Purpose
The insulin-like growth factor axis plays an important role in fibrogenesis. However, little is known about mannose-6-phosphate/Insulin-like growth factor-II receptor (M6P/IGF-IIR) expression during fibrosis. When expressed preferentially on fibrogenic cells, this receptor may be used to selectively deliver drugs to these cells. We investigated M6P/IGF-IIR expression in livers of bile duct-ligated (BDL) rats and in renal vascular walls of renin transgenic TGR(mRen2)27 rats. Both models are characterized by fibrogenic processes. Furthermore, we studied whether drug delivery via M6P/IGF-II-receptor-mediated uptake is possible in fibroblasts.
Materials and Methods/Results
M6P/IGF-IIR mRNA expression was investigated 3, 7 and 10 days after BDL. At all time-points hepatic M6P/IGF-IIR expression was significantly increased compared to healthy controls. Moreover, immunohistochemical staining revealed that α-sma-positive cells were M6P/IGF-IIR-positive. In kidneys of TGR(mRen2)27 rats, the number of M6P/IGF-IIR-positive arteries per microscopic field was increased 5.5 fold over healthy controls. To examine whether M6P/IGF-IIRs could be used as a port of entry for drugs, we coupled mycophenolic acid (MPA) to mannose-6-phosphate-modified human serum albumin (M6PHSA). M6PHSA-MPA inhibited 3T3-fibroblast proliferation dose-dependently, which was reversed by co-incubation with excess M6PHSA, but not by HSA.
Conclusions
M6P/IGF-IIRs are expressed by fibrogenic cells and may be used for receptor-mediated intracellular delivery of the antifibrogenic drug MPA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibrotic processes are characterized by an excessive deposition of extracellular matrix proteins and proliferation of fibroblasts in response to chronic tissue injury. Liver fibrosis and fibrotic vascular lesions (atherosclerosis, hypertension-induced vascular thickening) are examples of these disorders and many similarities exist between the processes (1). Both diseases are characterized by the activation of cells from the fibroblast-lineage, which are present in a resting state within healthy tissue. Within the liver, it is the hepatic stellate cell (HSC) that plays a central role in the initiation and perpetuation of fibrosis (2). Quiescent HSC transform into proliferating, α-sma-positive, collagen-producing cells after activation. In analogy to the HSC within the liver, in fibrotic vascular lesions vascular smooth muscle cells differentiate from a quiescent phenotype into a secretory and migrating cell type. This is also accompanied by a proliferation of these cells, associated with a narrowing of the vascular lumen (3–5).
Experimental pharmacological treatments therefore focus on the inhibition of proliferation of fibroblasts or vascular smooth muscle cells. Mycophenolic acid (MPA) is a drug with antiproliferative properties in fibroblast-like cells (6,7). The antiproliferative effect of MPA is the result of a depletion of guanine nucleotides via a non-competitive inhibition of inosine monophosphate dehydrogenase (IMPDH) type II. This enzyme catalyzes the conversion of inosine-5′-monophosphate into xanthine-5′-monophosphate, which is the first and rate-limiting step in de novo guanosine synthesis (8,9). In vascular lesions characterized by vascular smooth muscle cell proliferation, such as atherosclerosis and in-stent restenosis, beneficial effects of MPA have been reported via a direct effect on vascular smooth muscle cells (10,11). More recently, data from our group showed that MPA is capable of inhibiting the proliferation of cultured hepatic stellate cells, as well (12). Hence, patients with fibrosis in various organs may benefit from treatment with MPA. However, because MPA also is a potent immunosuppressive drug, systemic application of MPA would lead to immunosuppressive effects (8). Although immunosuppression may be very useful, for instance in patients that underwent organ transplantation, it can be considered a side effect in patients with liver fibrosis or fibrotic lesions of the renal vasculature. In these latter cases, no systemic immunosuppression is required. On the contrary, it will increase their risk for developing viral and bacterial infections and in the case of viral hepatitis-induced fibrosis will lead to a flare up of the virus infection (13). Moreover, a suppression of certain T cell subsets may even aggravate fibrosis (14–16). In addition, attenuation of fibroblast proliferation should occur locally, without interfering with this common process in healthy organs. A targeted delivery of MPA to proliferating cells may avoid these matters, while at the same time the antiproliferative effect of MPA is enhanced in fibrogenic cells.
