus or elastic modulus (G′), the loss modulus or viscous modulus (G″), the loss tangent (tan δ) and the yield stress (τ 0). They are given by the following equations:
where ω is the frequency (rad•s−1), γ 0 is the amplitude and δ is the phase angle.
Each rheological measurement was carried out at least three times. The variation coefficient did not exceed 9% for the steady-state measurements and 5% for the dynamic measurements (stress sweep and frequency sweep).
Freeze-Fracture Electron Microscopy and Phase Contrast Microscopy
Freeze-fracture electron microscopy and phase contrast microscopy were used to observe the organization of the droplets and their size distribution in the emulsions. The freeze-fracture electron microscopy also makes it possible to observe the emulsion structure. Neither technique required any dilution of the emulsions and so did not alter their structures.
For the freeze fracture technique, a drop of emulsion was deposited in a cell cooled in propane with liquid nitrogen (−196°C), which solidifies the structure of the emulsion. The sample was vacuum inserted (10−6 Torr) in a cryogenic enclosure and cut transversely. Then the sample was metallized by a deposit of Pt/C or “shadow” to give emerging relief to the structure of the planes of fracture. The fractured surfaces were examined using a Philips XL30-SFEG transmission electron microscope equipped with a motorized support.
The analyses of the “Montanov” emulsions were carried out using a optical phase contrast microscope (Nikon's Eclipse E600) equipped with a CDD camera (magnification X 40). The micrographs were collected and analysed using the Lucia G version 4.8 software to measure the size, size distribution, maximum diameter and average diameter of the droplets. Each value obtained for each emulsion was an average of ten measurements (from ten micrographs).
Results and Discussion
Storage Tests at 25 and 50°C
Macroscopic Observations
The stability of the emulsions was observed at two temperatures: 25 and 50°C. At definite time intervals (1, 2, 3, 4, 5 and 6 months), the stability was assessed at room temperature by visual observation of the possible separation of the emulsion phases. All emulsions studied were stable at 25°C during the 6 months of storage.
Destabilization was observed during the aging for emulsions stored at 50°C:
-
destabilization of the OC emulsion after 3 months at 50°C;
-
destabilization of the NC and ADC emulsions after 4 months at 50°C;
-
destabilization of the emulsions formulated with the “Montanov 68” emulsifier (OM68 and NM68) after 3 months at 50°C;
-
creaming of the emulsions formulated with the “Montanov 82” emulsifier (OM82 and NM82) after 1 month at 50°C and then destabilization after 3 months.
According to these macroscopic observations, the emulsions formulated with the “Montanov 82” emulsifier (OM82 and NM82) were the most unstable (they broke down earlier than the others). NC and ADC were the most stable emulsions at 50°C.
Rheological Measurements at 25°C
Steady-State Flow: Evolution of the Apparent Viscosity η 0 and of the Yield Stress τ 0
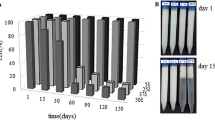
Figure 2 shows apparent viscosity η 0 versus storage time. ADC, formulated with sweet almond oil and the “Tween/Span” emulsifier, has the most significant viscosity at T 0 (after manufacture) and during its storage at 25°C. The viscosities of the oils (Table II) indicate that the most viscous oils do not give the most viscous emulsions. Figure 2 shows apparent viscosities of emulsions OM/NM68 higher than those of the Tween/Span emulsions. Moreover “Montanov 68” emulsions are well separated from “Montanov 82” emulsions (Fig. 2). Therefore the viscosities of these emulsions depend mainly on the emulsifier and the polymer because the viscosities of the oil phase and of the aqueous phase have very little impact on the emulsion viscosity (Table II). Figure 3 shows the yield stresses τ 0 versus storage time. τ 0 of emulsions OM/NM68 is lower than those of Tween/Span emulsions. From its microstucture, Montanov 68® is both a surfactant and a thickener. In emulsions, it forms lamellar liquid crystalline bilayers surrounding the oil droplets. Synergetic effects of viscoelastic hydrophilic gel of lamellar type and lipophilic gel network explain the high viscosities of emulsions OM/NM68 (23,24). Carbopol is a microgel, which is a collection of highly cross-linked polymer particles in continuous phase, which function as efficient thickeners and stabilizers for emulsions (25,26). This structure led to obtain emulsions with a significant yield stress that explains the high yield stresses of the Tween/Span emulsions.
