Aim
The objective of the study is to evaluate the mechanism behind alterations in passive and active transport of drugs in the presence of basolaterally applied extracellular protein using the Caco-2 cell model.
Methods
The permeation across Caco-2 monolayers of two groups of compounds, transported either solely by passive diffusion or partly also by active transport in the secretory direction, was studied at two donor concentrations in the absence or presence of bovine serum albumin (BSA, 0–4%). Each group contained compounds with high or low protein binding (PB) capabilities and high or low absorption in humans (fraction absorbed, f a). The unbound fraction (f u) of each compound was determined by ultrafiltration.
Results
The transport rate of the passively permeating compounds was the same in both apical-to-basolateral (absorptive) and basolateral-to-apical (secretory) directions in the absence of BSA. Basolaterally applied BSA increased the transport rate in the absorptive direction and decreased it in the secretory direction for all compounds, in direct proportion to the extent of PB. The efflux ratios for the actively transported compounds were reduced in the presence of BSA.
Conclusions
Basolaterally applied BSA, which mimics in vivo PB, alters both passive and active drug transport in the Caco-2 cell model. Using C u in the calculations of transport rate allowed elucidation of the different mechanisms behind these alterations. Our data also suggest that active secretory transport for highly protein-bound compounds might have less effect in vivo than predicted from traditional Caco-2 cell models (without BSA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To better mimic physiological conditions, bovine serum albumin (BSA) can be applied basolaterally, i.e., on the serosal side, in the Caco-2 cell system (1,2). This mimicking of extracellular tissue protein may improve the mass balance for lipophilic drugs, which will bind to the protein instead of the plastic wells (3,4). The extracellular protein will also help to maintain sink conditions during screening experiments by reducing back flux (1,2,5,6). Applying BSA only to the basolateral side would reflect the in vivo situation more closely than the traditional Caco-2 setup (without BSA). Depending on the extent of protein binding (PB) and the degree to which the compound permeates passively, addition of BSA basolaterally will increase transport in the absorptive (apical-to-basolateral, a-to-b) direction (2). This, in turn, can lead to higher predicted fraction of the drug absorbed from the human intestine after oral administration (f a). However, the transport in the secretory (basolateral-to-apical, b-to-a) direction will be reduced (7) because the unbound concentration of the drug (C u) available to permeate is lower due to PB, resulting in a lower efflux ratio. Nonetheless, this is considered to be a more accurate prediction of the in vivo situation regarding the relevance of secretory transport in the presence of BSA. However, to compare these transport rates with those obtained using the traditional experimental setup without BSA, the permeability values for both absorptive and secretory transport have to be adjusted for the reduced unbound concentration of the drug available to permeate.
Changes to the distribution of the drug caused by the addition of extracellular protein may also alter the intracellular drug concentration, as has been reported for the CYP3A4-metabolized drug midazolam (8). Because alteration of the intracellular concentration may also change the amount of compound at the binding site of an efflux system and, hence, the availability of the compound for active drug transport, both passive diffusion and (active) efflux processes need to be studied to understand how BSA influences drug transport in the Caco-2 cell model. To our knowledge, the extent of the effect of extracellular PB on concomitant passive and active drug transport has not been investigated to date.
Thus, to obtain more reliable information on physiological in vitro/in vivo relationships, it is important to consider PB in the Caco-2 cell model. The aims of this study were therefore to investigate the effect of extracellular PB on the passive and active transport of drugs and to investigate the influence of the inclusion of BSA on the basolateral side of Caco-2 monolayers on the interpretation of drug transport data. Because albumin is the most abundant protein in plasma, with a concentration of about 40 mg/ml (0.6 mM), and because it accounts for approximately 60% of the total protein content (9), we endeavored to mimic physiological conditions with a BSA concentration of 4% (w/v). BSA is less expensive than human serum albumin and is therefore commonly used as a replacement in in vitro studies (1,2,6).
Material and Methods
Experimental Design
Several criteria were used to select drugs for inclusion in this study. Because the majority, approximately 40%, of the 618 registered oral drugs (Physician Desk Reference, 1999) are amines and are thus weakly basic (10), we investigated mainly cationic compounds, although acidic drugs generally bind to albumin to a higher extent. The drugs were required to be chemically and metabolically stable under our experimental conditions in the Caco-2 cell model.