In isolated, culture-activated HSC there is evidence that the mannose-6-phosphate/Insulin-like growth factor-II receptor (M6P/IGF-IIR) is upregulated (17,18). Also, after acute liver damage due to a single CCl4 administration to rats, enhanced M6P/IGF-II receptor expression has been documented (19). Therefore, this receptor may be used as a target receptor for drug targeting strategies and indeed we have shown that coupling mannose-6-phosphate (M6P) groups to human serum albumin (HSA) leads to selective accumulation of this modified albumin in HSC (20). However, when designing an effective pharmacological therapy based on receptor-mediated drug targeting principles, it is pivotal to know at which time points the M6P/IGF-II receptor is expressed during ongoing experimental liver fibrosis in vivo. Additionally, if the M6P/IGF-II receptor is also over-expressed in blood vessels that are at risk for fibrotic vascular lesions, this strategy may also be applied to treat this disease. Yet, to our knowledge M6P/IGF-II receptor expression has not been investigated with respect to this.
In the present study, we therefore explored the time-course of M6P/IGF-II receptor expression during experimental liver fibrosis, induced by ligation of the common bile duct. In addition, we investigated whether this receptor is expressed in the renal vasculature of homozygous TGR(mRen2) rats, that display enhanced DNA synthesis in vascular smooth muscle cells and exhibit mild thickening of renal capillary walls (21,22). Furthermore, we studied whether delivery of MPA to M6P/IGF-II receptor-expressing fibroblasts is possible via receptor-mediated uptake in vitro, and whether this leads to pharmacological effects within these cells.
Materials and Methods
Experimental Animals
Liver fibrosis was induced in male Wistar rats (220–240 g, Harlan, Horst, The Netherlands) by ligation of the common bile duct according to standard procedures (20). The resulting fibrotic process in the liver is characterized by increased proliferation of bile duct epithelial cells, which are surrounded by collagens, mainly of type I and III and increased numbers of α-sma-positive cells (23). Livers were harvested 3, 7 and 10 days after surgery and were frozen in isopentane at −80°C. The livers from healthy animals served as controls.
Kidneys from 11-week old, male homozygous Ren2 transgenic rats (Max Delbrueck Centre for Molecular Medicine, Berlin, Germany) were used to study receptor expression in the renal vasculature. These transgenic animals are characterized by an increased activity of the renin angiotensin system and vascular hypertrophy (21,22). Kidneys from age-matched male Sprague–Dawley rats (Max Delbrueck Centre for Molecular Medicine) were used as controls.
The animals had free access to tap water and standard lab chow. All experiments were approved by the local committee for care and use of laboratory animals and were performed according to strict governmental and international guidelines for the use of experimental animals.
Immunohistochemical Staining Procedures
Cryostat liver and kidney sections of 4 μm thickness were prepared, and after acetone fixation the sections were stained according to standard indirect immunohistochemical techniques. The presence of M6P/IGF-II receptor was demonstrated with a goat polyclonal antibody purchased from Santa Cruz Biotechnology, Santa Cruz, CA and immunohistochemical staining for α-smooth muscle actin (α-sma) was performed with a mouse monoclonal antibody obtained from Sigma, Gillingham, UK.
RT-PCR and Gel Electrophoresis
RNA isolation from whole liver and kidney samples and the synthesis of cDNA were performed according to standard procedures. To assess the expression levels of M6P/IGF-II receptor mRNA relative to GAPDH, reverse transcriptase PCR was performed using the following primers in a concentration of 50 μM. FW 5′-GTGTCCTCTGGGTGTGGACT, RV 5′-CTCCTCCTTGCTGACCTTTG (M6P/IGF-IIR); FW 5′-GCTGGTGCTGAGTATGTCG, RV 5′-CTGTGGTCATGAGCCCTTCC (GAPDH). After the PCR reaction, products were run on a 2% agarose gel, at 90 V charge over a period of 45 min, and the bands were subsequently quantified by image analysis.