After a six-month storage at 25°C, only OC (with olive oil and the “Tween20/Span60” emulsifier) and “Montanov” emulsions show a decrease in viscosity. Figure 2 reveals a 50% decrease for OC, a decrease of approximately 30% for the emulsions formulated with the “Montanov 68” emulsifier and of 60% for those formulated with the “Montanov 82” emulsifier. NC (with apricot core oil) and ADC (with sweet almond oil) emulsions can be considered stable according to this parameter since the variations are not significant (<10%).
A decrease in the yield stress τ 0 was measured for the OC and “Montanov” emulsions (Fig. 3). τ 0 decreased by ≈43% for OC, by ≈23% for the M68 emulsions and by ≈40% for the M82 emulsions while it was stable for ADC and NC (non-significant variations of τ 0). These decreases correspond to the aging of the emulsions according to the studies of Pal (7), Py et al. (13) and Tadros (27), who showed that the viscosity, the storage modulus and the yield stress values decreased with aging. Therefore, OC emulsions were more unstable than ADC and NC formulated with the same “Tween20/Span60” emulsifier and the emulsions formulated with “Montanov 82” were more affected by the storage time than those formulated with “Montanov 68”. Moreover, the stability of ADC and NC could not be distinguished by η 0 and τ 0.
Steady-State Flow: The Sensitivity of Emulsions to Shearing
The sensitivity of these emulsions to shearing was tested by carrying out two successive tests in steady-state flow separated by a two-minute interval. Each measurement was repeated three times for each sample. The greater the variation of the yield stress τ 0 (measured between the two tests), the more brittle the emulsion, and this leads to believe that the emulsion either undergoes disorganization of its structure or takes longer to recover its initial state. Figure 4 summarizes the yield stress variations between the first and the second flow tests. The average variation of τ 0 is ≈30% for OC and >50% for the emulsions formulated with “Montanov 82”. These emulsions were very sensitive to shearing and thus the most unstable. NC, ADC, OM68 and NM68 did not show significant variations (<10%). They were not sensitive to shearing and were stable.
The sensitivity of the emulsions to shearing can be explained either by disorganization of the system (modification of the interactions between the polymer network and the emulsifier for the “Tween/Span” emulsions), or by the oil droplets having not enough time to reorganize.
The shearing dispersed the droplets very easily, as the strength of the interdroplet interactions was very low (28). Consequently, the capacity of droplets, deformed during flow, to reorganize and to recover their initial state, can be evaluated by this rheological test. According to the chemical formulae of Carbopol® 980, Tween 20 and Span 60 (which compose the “Tween/Span” emulsions), hydrogen-bonding interactions can occur between the oxyethylenic groups of Tween 20 and the carboxylic groups of Carbopol® (29). NC and ADC differ from OC only by the oil used. Consequently, the decrease in rheological parameters of OC can be partially explained by a destruction of the network or a weakening of the hydrogen-bonding interactions with aging. The emulsions formulated with the “Montanov 68” emulsifier were not sensitive to shearing and had a very stable structure, unlike those formulated with “Montanov 82.” In this case, the oil had very weak influence on the sensitivity of the emulsions to shearing.
This innovative method made it possible to determine quickly (a two-minute interval between two successive flow tests) the sensitivity to shearing of the emulsion structure.