Two groups of drugs were investigated. Group 1 included compounds transported by passive diffusion only, and group 2 comprised compounds whose transport is influenced by active apical efflux systems such as P-glycoprotein (P-gp) and/or the breast cancer resistance protein. Each group contained compounds with either high or low PB and high or low f a, as ascertained from the literature (11–13). To cover a wide variety of compounds and to fulfill the above criteria for ourin vitro experiments, the “low” and “high” limits for both PB and f a were set as 0–30 and 70–100%, respectively (see Table I). This was despite generally held views that a compound is highly protein-bound in vivo when over 95% of the compound is plasma protein bound, and that a compound is considered to be easily absorbed when the extent of absorption in humans is90% or more of an administered dose (14,15).
Compounds and Radiolabeled Markers
[14C]-Mannitol (1.9 GBq/mmol), S-[3H]-propranolol (703 GBq/mmol), and [3H]-digoxin (629 GBq/mmol) were obtained from NEN™ Life Science Products Inc. (Boston, MA, USA). Quinidine [9-3H] (740 GBq/mmol) was purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO, USA). [14C]-Doxorubicin (2.04 GBq/mmol) waspurchased from Amersham Pharmacia Biotech UK Limited (Buckinghamshire, UK). Inogatran, [3H]-inogatran (58.5 GBq/mmol), metoprolol, and [3H]-metoprolol (31.5 GBq/mmol) were synthesized at AstraZeneca (Mölndal, Sweden). SOLUENE®-350 tissue solubilizer solution and OptiPhase “Highsafe” 3 were purchased from PerkinElmer™ (Boston, MA, USA) and Wallac (Loughborough, UK), respectively. Solvents used were of analytical grade and were purchased from Skandinaviska Gentech (Kungsbacka, Sweden). All other chemicals were obtained from Sigma-Aldrich Co. (Stockholm, Sweden). The quality of the BSA determines the binding capacity as well as the binding affinity to a drug; we used BSA from Sigma-Aldrich Co., which was a Fraction V (Cat. No.: A-8022) of the following lot No's.: 78H1090, 107H0558, and 109H1051.
Growth Conditions for Caco-2 Cells
Caco-2 cells (ATCC, Rockville, MD, USA) were maintained as described previously (10). All experiments were performed with Caco-2 cells in the same condition as those used for screening at AstraZeneca. Cells (passage 26–39) were seeded on Transwell® 12-well plates (Cat No. 3401; Costar; 1.13 cm2, 0.4-μm pore size) and used after 17–20 days in culture for transport experiments.
Transport Experiments
Transport studies were performed as described previously (10) using the following sampling times: 0, 5, 15, 25, 50, 80, and 120 min. The incubation medium was Hank's balanced salt solution supplied with 25 mM HEPES (HBSS–HEPES). All experiments were performed at a basolateral pH of 7.4 to take the possible effect of pH in the blood on the binding rate into account. To avoid the impact of a pH gradient on drug transport (10,16), the apical pH was also set to 7.4. The influence of basolaterally applied BSA (1, 2, and 4%) on the permeability of the monolayers tothe drugs was determined bidirectionally. Each experiment was performed at least in triplicate, and each compound was investigated at two donor concentrations. The radioactivity in the samples was determined by liquid scintillation counting in a counter from Wallac (Turku, Finland), using a quench curve prepared for the presence of BSA. The transepithelial electrical resistance (TER) of the monolayers and the permeability to mannitol were taken as indicators of the monolayer integrity. Only cell monolayers with TER values over 250 Ω cm2 were used. The threshold for the permeability to mannitol for omitting monolayers wasset at < 0.4 × 10−6 cm s−1.
Determination of the Cellular and Filter Concentration
After the final receiver and donor samples were collected, the filters containing the cell monolayers were washed twice with ice-cold HBSS–HEPES buffer (pH 7.4). The filter supports were then cut out with a scalpel and transferred into 20-ml glass vials. The cell monolayers were dissolved together with the filter support in 2-ml SOLUENE®-350 tissue solubilizer solution, and thereafter, 15-ml OptiPhase “Highsafe” 3 solution was added to each vial. The radioactivity content of the well-mixed samples was measured using a liquid scintillation counter (Wallac, Turku, Finland). The radioactivity associated with the cells or adsorbed onto the filter was then determined.