Synthesis and Characterization of the Conjugate
The synthesis of M6PHSA was performed as described earlier (20). The characterization of this neo-glycoprotein was done by analysis of mannose-, phosphate- and protein content of the construct according to standard procedures (20). MPA (Sigma) was coupled to M6PHSA via an ester bond by activation with 2-iodoethanol. To 20 mg of MPA dissolved in 800 μl of dichloromethane (Merck, Darmstadt, Germany), 20 μl of SOCl2 (Sigma) and 0.4 μl of dimethylformamide (Merck) were added and stirring was maintained at room temperature for 5 h. The reaction mixture was evaporated to dryness, redissolved in 800 μl of dichloromethane and reacted with 200 μl of 2-iodoethanol (Sigma) for 90 min on ice, protected from light. The 2-iodoethanol ester of MPA was separated from the excess of 2-iodoethanol by elution of the reaction mixture with dichloromethane:acetone:acetonitrile = 8:1:1 on a Silica G column (Merck). The identity of the reaction product was confirmed by mass spectrometry. MPA-2-iodoethanol ester was coupled to the sulfhydryl groups of M6PHSA which were incorporated via S-acetylthioglycolic acid N-hydroxysuccinimide ester derivatization (SATA, Sigma) according to Duncan et al. (24). The reaction mixture was purified by filtration and dialysis against water, subsequently freeze dried and stored at −20°C. The drug:protein ratio was assessed by HPLC using a Zorbax SB-AQ 3.5 μm; 4.6 × 150 mm column (Agilent technologies, USA), after hydrolysis of the ester bond between MPA and the carrier at pH = 12. Samples were eluted with acetonitrile:PBS = 25:75, flow 1.0 ml/min and detected with a Waters UV detector (model 441) at 254 nm. Protein content was assessed by the Biorad protein assay. Drug loading was then calculated by determination of the molar ratio of MPA to protein. The monomeric protein content was assessed by size exclusion chromatography on an FPLC HR 10/30 Superdex 200 column.
Culture of 3T3-Fibroblasts
NIH/3T3-fibroblasts were cultured at 37°C in Dulbecco's modified Eagles Medium (Biowithaker, Verviers, Belgium) containing 5% FCS, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM l-glutamine (Gibco, Paisley, UK). One day prior to proliferation experiments, cells were seeded in 96-well plates at a density of 5,000 cells/well.
Effects on Cell Proliferation
The effect of test compounds on cell proliferation was assessed in 3T3-fibroblasts, and was measured by bromodeoxyuridine (BrdU)-incorporation assays, using standard ELISA techniques. During proliferation experiments, 3T3-fibroblasts were cultured in the described medium, supplemented with 10 μM BrdU and treated with MPA, HSA, M6PHSA, M6PHSA-MPA or vehicle for 4 h. In competition experiments, 120 μg/ml of the conjugate was co-incubated for 4 h with M6PHSA or HSA (1 mg/ml) to establish whether the effect of the (targeted) drug could be inhibited by an excess of receptor ligand.
Effects on Cell Viability
Cell viability of 3T3-fibroblasts was assessed by the Alamar Blue assay for mitochondrial activity according to the instructions of the manufacturer (Serotec, Kidlington, UK). Cells were seeded in 96-well plates as described above, and were incubated with MPA and Alamar Blue simultaneously for 4 h. Alamar Blue conversion was measured fluorimetrically at an excitation wavelength of 560 nm and an emission wavelength of 590 nm.
Statistical Analysis
Data were expressed as the average ± SD and subjected to a two-tailed Student's t-test. When multiple means were compared, one-way analysis of variance was performed, followed by the LSD post hoc test. Differences were considered statistically significant at P < 0.05.
Results
M6P/IGF-IIR Expression on mRNA and Protein Level in the Liver after BDL
RT-PCR analysis of liver specimens revealed that M6P/IGF-IIR mRNA expression in the livers was already increased during the early stages after bile duct ligation. At 3, 7 and 10 days after BDL, mRNA expression in the livers increased to 1.5 ± 0.2, 1.8 ± 0.4 and 1.8 ± 0.1 fold over control, respectively (Fig. 1; P < 0.05).
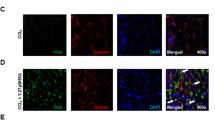
Immunohistochemical staining of liver sections revealed that cells with spindle-shaped nuclei, which were mainly found around the proliferating bile duct epithelial cells, clearly stained positive for the M6P/IGF-II receptor. In order to verify the specificity of the observed staining, we co-incubated the antibody with a blocking peptide prior to application to the sections. This procedure completely abolished staining, confirming the M6P/IGF-IIR specificity of the procedure. Immunohistochemical analysis of consecutive sections for α-sma revealed that the M6P/IGF-II receptor-expressing cells were also positive for this myofibroblast marker. Besides expression in the non-parenchymal α-sma-positive cells, the M6P/IGF-II receptor could also be detected in hepatocytes of both healthy livers and livers from bile duct-ligated rats (Fig. 2).