Oscillatory Measurements: Stress Sweep
The oscillatory measurements performed on the seven emulsions showed that they have a predominantly elastic behaviour (or solid-like behaviour) after their manufacture (T 0): the storage modulus G′ (also called “elastic modulus”) was always greater than the loss modulus G″ (also called “viscous modulus”) and thus the loss tangent (tan δ = G″/G′) was lower than 1. Table III summarizes the variations of the rheological parameters measured in dynamic mode after 6 months of storage at 25°C. According to this table, a decrease in these parameters was measured for all the emulsions.
ADC and NC showed a similar weak decrease in parameters (that is why it is difficult to discriminate between them) while higher decreases of these parameters were measured for OC—formulated with olive oil. The emulsions formulated with “Montanov 82” (OM82 and NM82) showed the highest variations. According to the results presented in Table III, a stability order is proposed: the emulsions are classified from the most stable (NC and ADC) to the most unstable (OM82 and NM82) as follows:
NC, ADC > OM68, NM68 > OC > OM82, NM82
These results suggest that OC, OM82 and NM82 were the most unstable during storage. Many authors have noticed that the aging of the emulsions affect the storage modulus G′, the viscosity and the yield stress (7,12–16). The variations of these rheological parameters (either increase or decrease) differ and depend on the destabilization process involved (flocculation, creaming, coalescence, Oswald ripening) and the type of interaction between droplets (Brownian movements...). A decrease in the storage modulus G' versus storage time has been measured in several studies (7,13). Paraskevopoulo et al. (30) observed a similar decrease in the value of the storage modulus in food emulsions and attributed it to the increase in droplet size. However, data presented in this study did not reveal any increase in droplet size during storage, whatever the emulsion. Thus, this behaviour could be attributed to a modification of the interdroplet interactions due to aging of the polymeric film, resulting in weaker interactions in the case of “Tween/Span” emulsions. Diftis et al. (28) explained that the decrease in viscoelasticity parameters with aging was due to droplet rearrangements, occurring continuously after preparation and leading to a weaker network structure.
The variations of the loss tangent (tan δ = G″/G′), presented in Table III, were significant only for the “Tween/Span” emulsions (>5%, if we consider the mean of two replicates for each emulsion type). The “Montanov” emulsions did not show any significant variation of tan δ with aging time because their viscoelastic moduli G′ and G″ decrease similarly with time. The OC emulsions were also different from the other “Tween/Span” emulsions: their tan δ increased with storage time. This weak increase in tan δ corresponds to a faster decrease in the elastic modulus G′ than in the viscous modulus G″. This suggests a decrease in elastic properties during storage that may be explained by a weakening of the interactions between droplets and consequently a destabilization of the system. It was observed that the more elastic the emulsion is (G′ ≫ G″), the more stable it is (31,38). The decrease in tan δ, measured for NC and ADC emulsions, corresponds to a faster decrease in G″ than in G′ and illustrates a more elastic behaviour after 6 months at 25°C.
Therefore these results confirm that the OC emulsions and those made with “Montanov 82” have the most unstable structures (modification of the viscoelastic parameters).
Oscillatory Measurements: Frequency Sweep (FS)
To validate the parameter values obtained by stress sweep tests (SS), the viscoelastic behaviour of the emulsions subjected to a frequency sweep (FS) was studied. The rheological parameters measured on the FS curves at 1 Pa were compared with those obtained on the SS curves in the linear viscoelastic region. A good similarity between the values obtained by these two oscillatory tests (SS and FS) was observed: the standard deviations of the G′, G″ and tan δ values were lower than 5%. Thus, the parameter values calculated in oscillatory mode were validated for these emulsions.