Donor Concentrations of the Model Compounds
To cover a wide concentration range in the study of passively and actively transported compounds, the lowest concentrations to be investigated were determined by the specific activity of the radiolabeled compound, and the highest concentrations were, in general, determined by the drug solubility or toxicity to the monolayers. For quinidine, the highest concentration was that at which the bidirectional transport rates were not significantly different, i.e., 10 μM.
Determination of the Protein Binding
We defined standardized conditions for binding experiments as close as possible to physiological conditions, using an assay temperature of 37°C, an albumin concentration of 40 g/l, and a pH of 7.4.
Centrifree® micropartition devices from Millipore (Bedford, MA, USA) were prewarmed to 37°C, and 400-μl samples were loaded into the reservoir. Each PB value was determined twice using two micropartition devices. The samples were always kept at 37°C and pH 7.4. After centrifuging in a Hettich Rotixa/KS centrifuge (Tutlingen, Germany) with a fixed-angle bucket at 3080 rpm (2000 × g) for 20 min, radioactivities in 100-μl ultrafiltrate were determined using a liquid scintillation counter (Wallac, Turku, Finland). The initial total concentration (C initial) was also determined from the radioactivity content in the initial samples. The unbound fraction (f u) of the compound was then determined according to the following equation:
where C u is the free/unbound concentration available to permeate. An assumption was made that only the unbound drug is able to move across membranes.
Low recovery from either protein-free ultrafiltrate or buffer could indicate adsorption to and/or membrane rejection of the ligand. Thus, comparable concentrations of drug in HBSS–HEPES buffer (pH 7.4) without protein were analyzed to ensure that no binding to the filter material occurred. No significant losses were observed for any of the compounds.
Data Treatment
All experiments were performed under “sink” conditions, and, consequently, the permeability (P app) values were calculated as described previously (3,10,13,17). All permeability rates were calculated by linear regression from concentration-time profiles (r 2 ≥ 0.97).
The ratio of the secretory to absorptive transport rates was calculated to obtain information regarding any asymmetry in the transport of the drug. Thus, (apical) drug efflux ratios were calculated using the following equation:
The recovery (R) is the percentage of original drug mass accounted for at the end of the experiment (the sum of the amounts on the apical and basolateral sides) in relation to the initial amount added. The recovery was calculated using the following equation:
where C a(t) and C b(t) are the drug concentrations on the apical and basolateral sides of the monolayer at time (t), respectively, C 0 is the concentration of the drug in the donor solution at time zero (t = 0), V a and V b are the volumes of the apical and basolateral compartments, respectively, and V d is the volume of the donor solution added to the appropriate side of the monolayer. The recovery for all transport experiments was higher than 92%, and no adsorption to the plastic of the cell culture plates was observed for any of the investigated compounds; therefore, the amount of compound that was unaccounted for was considered to be associated with the cell monolayer.
Statistics
Values are expressed as the mean ± standard deviation (SD). All cell culture experiments were performed at least in triplicate. The PB was determined in duplicate. The statistical difference between the permeabilities of the cells to the drugs in the absence or presence of 4% BSA was calculated using an unpaired t test with a two-tailed distribution for comparison of two mean values. Analysis of variance was used when more than two mean values were compared, i.e., when the statistical difference between the permeabilities of the cell monolayers to the drug at different BSA concentrations was calculated (0, 1, 2, and 4%). A p value of less than 0.01 was considered statistically significant.
Results
Selection of Model Drugs
One poorly permeating model drug with low PB, mannitol, and one easily permeating drug with low PB, metoprolol, were selected for group 1. Unfortunately, a highly protein bound, poorly permeating compound that is not transported via an active process could not be identified for group 1, consistent with the fact that hydrophilic compounds, which are poorly absorbed, do not bind extensively to BSA (18). However, an easily absorbed, highly protein-bound compound that is passively transported, S-propranolol, was included in group 1. The S-enantiomer was chosen because this enantiomer of propranolol is known to bind to plasma proteins to a higher extent than the R-enantiomer (19).