Representative microphotographs of sequential sections of rat livers, stained for the myofibroblast marker α-sma and for the presence of M6P/IGF-II receptor in normal liver and at day 3, 7 and 10 after BDL (original magnification 200×). Arrows indicate the M6P/IGF-II receptor-positive non-parenchymal cells.
M6P/IGF-IIR Expression on Protein and mRNA Level in Vessels of TGR(mRen2)27 Rats
Immunohistochemical staining of cryostat sections of kidney for the M6P/IGF-IIR revealed that in Ren2 transgenic rats, the receptor expression in the arterial vessel walls was increased compared to SD controls (Fig. 3a). To quantify this, the number of M6P/IGF-II receptor-positive vessels was counted per microscopic field and related to the total number of arteries that were identified by staining for α-sma. Results showed an increase from 0.8 ± 0.4 M6P/IGF-II-positive arteries in controls to 4.4 ± 1.3 in transgenic animals, a five-fold induction (Fig. 3b). The presence of the receptor was demonstrated in glomeruli and tubular epithelial cells. Although in glomeruli no differences between control and Ren2 rats could be observed with respect to receptor expression, within tubular epithelial cells, M6P/IGF-IIR staining appeared to be reduced in Ren2 animals. Evaluation of M6P/IGF-IIR mRNA expression levels in whole kidney samples revealed no significant differences between normal and Ren2 rats (Fig. 3c).
A Representative microphotographs of kidney sections stained for M6P/IGF-IIR. G=glomerulus, V=blood vessel (Original magnification 200×). B Quantification of the number of M6P/IGF-IIR-positive blood vessels per microscopic field, counted at a magnification of 100×. Data represent the average±SD of five animals per group. * indicates P<0.05. C No significant differences could be observed in M6P/IGF-IIR mRNA expression between Ren2 rats and SD controls (at least four animals per group).
Synthesis and Characterization of M6P Constructs
Mannose-6-phosphate was successfully coupled to HSA. Assays for the amounts of sugar, phosphate and protein showed that a total of 29 mannose-6-phosphate groups were coupled per HSA core protein. After conjugation of MPA and subsequent purification, HPLC-analysis revealed that coupling of MPA to M6PHSA resulted in a drug to protein ratio of 0.4:1. This low coupling ratio was the result of a relatively inefficient reaction between the 2-iodoethanolester of MPA and the –SH groups on the protein backbone, which could not be enhanced. Still, drug loading was sufficient for pharmacological evaluation of the targeting concept. FPLC analysis showed that more than 70% of the total amount of the protein was present in the monomeric form.
Pharmacological Effect of MPA on Fibroblasts
Immunohistochemical staining of 3T3-fibroblasts revealed that this cell type expressed the M6P/IGF-II receptor (data not shown). In Fig. 4a, the effect of MPA in cultures of 3T3-fibroblasts is shown. The drug affected fibroblast proliferation in a dose-dependent manner, inhibiting BrdU-incorporation by 72 ± 17% at a concentration of 750 nM after 4 h of incubation. Cell viability, as assessed by measurement of mitochondrial activity with the Alamar Blue assay, was not affected (Fig. 4b) and also cell morphology, as assessed with a phase contrast microscope, was similar to vehicle-treated control cultures.
Effect of 4 h incubations with increasing concentrations of MPA on the BrdU-incorporation in cultures of 3T3-fibroblasts (A). MPA decreases the proliferation of these cells dose-dependently, whereas cell viability is not affected after 4 or 24 h incubation with MPA (B). The data represent the average±SD of three independent experiments. * indicates P<0.05 compared to control.
Pharmacological Effect of M6PHSA-MPA on Fibroblasts
In Fig. 5 it can be seen that increasing concentrations of the conjugate inhibited BrdU-incorporation in cultures of 3T3-fibroblasts in a dose dependent manner. BrdU-incorporation was inhibited by 82 ± 17% at the highest concentration of 480 μg/ml M6PHSA-MPA, which corresponds to 2.56 μM MPA (P < 0.05). HSA and M6PHSA alone did not affect cell division at all.
The effect of M6PHSA-MPA on the proliferation of 3T3-fibroblasts. Note that M6PHSA-MPA induces a dose-dependent decrease in BrdU-incorporation, whereas HSA and M6PHSA do not inhibit cell proliferation. The effect of free MPA is shown as a reference. Data represent the average±SD of at least three independent experiments. * indicates P<0.05 compared to control.