Figure 5 shows an example of the frequency plots of G′, G″ and tan δ for a stable emulsion (NC) and an unstable one (OC) at T 0 and T6 months. Both G′ and G″ increased slightly with the frequency for all emulsions (“Montanov” and “Tween/Span”). G′ was greater than G″ at all frequencies. Therefore, the emulsions had a solid-like behaviour (predominantly elastic behaviour). Moreover the value of G′ was ten times higher than that of G″, which characterizes the “gel” or “network” structure. After 6 months, G′ was still higher than G″ and thus the elastic behaviour was preserved. The influence of the frequency on the viscoelastic properties of the emulsions was measured: they were always predominantly elastic (“gel” structure). Neither G′ nor G″ for NC and ADC vary significantly with aging time. This illustrates a more significant stability of these emulsions. “Montanov” emulsions showed a decrease in their moduli with aging at all frequencies. Like in the SS tests, the variations of G′ and G″ were higher for the emulsions formulated with “Montanov 82” than for those formulated with “Montanov 68” at all frequencies. The value of tan δ was constant up to 4 Hz for the seven emulsions. It was always much lower than 1 (≈0.1), which confirms the elastic behaviour of these emulsions. Above 4 Hz, tan δ increased strongly with the frequency. This suggests that G″ increased more than G′. These emulsions lost their elastic properties when they were submitted to high frequencies (similar to high shearing).
Rheological Measurements at 50°C
The rheological parameters were also studied at 50°C to verify that the variations measured at 25°C were representative of the aging phenomenon. Figure 6 shows the evolution of η 0, τ 0, G′ and G″ with time for ADC (stable emulsion) and OC (unstable). The graphs show a decrease in all parameters with time for these emulsions stored at 50°C. This demonstrates the influence of aging. Therefore the decrease in these parameters measured at 25°C for OC and “Montanov” emulsions is representative of aging. Moreover a more significant decrease in these parameters was measured for the unstable emulsion than for the stable one. The same results were obtained for “Montanov” emulsions, for which the OM82 and NM82 parameters evolved faster than the OM68 and NM68 ones. Consequently, it was possible to determine the stability of the emulsions by monitoring their rheological parameters.
Microscopic Measurements
All emulsions were studied by microscopy every month during storage at 25 and 50°C. The droplet parameters (droplet diameter and number) for all emulsions measured by microscopy (freeze-fracture electron microscopy or phase contrast microscopy) did not change during storage at 25°C (Fig. 7a, b and c). The “Tween/Span” emulsions could not be observed by contrast phase microscopy because the droplet mean diameter was below the detection threshold of the optical microscope. Therefore, these emulsions were analysed by freeze-fracture electron microscopy and their droplet parameters were not calculated using the Lucia G software (like for “Montanov” emulsions) but evaluated manually. According to several authors (33–35) this technique is ideally suitable to visualize the three-dimensional structure of the emulsion and the distribution of the droplets.
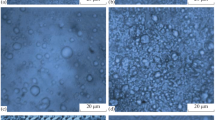
No modification of the droplet size versus storage time was observed on the micrographs at 25°C. The micrographs obtained at T 0 by contrast phase microscopy showed a similarity in droplet size and organization for emulsions formulated with the same “Montanov” emulsifier (Fig. 8). The droplet size for OM68 and NM68 (around 9 μm) was bigger than that for OM82 and NM82 (around 2 μm), as shown on Figs. 7a and 8. The type of oil did not influence the droplet structure (diameter, organization).
The droplet parameters evolved with time when the emulsions were submitted to accelerated aging at 50°C:
-
slight increase in the mean diameter after 3 months of storage at 50°C;
-
increase in the maximum diameter after a three-month storage for all emulsions;
-
increase in the number of droplets in OM82 and NM82 after 1 month at 50°C (Fig. 7c) corresponding to a creaming phenomenon. The samples for the microscopic analyses were taken at the surface of the emulsions. When creaming occurs, the oil droplets rise to the surface. Therefore, the droplet number increases at the surface during creaming of the emulsion and decreases down to its initial value (and even below for “Montanov 82” emulsions) after 3 months. This corresponds to the coalescence of the oil droplets and the destabilization of these emulsions. The emulsions formulated with “Montanov 68” did not show any significant variation of the droplet number but the maximum droplet diameter increased with aging time, which also suggests a coalescence of the oil droplets.