Group 2 comprised four drugs that are transported by both passive and active processes (Table I). For the poorly protein-bound compounds, two drugs that are actively apically effluxed from cells were selected: inogatran, which is poorly absorbed, and digoxin, which is easily absorbed. The two highly protein-bound compounds were doxorubicin, a poorly absorbed drug, and quinidine, an easily absorbed drug (Table I).
Integrity of Caco-2 Cell Monolayers
No significant difference in TER was found between Caco-2 systems with or without basolaterally applied BSA (0, 1, 2, or 4%; p > 0.1; n = 40) in our study. In addition, all permeability values for mannitol were lower than 0.4 × 10−6 cm s−1 (0.27 ± 0.07 × 10−6 cm s−1, n = 458). Thus, in contrast to Raub et al. (20) using MDCK cells, TER values and mannitol fluxes across the Caco-2 cell monolayers were not affected by extracellular BSA in our study.
Caco-2 Cell Permeability of the Passively Transported Drugs (Group 1)
The transport rates were determined in the absorptive and secretory directions at two donor concentrations for the passively transported compounds in group 1 (Table I): mannitol, metoprolol, and S-propranolol.
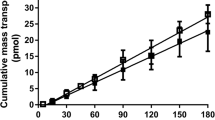
An example of the bidirectional permeation of metoprolol at basolaterally applied 0, 1, 2, and 4% BSA and the corresponding recalculated P app values is shown in Fig. 1. In the presence of 1, 2, or 4% BSA, the transport rate in the absorptive direction increased, and the transport rate in the secretory direction decreased with increased amounts of basolaterally applied BSA. The amounts of metoprolol bound to protein were 1, 7, and 8% for the three BSA concentrations, respectively (Table II). To account for the unbound substance in the basolateral compartment, the unbound fraction (f u) was determined and the unbound concentration (C u) of each of the compounds was calculated, according to Eq. (1). When the unbound concentrations in the final receiver solution (in a-to-b transport studies) and initial donor solution (in b-to-a transport studies) were accounted for, none of the adjusted transport rates were significantly different from the bidirectional transport rates in the absence of BSA (p > 0.1; Fig. 1). The same procedure was then applied to all compounds. The fractions of the compounds that were bound to 1, 2, and 4% BSA at the two investigated donor concentrations were measured and are listed in Table II. Figures 2, 4, and 5 illustrate the transport rates obtained in the presence and absence of 4% BSA and the corresponding recalculated transport rates for the experimentally determined PB.
Caco-2 monolayer permeability (P app) values for metoprolol in the apical-to-basolateral (a-to-b) direction (black symbols) and basolateral-to-apical (b-to-a) direction (white symbols) in the presence of basolaterally applied bovine serum albumin (BSA, w/v). The corresponding permeability values were recalculated using the unbound concentration of metoprolol available at the various BSA concentrations and are also shown. Values are means ± SD (n ≥ 3).
Transport of the passively transported drugs: (A) mannitol, (C) metoprolol, and (D) propranolol across Caco-2 monolayers in the apical-to-basolateral (a-to-b) direction (black bars) and basolateral-to-apical (b-to-a) direction (white bars) in the absence and presence of 4% BSA (w/v) on the basolateral side of the monolayer. The corresponding permeability (P app) values that account for the PB by correcting for the unbound concentration are also given for each compound. (B) A passively transported compound fulfilling the criterion of high PB and poor permeation could not be identified (see text). Values are means ± SD (n ≥ 3). PB, protein binding.
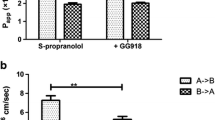
For mannitol, which is a poorly absorbed drug without PB, the influence of basolateral BSA was negligible, and the absorptive transport rate equaled the secretory rate under all experimental conditions (n > 30; Fig. 2A). In contrast, the easily absorbed and poorly protein-bound drug metoprolol (Tables I and II) showed a slight increase in the absorptive transport rate and a decrease in the secretory rate with increasing concentrations of basolaterally applied BSA (0 → 4%; Fig. 2C). The effect of BSA was more pronounced for the easily absorbed, weakly basic S-propranolol, which has higher PB than metoprolol (Table I). A significant increase in the transport rates in the absorptive direction of over 30% and a significant reduction in S-propranolol transport in the secretory direction of almost 60% were observed when 4% BSA was added basolaterally (Fig. 2D). For passively transported compounds, the relative increase in the permeation of the compound across the Caco-2 cell monolayers in the absorptive direction was directly proportional to the extent of PB for each compound (slope of 0.78, Fig. 3). Thus, in group 1, the most obvious impact on drug transport of 4% basolateral BSA occurred with the highly protein-bound and easily absorbed compound S-propranolol.