Competition Experiments
To assess whether the M6PHSA-MPA construct is specifically internalized by receptors, the effect of M6PHSA, as a competitive receptor ligand, on the antiproliferative action of M6PHSA-MPA was tested. In Fig. 6 it is shown that an excess of M6PHSA strongly reduced the effect of the conjugate (P < 0.05) whereas HSA alone had no significant effect. This excess of M6PHSA did not influence the effect of free MPA (data not shown). These experiments indicate that the effect of M6PHSA-MPA is the result of drug release after receptor-mediated uptake of the conjugate.
Discussion
In this paper we show that the M6P/IGF-II receptor is upregulated already in the early stages after bile duct ligation and increased expression is also found in renal blood vessels of homozygous Ren2 transgenic rats. This is in line with reports that show that the insulin-like growth factor axis plays an important role in development of liver fibrosis and the development of fibrotic vascular lesions (25–29). Although the role of the IGF-axis in fibrogenesis is generally accepted, to our knowledge no reports exist that describe M6P/IGF-II receptor expression and upregulation in an early stage in the animal models that we investigated. In both animal models the receptor is present on cells with spindle-shaped nuclei, which both are α-sma-positive, two typical features of fibroblast-like cells and vascular smooth muscle cells.
The M6P/IGF-II receptor plays a role in the shuttling of proteins to the early and late endosomes (30). In line with this, the receptor is also found in hepatocytes and in tubular epithelial cells. Importantly, previous studies from our group showed no binding of M6PHSA at all to these cells after iv administration of the protein, whereas a co-localization with markers for hepatic stellate cells was clearly notable (20). This indicates that M6P/IGF-II receptors on the cell surface of fibroblasts are accessible, but M6P/IGF-II receptors within hepatocytes are not accessible for iv administered M6P-containing proteins.
The physiological function of M6P/IGF-II receptor upregulation during fibrosis is still unclear. In contrast to the IGF-I receptor, the M6P/IGF-II receptor has no well described signalling function upon binding of insulin-like growth factor. However, it can regulate local levels of insulin-like growth factor by binding and subsequent lysosomal degradation (30). In addition, M6P/IGF-II receptor-mediated cleavage of latent TGF-beta into the active profibrogenic cytokine has been reported in cultures of activated HSC. This would allow activation of this potent fibrogenic cytokine in the direct surroundings of cells of the fibroblast lineage (31). Moreover, with respect to the vascular pathology that develops in renin transgenic rats, and the recent understanding that angiotensin II plays an important role in liver fibrosis as well (32), it is of note that pro-renin can also bind to M6P/IGF-II receptors (33,34). The early upregulation of M6P/IGF-II receptor expression on HSC and vascular smooth muscle cells in Ren2 and BDL rats may therefore also point to a role of this receptor in the regulation of renin levels in the direct vicinity of these cells during fibrogenesis, thus indirectly influencing local angiotensin II levels.
Although a clearly enhanced staining for M6P/IGF-II receptors was found by using immunohistochemical methods in fibrotic livers as well as in renal arteries, mRNA levels for this receptor were only increased in liver samples and not in kidney samples. This is probably related to the fact that M6P-IGF-IIR expression in the kidney is only very locally enhanced so that these local changes cannot be clearly measured against the background of the expression levels of the surrounding tubular epithelial cells.
As an in vitro model for the testing of drug targeting preparations directed to cells that express the M6P/IGF-II receptor, we used 3T3-fibroblasts (35). The sensitivity of this cell-line to treatment with MPA described here, confirms a previous report. This report demonstrated that high concentrations of MPA in cultures of 3T3 cells resulted in cytotoxicity, mainly in proliferating cells (36). We now show that MPA affects cell proliferation at low concentrations without significant loss of cell viability. Using this model system, we demonstrated that our M6PHSA-MPA conjugate inhibits fibroblast proliferation dose-dependently (Fig. 5). Furthermore, data from competition experiments indicated that the effect of M6PHSA-MPA is mediated by receptor-mediated endocytosis, a process which can be saturated by competitive ligands. M6PHSA attenuated the anti-proliferative effect of MPA-constructs significantly, in contrast to HSA itself (Fig. 6), indicating that the effect of this MPA-construct is mediated by receptors that recognize M6P-ligands, most likely M6P/IGF-II receptors. This in vitro finding provides a basis for the effective targeting in vivo, since it shows that passive diffusion, which is the major mechanism by which MPA accumulates in leukocytes and other non-target cells, is now largely disqualified. In addition, the rapid metabolism of MPA in hepatocytes after systemic injection (37) would also be prevented by the delivery of pharmacologically active MPA to fibrogenic cells. The current observations support and extend previous data from our lab on the binding and uptake of 125I-labeled M6PHSA-MPA in cultures of rat HSC (12).