Consequently, the emulsions formulated with the same emulsifier evolved similarly during storage at 50°C.
The droplet diameter for the “Tween/Span” emulsions was evaluated by freeze-fracture electron microscopy. Figure 9 shows a detailed view of OC and NC emulsions that helps visualize the organization of the droplets in the continuous phase at T 0 and T4 months at 50°C. The micrographs for NC and ADC are similar. Therefore only NC is presented in Fig. 9 as a stable emulsion. The maximum droplet size measured on this figure is assessed to ≈2 μm for all emulsions. The type of oil used does not seem to influence the droplet size, but their organization in the continuous phase is different. Micrographs of NC and ADC show a homogeneous distribution of the oil droplets in the continuous phase at T 0. The structure of OC is different from that of the other two emulsions: a network structure is observed on the micrograph at T 0. The Carbopol® polymer formed a network in the aqueous phase surrounding the oil droplets only for OC. This network could be explained by the formulation of the OC emulsions.
The formulation of the “Tween/Span” emulsions required the neutralization of Carbopol® (which is a reticulated polymer of acrylic acid) with a sodium hydroxide solution to obtain a pH equal to 7. The initial pH of the emulsions depends on the oil, as shown on Table IV. Therefore, the quantity of NaOH necessary to neutralize the polymer was not the same for each emulsion. The initial pH of OC was the lowest, so the quantity of NaOH was the greatest and the molecule of polymer tended to uncoil into a more extended structure (36), leading after neutralization to a more viscous emulsion (Table IV). The polymer chains are initially coiled into a spiral (i.e., at pH = 3), thus affording a relatively low viscosity. As neutralization progresses, the carboxyl groups of the acrylic acid become ionized, leading to an increasing repulsion of the negative charges that causes the molecular structure to uncoil, thus inducing a gradual rise in viscosity (37). It was also reported (38) that the interaction of Carbopol® with a non-ionic surfactant like Tween 20 could also alter the polymer structure and the viscosity of the system. The surfactant can act as a binding bridge among different polymer chains (interpolymeric complex), which promotes the appearance of a three-dimensional network and thus increases the viscosity. According to these authors, the more significant uncoiling of the polymer structure for OC might explain the network observed at T 0 on the OC micrograph (Fig. 9a). The Carbopol® polymer in NC and ADC might not be sufficiently uncoiled to allow the development of a network around the droplets. Moreover this assumption may also explain the low viscosities of these emulsions.
Micrographs of aged OC emulsions at 50°C show the disappearance of the polymer network observed at T 0. This explains the decrease in rheological parameters with aging time.
Consequently, the microscopic analyses of the emulsions stored at 25 and 50°C confirm the results obtained by rheological methods at 25°C.
Conclusion
The study of the yield stress variation between two successive steady state flow tests made it possible to determine the most structurally unstable emulsion. The results showed that the emulsions formulated with the “Montanov 82” emulsifier were the most sensitive to shear. The rheological assumption of stability classification, done for the emulsions stored at 25°C, was confirmed by rheological and microscopic measurements at 50°C. The micrographs obtained by electronic microscopy supported and completed the assumptions made about the sensitivity of the OC structure to shearing. The freeze-fracture electron microscopy made it possible to study the organization of the emulsion structure and to show the presence of a network that is destroyed during storage in the case of OC. Thus, our study revealed that rheological measurements turn out to be a powerful tool to investigate the stability of emulsions, to follow the evolution of their viscoelastic properties with aging and to optimize their formulations.
References
A. T. Florence and F. Rieg. L'instabilité des émulsions. In: F. Puissieux and M. Seiller (eds.), Agents de Surface et Émulsions: Les Systèmes Dispersés I. Lavoisier, Tec & Doc, Paris, 1983, pp. 321–342, (Galenica 5 vol. 5).