The relative change in the apparent permeability coefficients (P
app) in the absorptive direction caused by the sink conditions created by basolaterally applied 1, 2, or 4% BSA (w/v) is correlated to the fraction of compound bound to BSA. A good correlation for the passively transported compounds (■ mannitol,  metoprolol, □ S-propranolol) was obtained (r
2 = 0.9). Values are for each compound from two initial donor concentrations. The trendline for the passively (solid line) transported compounds is shown.
metoprolol, □ S-propranolol) was obtained (r
2 = 0.9). Values are for each compound from two initial donor concentrations. The trendline for the passively (solid line) transported compounds is shown.
The permeation rates of mannitol (10–100 μM) and metoprolol (1–1000 μM) were not concentration dependent. S-Propranolol was investigated at 50 nM and 1000 μM. A decrease in binding to BSA from 56 to 38% was observed with the higher concentration of S-propranolol (Table II), which was in agreement with the increase in the corresponding efflux ratios at the higher donor concentration in the presence of BSA (Table III). However, when the unbound concentration based on the fraction bound to 4% BSAwas taken into account, there were no significant differences in permeation between the 50-nM and 1000-μM S-propranolol concentrations. Therefore, Fig. 2 shows the results obtained for mannitol 100 μM, metoprolol 1000 μM, and S-propranolol 1000 μM (Fig. 2). Thus, transport rates recalculated to accommodate C u (by using f u) revealed that BSA maintained the sink conditions, without changing the passive (intrinsic) permeation of the drug. To our knowledge, this is the first time that the extent of the effect of extracellular BSA in the Caco-2 cell model has been systematically evaluated and that the effect of C u has been accounted for.
Caco-2 Cell Permeability of Drugs that are Actively Secreted (Group 2)
The bidirectional transport rates were then determined for the compounds in group 2, which were able to permeate passively and were also transported actively in the secretory direction: inogatran, doxorubicin, digoxin, and quinidine.
For inogatran, which is a poorly permeating drug with a low PB capacity, the effect on the transport rates of basolaterally applied BSA was more pronounced in the secretory than in the absorptive direction, at both the low (0.65 μM; Fig. 4A) and high (250 μM; Fig. 5A) donor concentrations. The permeation of inogatran (0.65 μM) in thesecretory direction decreased significantly when 4% BSA was applied to the basolateral side of the monolayers, whereas the change in transport rate for the absorptive direction was within the standard deviation of the calculated P app values (Fig. 4A). In the presence of 1, 2, or 4% BSA, the fraction of inogatran bound to the protein was independent of the drug concentration (Table II). At the higher donor concentration of 250 μM, the secretory transport rate of inogatran (0.90 ± 0.07 × 10−6 cm s−1) still exceeded the corresponding absorptive transport rate (0.34 ± 0.07 × 10−6 cm s−1, Fig. 5A). These transport rates gave similar but not identical efflux ratios of 3.3 ± 0.7 and 2.7 ± 0.6 at 0.65 and 250 μM, respectively (Table III).
Transport of the actively transported drugs: (A) inogatran, (B) doxorubicin, (C) digoxin, and (D) quinidine across Caco-2 monolayers in the apical-to-basolateral (a-to-b) direction (black bars) and basolateral-to-apical (b-to-a) direction (white bars) at the lowest investigated donor concentrations in the absence and presence of 4% BSA (w/v) on the basolateral side of the monolayer. The corresponding permeability (P app) values that account for the unbound fraction are also given for each compound. Values are means ± SD (n ≥ 3). PB, protein binding.
Transport of the actively transported drugs: (A) inogatran, (B) doxorubicin, (C) digoxin, and (D) quinidine across Caco-2 monolayers in the apical-to-basolateral (a-to-b) direction (black bars) and basolateral-to-apical (b-to-a) direction (white bars) at the highest investigated donor concentrations in the absence and presence of 4% BSA (w/v) on the basolateral side of the monolayer. The corresponding permeability (P app) values that account for the unbound fraction are also given for each compound. Values are means ± SD (n ≥ 3). PB, protein binding.