We did not measure the release of native MPA from the carrier within target cells. However, the fact that a pharmacological effect was found suggests that this is the case. In a previous study we showed that MPA, which was conjugated via an acid-stable amide bond to the drug carrier, did not exert any antiproliferative effect (12). This strengthens the notion that MPA must be released from the carrier in its native form in order to exert its growth-inhibiting properties.
The feasibility of using M6P/IGF-IIR-mediated uptake for the selective intracellular delivery of therapeutic modalities is further exemplified by a paper written by the group of Sly (38). They demonstrated that it is possible to enhance the lysosomal delivery of human β-glucuronidase into fibroblasts in a murine model for mucopolysaccharidosis by fusing the enzyme at its C-terminus with an M6P/IGF-IIR-recognizing peptide. In addition to M6P/IGF-IIR targeting, data from our group indicate that targeting of the Platelet Derived Growth Factor receptor or collagen VI receptor also holds promise for the selective delivery of drugs to fibrogenic cells (39,40).
In conclusion: the M6P/IGF-II receptor is upregulated on fibroblast-like cells in fibrotic livers in an early stage after bile duct ligation and in renal vascular walls of homozygous Ren2 transgenic rats. It may also provide a relevant port of entry for antifibrogenic drugs, thus enabling selective drug delivery to pathogenic cells at an early stage of fibrotic diseases.
Abbreviations
- BrdU:
-
bromodeoxy uridine
- HSC:
-
hepatic stellate cell
- MPA:
-
mycophenolic acid
- M6PHSA:
-
mannose-6-phosphate-modified human serum albumin
- M6P/IGF-IIR:
-
mannose-6-phosphate/insulin-like growth factor-II receptor
- PBS:
-
phosphate buffered saline
- Ren2:
-
TGR(mRen2)27 transgenic rats
- α-sma:
-
α-smooth muscle actin
References
D. W. Powell, R. C. Mifflin, J. D. Valentich, S. E. Crowe, J. I. Saada, and A. B. West. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. 277:C1–C9 (1999).
S. L. Friedman. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275:2247–2250 (2000).
M. N. Babapulle and M. J. Eisenberg. Coated stents for the prevention of restenosis. Part I. Circulation 106:2734–2740 (2002).
M. N. Babapulle and M. J. Eisenberg. Coated stents for the prevention of restenosis. Part II. Circulation 106:2859–2866 (2002).
D. P. Faxon, V. Fuster, P. Libby, J. A. Beckman, W. R. Hiatt, R. W. Thompson, J. N. Topper, B. H. Annex, J. H. Rundback, R. P. Fabunmi, R. M. Robertson, and J. Loscalzo. Atherosclerotic vascular disease conference: Writing Group III: pathophysiology. Circulation 109:2617–2625 (2004).
I. A. Hauser, L. Renders, H. H. Radeke, R. B. Sterzel, and M. Goppelt-Struebe. Mycophenolate mofetil inhibits rat and human mesangial cell proliferation by guanosine depletion. Nephrol. Dial. Transplant. 14:58–63 (1999).
C. Heinz, T. Hudde, K. Heise, and K. P. Steuhl. Antiproliferative effect of mycophenolate mofetil on cultured human Tenon fibroblasts. Graefes Arch. Clin. Exp. Ophthalmol. 240:408–414 (2002).
A. C. Allison and T. Eunson. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology 47:85–118 (2000).
Y. Ji, J. Gu, A. M. Makhov, J. D. Griffith, and B. S. Mitchell. Regulation of the interaction of inosine monophosphate dehydrogenase with mycophenolic acid by GTP. J. Biol. Chem. 281:206–212 (2006).
H. Shimizu, M. Takahashi, S. Takeda, S. Inoue, J. Fujishiro, Y. Hakamata, T. Kaneko, T. Murakami, K. Takeuchi, I. Takeyoshi, Y. Morishita, and E. Kobayashi. Mycophenolate mofetil prevents transplant arteriosclerosis by direct inhibition of vascular smooth muscle cell proliferation. Transplantation 77:1661–1667 (2004).
F. Romero, B. Rodriguez-Iturbe, H. Pons, G. Parra, Y. Quiroz, J. Rincon, and L. Gonzalez. Mycophenolate mofetil treatment reduces cholesterol-induced atherosclerosis in the rabbit. Atherosclerosis 152:127–133 (2000).