R. P. Borwankar, L. A. Lobo, and D. T. Wasan. Emulsion stability—kinetics of flocculation and coalescence. Colloids Surf. 69:135–146 (1992).
E. Dickinson. Les Colloïdes Alimentaires. Masson, Paris, 1992, pp. 85–121.
P. C. Hiemenz and R. Rajagopalan. Principles of Colloid and Surface Chemistry, 3rd ed. Dekker, New York, 1997.
J. Mewis and J. H. Macosko. Rheology: Principles, Measurements and Applications. VCH, New York, 1994, Chapter 10.
T. F. Tadros. Fundamental principles of emulsion rheology and their applications. Colloids Surf. A: Physicochem. Eng. Asp. 91:39–55 (1994).
R. Pal. Effect of droplet size on the rheology of emulsions. AIChE J. 42:3181–3190 (1996).
H. A Barnes. Rheology of emulsions—a review. Colloids Surf. A: Physicochem. Eng. Asp. 91:89–95 (1994).
T. F. Tadros. Correlation of viscoelastic properties of stable and flocculated suspensions with their interparticle interactions. Adv. Colloid Interface Sci. 68:97–200 (1996).
T. G. Mason. New fundamental concepts in emulsion rheology. Curr. Opin. Colloid Interface Sci. 4:231–238 (1999).
R. Chanamai and D. J. McClements. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surf. A: Physicochem. Eng. Asp. 172:79–86 (2000).
I. Terrisse, M. Seiller, A. Rabaron, J. L. Grossiord, A. Magnet, and C. Le Hen-Ferrenbach. Rheology: how to characterize and to predict the evolution of W/O/W multiple emulsions. Int. J. Cosmet. Sci. 15:53–62 (1993).
C. Py, J. Rouvière, P. Loll, M. C. Taelman, and T. F. Tadros. Investigation of multiple emulsion stability using rheological measurements. Colloids Surf. A: Physicochem. Eng. Asp. 91:215–225 (1994).
S. Tamburic. The aging of polymer-stabilized creams: a rheological viewpoint. Cosmet. Toiletries 15:43–49 (2000).
M. Korhonen, L. Hellen, J. Hirvonen, and J. Yliruusi. Rheological properties of creams with four different surfactant combinations—effect of storage time and conditions. Int. J. Pharm. 221:187–196 (2001).
V. Kontogiorgos, C. G. Biliaderis, V. Kiosseoglou, and G. Doxastakis. Stability and rheology of egg-yolk-stabilized concentrated emulsions containing cereal β-glucans of varying molecular size. Food Hydrocoll. 18:987–998 (2004).
H. Masmoudi, Y. Le Dréau, P. Piccerelle, and J. Kister. The evaluation of cosmetic and pharmaceutical emulsions aging process using classical techniques and a new method: FTIR. Int. J. Pharm. 289:117–131 (2005).
J. Roussos. Formulation des émulsions. In: F. Puissieux, M. Seiller (eds.), Agents de Surface et Émulsions: Les Systèmes Dispersés I. Lavoisier, Tec & Doc, Paris, 1983, pp. 407–421, (Galenica 5 vol. 5)
J. Surh, E. A. Decker, and D. J. McClements. Properties and stability of oil-in-water emulsions stabilized by fish gelatine. Food Hydrocoll. 20:596–606 (2006).
V. André, N. Willenbacher, H. Debus, L. Börger, P. Fernandez, T. Frechen, and J. Rieger. Prediction of emulsion stability: facts and myth. Cosmetics and Toiletries Manufacture Worldwide 102–109 (2003).
D. Vasiljevic, J. Parojcic, M. Primorac, and G. Vuleta. An investigation into the characteristics and drug release properties of multiple W/O/W emulsion systems containing low concentration of lipophilic polymeric emulsifier. Int. J. Pharm. 309:171–177 (2006).