The transport rates of the highly protein-bound drug doxorubicin (Tables I and II) were significantly influenced by basolateral BSA. At the lower donor concentration of 18 μM, a significant increase in the absorptive transport rate and a decrease in the secretory transport rate were observed with increased concentrations of basolateral BSA (0 → 4%; Fig.4B). A similar pattern was obtained at the higher donor concentration of 120 μM (Fig. 5B). In the presence of 1, 2, or 4% BSA, the bound fraction of doxorubicin was 18, 33, and 46%, respectively; these values were only slightly affected by the initial drug concentration (Table I). When the (initial) donor concentration of doxorubicin was increased from 18 to 120 μM, the secretory transport rates decreased (from 5.9 ± 0.6 × 10−6 to 4.2 ± 0.3 × 10−6 cm s−1), and the absorptive transport rates also decreased slightly (from 0.69 ± 0.07 × 10−6 to 0.53 ± 0.03 × 10−6 cm s−1). The resulting efflux ratios at low (18 μM) and high (120 μM) concentrations were 8.5 ± 1.2 and 8.0 ± 0.7, respectively (Figs. 4C and 5C, Table III). These ratios reflect the probable presence of an (active) efflux system. Because the change in the intracellular concentration due to BSA is expected to be most pronounced for poorly permeating and highly protein-bound compounds, we have only shown the results for doxorubicin (Fig. 6). BSA reduced the amount of doxorubicin remaining associated with the monolayer after the experiment and thus the amount of doxorubicin available at the binding site of the carrier (Fig. 6).
The easily permeating, well-known P-gp substrate digoxin was investigated as an actively transported compound with a low PB capacity. The rate of the bidirectional permeation of digoxin was determined for a low donor concentration of 59 nM (Fig. 4C) and a high concentration of 625 μM (Fig. 5C), which was the solubility limit of digoxin in the presence of 1% dimethyl sulfoxide. The absorptive transport rate was not significantly different between the two concentrations in the absence of BSA (from 3.4 ± 0.2 × 10−6 cm s−1 at 59 nM to 2.9 ± 0.2 × 10−6 cm s−1 at 625 μM). Incontrast, the transport rates in the secretory direction decreased significantly as the donor concentration increased (from 26.3 ± 0.7 × 10−6 cm s−1 at the lowest donor concentration to 5.3 ± 0.3 × 10−6 cm s−1 at the highest concentration; Figs. 4C and 5C). The transport rate in the absorptive direction was always slower than that in the secretory direction, resulting in efflux ratios of 7.7 ± 0.5 and 1.8 ± 0.1 for the low and high concentrations, respectively (Table III). Digoxin was poorly bound to BSA (Table II); however, in the presence of 4% BSA in the basolateral medium, the transport rate of digoxin (59 nM) in the secretory direction was significantly reduced (Fig. 4C), whereas the rate in the absorptive direction was slightly increased in comparison with control experiments (without BSA; Fig. 4C). At the higher donor concentration of 625 μM, the bidirectional transport rates of digoxin were only slightly altered in the presence of 4% (w/v) BSA (Fig. 5C).
A similar, but more pronounced, effect was observed for the highly permeating, highly protein-bound drug quinidine (Fig. 4D). More than 40% of the applied quinidine (50 nM) was bound to 4% BSA (Table II). A significant increase in the transport rate in the absorptive direction and a significant reduction in the secretory rate were observed when 4% BSA was added basolaterally (Fig. 4D). Accounting for C u led to acalculated transport rate of 25 ± 2 × 10−6 cm s−1 in the absorptive direction and 100 ± 4 × 10−6 cm s−1 in the secretory direction. With an unbound fraction of about 60% (Table II), the concentration of the remaining unbound drug is 30 nM. Therefore, as a control experiment, the bidirectional transport rate for quinidine was also investigated at an initial donor concentration of 30 nM in the absence of BSA. The transport rates obtained at this donor concentration were comparable to those at 50 nM in the presence of 4% BSA adjusted for C u, indicating that our approach for taking into account the unbound concentration and recalculating the permeability values was sound (Fig. 7). An analogous effect of BSA was observed for the higher donor concentration of 10 μM (Fig. 5D). At this concentration, however, the rate of transport of quinidine was equal in both directions, and this was therefore the highest concentration investigated. At a lower concentration of 50 nM, an efflux ratio of 3.0 ± 0.3 was obtained (Table III).