R. Greupink, H. I. Bakker, C. Reker-Smit, A. M. Loenen-Weemaes, R. J. Kok, D. K. Meijer, L. Beljaars, and K. Poelstra. Studies on the targeted delivery of the antifibrogenic compound mycophenolic acid to the hepatic stellate cell. J. Hepatol. 43:884–892 (2005).
D. R. Nelson, Z. Tu, C. Soldevila-Pico, M. Abdelmalek, H. Zhu, Y. L. Xu, R. Cabrera, C. Liu, and G. L. Davis. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology 38:859–868 (2003).
J. J. Maher. Interactions between hepatic stellate cells and the immune system. Semin. Liver Dis. 21:417–426 (2001).
T. Poynard, P. Mathurin, C. L. Lai, D. Guyader, R. Poupon, M. H. Tainturier, R. P. Myers, M. Muntenau, V. Ratziu, M. Manns, A. Vogel, F. Capron, A. Chedid, and P. Bedossa. A comparison of fibrosis progression in chronic liver diseases. J. Hepatol. 38:257–265 (2003).
Z. Shi, A. E. Wakil, and D. C. Rockey. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc. Natl. Acad. Sci. U.S.A. 94:10663–10668 (1997).
P. J. de Bleser, P. Jannes, S. C. van Buul-Offers, C. M. Hoogerbrugge, C. F. van Schravendijk, T. Niki, V. Rogiers, J. L. van den Brande, E. Wisse, and A. Geerts. Insulinlike growth factor-II/mannose 6-phosphate receptor is expressed on CCl4-exposed rat fat-storing cells and facilitates activation of latent transforming growth factor-beta in cocultures with sinusoidal endothelial cells. Hepatology 21:1429–1437 (1995).
J. A. Weiner, A. Chen, and B. H. Davis. E-box-binding repressor is down-regulated in hepatic stellate cells during up-regulation of mannose 6-phosphate/insulin-like growth factor-II receptor expression in early hepatic fibrogenesis. J. Biol. Chem. 273:15913–15919 (1998).
P. J. de Bleser, C. D. Scott, T. Niki, G. Xu, E. Wisse, and A. Geerts. Insulin-like growth factor II/mannose 6-phosphate-receptor expression in liver and serum during acute CCl4 intoxication in the rat. Hepatology 23:1530–1537 (1996).
L. Beljaars, G. Molema, B. Weert, H. Bonnema, P. Olinga, G. M. Groothuis, D. K. Meijer, and K. Poelstra. Albumin modified with mannose 6-phosphate: a potential carrier for selective delivery of antifibrotic drugs to rat and human hepatic stellate cells. Hepatology 29:1486–1493 (1999).
M. H. de Borst, G. Navis, R. A. de Boer, S. Huitema, L. M. Vis, W. H. van Gilst, and H. van Goor. Specific MAP-kinase blockade protects against renal damage in homozygous TGR(mRen2)27 rats. Lab. Invest. 83:1761–1770 (2003).
M. J. Brosnan, A. M. Devlin, J. S. Clark, J. J. Mullins, and A. F. Dominiczak. Different effects of antihypertensive agents on cardiac and vascular hypertrophy in the transgenic rat line TGR(mRen2)27. Am. J. Hypertens. 12:724–731 (1999).
L. Beljaars, K. Poelstra, G. Molema, and D. K. Meijer. Targeting of sugar- and charge-modified albumins to fibrotic rat livers: the accessibility of hepatic cells after chronic bile duct ligation. J. Hepatol. 29:579–588 (1998).
R. J. Duncan, P. D. Weston, and R. Wrigglesworth. A new reagent which may be used to introduce sulfhydryl groups into proteins, and its use in the preparation of conjugates for immunoassay. Anal. Biochem. 132:68–73 (1983).
S. Zaina and J. Nilsson. Insulin-like growth factor II and its receptors in atherosclerosis and in conditions predisposing to atherosclerosis. Curr. Opin. Lipidol. 14:483–489 (2003).
S. Zaina, L. Pettersson, B. Ahren, L. Branen, A. B. Hassan, M. Lindholm, R. Mattsson, J. Thyberg, and J. Nilsson. Insulin-like growth factor II plays a central role in atherosclerosis in a mouse model. J. Biol. Chem. 277:4505–4511 (2002).