P. B. Laxton and J. C. Berg. Gel trapping of dense colloids. J. Colloid Interface Sci. 285:152–157 (2005).
S. Savic, G. Valuta, R. Daniels, and C. C. Mueller-Goymann. Colloidal microstructure of binary systems and model creams stabilized with an alkylpolyglucoside non-ionic emulsifier. Colloid. Polym. Sci. 283:439–451 (2005).
L. Baudonnet, J. L. Grossiord, and F. Rodriguez. Physicochemical characterization and in vitro release of salicylic acid from O/W emulsions prepared with Montanov 68®: effect of formulation parameters. Drug Dev. Ind. Pharm. 30:975–984 (2004).
G. P. Roberts and H. A. Barnes. New measurements of the flow-curves for Carbopol dispersions without slip artefacts. Rheol. Acta. 40:499–503 (2001).
H. Zhu, Y. D. Kim, and D. De Kee. Non-Newtonian fluids with a yield stress. J. Non-Newtonian Fluid Mech. 129:177–181 (2005).
T. F. Tadros. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 108–109:227–258 (2004).
N. G. Diftis, C. G. Biliaderis, and V. D. Kiosseoglou. Rheological properties and stability of model salad dressing emulsions prepared with a dry-heated soybean protein isolate-dextran mixture. Food Hydrocoll. 19:1025–1031 (2005).
T. Ó. Thorgeirsdóttir, A. L. Kjøniksen, K. D. Knudsen, T. Kristmundsdóttir, and B. Nyström. Viscoelastic and structural properties of pharmaceutical hydrogels containing monocaprin. Eur. J. Pharm. Biopharm. 59:333–342 (2005).
A. Paraskevopoulou, V. Kiosseoglou, S. Alevisopoulos, and S. Kasapis. Small deformation properties of model salad dressings prepared with reduced cholesterol egg yolk. J. Texture Stud. 28:221–237 (1997).
N. Jager-Lézer, J. F. Tranchant, V. Alard, C. Vu, P. C. Tchoreloff, and J. L. Grossiord. Rheological analysis of highly concentrated W/O emulsions. Rheol. Acta. 37:129–138 (1998).
G. K. Moates, A. D. Watson, and M. M. Robins. Creaming and oscillation rheology of weakly flocculated concentrated emulsions. Colloids Surf. A: Physicochem. Eng. Aspects. 190:167–178 (2001).
J. Dubochet, J. Lepault, R. Freeman, J. A. Berriman, and J. C. Homo. Electron microscopy of frozen water and aqueous solutions. J. Microscopy. 128:219–237 (1982).
J. A. Zasadzinski and S. M. Bailey. Applications of freeze-fracture replication to problems in materials and colloid science. J. Electron Microsc. Tech. 13:309–334 (1989).
E. Dumay, C. Lambert, S. Funtenberger, and J. C. Cheftel. Effects of high pressure on the physico-chemical characteristics of dairy creams and model oil/water emulsions. Lebensmittel-Wissenschaft und-Technologie. 29:606–625 (1996).
T. Sanz Taberner, A. Martín-Villodre, J. M. Pla-Delfina, and J. V. Herráez. Consistency of Carbopol 971-P NF gels and influence of soluble and cross-linked PVP. Int. J. Pharm. 233:43–50 (2002).
R. Barreiro-Iglesias, C. Alvarez-Lorenzo, and A. Concheiro. Poly(acrylic acid) microgels (carbopol® 934)/surfactant interactions in aqueous media: Part I: Nonionic surfactants. Int. J. Pharm. 258:165–177 (2003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masmoudi, H., Piccerelle, P., Le Dréau, Y. et al. A Rheological Method to Evaluate the Physical Stability of Highly Viscous Pharmaceutical Oil-in-Water Emulsions. Pharm Res 23, 1937–1947 (2006). https://doi.org/10.1007/s11095-006-9038-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9038-x