R. Novosyadlyy, K. Tron, J. Dudas, G. Ramadori, and J. G. Scharf. Expression and regulation of the insulin-like growth factor axis components in rat liver myofibroblasts. J. Cell Physiol. 199:388–398 (2004).
J. G. Scharf, T. Knittel, F. Dombrowski, L. Muller, B. Saile, T. Braulke, H. Hartmann, and G. Ramadori. Characterization of the IGF axis components in isolated rat hepatic stellate cells. Hepatology 27:1275–1284 (1998).
G. Pugliese, F. Pricci, N. Locuratolo, G. Romeo, G. Romano, S. Giannini, B. Cresci, G. Galli, C. M. Rotella, and U. Di Mario. Increased activity of the insulin-like growth factor system in mesangial cells cultured in high glucose conditions. Relation to glucose-enhanced extracellular matrix production. Diabetologia 39:775–784 (1996).
N. M. Dahms and M. K. Hancock. P-type lectins. Biochim. Biophys. Acta 1572:317–340 (2002).
P. A. Dennis and D. B. Rifkin. Cellular activation of latent transforming growth factor beta requires binding to the cation-independent mannose 6-phosphate/insulin-like growth factor type II receptor. Proc. Natl. Acad. Sci. U. S. A. 88:580–584 (1991).
R. Bataller, E. Gabele, C. J. Parsons, T. Morris, L. Yang, R. Schoonhoven, D. A. Brenner, and R. A. Rippe. Systemic infusion of angiotensin II exacerbates liver fibrosis in bile duct-ligated rats. Hepatology 41:1046–1055 (2005).
P. J. Admiraal, C. A. van Kesteren, A. H. Danser, F. H. Derkx, W. Sluiter, and M. A. Schalekamp. Uptake and proteolytic activation of prorenin by cultured human endothelial cells. J. Hypertens. 17:621–629 (1999).
M. M. van den Eijnden, J. J. Saris, R. J. de Bruin, E. de Wit, W. Sluiter, T. L. Reudelhuber, M. A. Schalekamp, F. H. Derkx, and A. H. Danser. Prorenin accumulation and activation in human endothelial cells: importance of mannose 6-phosphate receptors. Arterioscler. Thromb. Vasc. Biol. 21:911–916 (2001).
T. Braulke and G. Mieskes. Role of protein phosphatases in insulin-like growth factor II (IGF II)-stimulated mannose 6-phosphate/IGF II receptor redistribution. J. Biol. Chem. 267:17347–17353 (1992).
D. F. Smee, M. Bray, and J. W. Huggins. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir. Chem. Chemother. 12:327–335 (2001).
H. Tedesco-Silva, M. C. Bastien, L. Choi, C. Felipe, J. Campestrini, F. Picard, and R. Schmouder. Mycophenolic acid metabolite profile in renal transplant patients receiving enteric-coated mycophenolate sodium or mycophenolate mofetil. Transplant. Proc. 37:852–855 (2005).
J. H. LeBowitz, J. H. Grubb, J. A. Maga, D. H. Schmiel, C. Vogler, and W. S. Sly. Glycosylation-independent targeting enhances enzyme delivery to lysosomes and decreases storage in mucopolysaccharidosis type VII mice. Proc. Natl. Acad. Sci. U. S. A. 101:3083–3088 (2004).
L. Beljaars, G. Molema, D. Schuppan, A. Geerts, P. J. de Bleser, B. Weert, D. K. Meijer, and K. Poelstra. Successful targeting to rat hepatic stellate cells using albumin modified with cyclic peptides that recognize the collagen type VI receptor. J. Biol. Chem. 275:12743–12751 (2000).
L. Beljaars, B. Weert, A. Geerts, D. K. Meijer, and K. Poelstra. The preferential homing of a platelet derived growth factor receptor-recognizing macromolecule to fibroblast-like cells in fibrotic tissue. Biochem. Pharmacol. 66:1307–1317 (2003).
Acknowledgments
This study was financially supported by the Dutch Foundation for Technical Sciences (STW), grant no GFA.5460. Prof. D.K.F. Meijer is gratefully acknowledged for valuable scientific discussion and review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greupink, R., Bakker, H.I., van Goor, H. et al. Mannose-6-Phosphate/Insulin-Like Growth Factor-II Receptors may Represent a Target for the Selective Delivery of Mycophenolic Acid to Fibrogenic Cells. Pharm Res 23, 1827–1834 (2006). https://doi.org/10.1007/s11095-006-9025-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9025-2