Transport of the actively transported drug quinidine across Caco-2 monolayers in the apical-to-basolateral (a-to-b) direction (black bars) and basolateral-to-apical (b-to-a) direction (white bars) at 50 nM in the absence and presence of 4% BSA (w/v) on the basolateral side of the monolayer. The corresponding permeability (P app) values that account for the unbound fraction (58%) and, as comparison, the P app values at a concentration of 30 nM in the absence of BSA are also shown. Values are means ± SD (n ≥ 3). PB, protein binding.
In summary, at the lower concentrations of all the investigated actively transported compounds in group 2, the transport rates in the secretory direction always exceeded those in the absorptive direction in the absence of BSA. In addition, the actively transported compounds could be identified under all experimental conditions when BSA was applied basolaterally and when the f u had been accounted for. The transport rate in the secretory direction decreased when 4% BSA was present on the basolateral side of the monolayers, compared with the transport rates when BSA was absent. This was reflected in a decrease in the corresponding efflux ratios (Table III).
Discussion
Protein binding is known to affect the effective concentration of the drug and the equilibrium between tissue and plasma concentrations. As expected, the rates of mannitol transport in the absorptive and secretory directions were equal under all experimental conditions using BSA, reflecting the fact that mannitol is not bound to plasma proteins (Figs.2A and 3, Table I). Propranolol has been investigated earlier in Caco-2 cell assays in the presence of BSA (5,7). In contrast to our findings, however, neither of these two publications showed a significant impact of 4% albumin on the absorptive transport rate for propranolol. This may have been because, in one of the publications (5), the first sample was taken after 30 min, which is considerably later than in our experimental approach, where the first sample was taken after 5 min. In the other publication (7), a large variability in the experimental data was reported. In our study, the rate of absorptive transport of metoprolol and S-propranolol increased with the extent of their binding to albumin as described in Fig. 3 with a slope of 0.78. The slope of 0.78 can beused to estimate the maximal expected impact of 78% change if a protein binding of 100% is assumed using the experimental setup with basolaterally applied BSA and the limited set of compounds (mannitol, metoprolol, and propranolol; Fig. 3).
The measurements of f u, made without destroying the equilibrium in the sample solution, enabled the calculation of C u. This, in turn, provided an opportunity to elucidate the mechanisms behind the alterations of drug transport due to BSA. Whereas the in vitro fractions bound to BSA of the poorly protein-bound compounds inogatran and metoprolol in our studies were in good agreement with the in vivo PB data published in the literature, the values for the highly protein-bound compounds were, in general, lower in our in vitro assays (Tables I and II). In addition, BSA behaved as a saturable reservoir, as seen from the concentration-dependent reduction in binding, from 56 to 38%, for S-propranolol (Table II). By accounting for the unbound fraction of drug in our experiments, we showed that it is possible to estimate the rate of passive diffusion of drugs in the presence of extracellular protein from experimental transport studies. Importantly, under these conditions, albumin (BSA) maintained sink conditions and thus retained the concentration gradient needed as a driving force for drug transport, while providing more physiologically realistic conditions than would occur in the absence of BSA. Under this hypothesis, the data obtained from the proposed setup may be closer to the actual relationship between P app and f a because compounds with high PB have access to the sink conditions in plasma in vivo. Thus, the inclusion of basolateral BSA can be suggested as a secondary screening tool offering more physiological conditions for the compound with regard to its absorptive transport.
Because BSA has been introduced to the experimental design, it might be desirable to know the maximal impact of the protein on the permeation of added compounds through the membranes. One approach would be to use the following equation:
where P app(a–b) (0% BSA) is the apparent permeability value in the absorptive direction in the absence of BSA, P app(a–b) (4% BSA) is the apparent permeability value in the absorptive direction in the presence of 4% BSA, and f u (4% BSA) is the percentage of unbound compound in the presence of 4% BSA.
If the recovery of the compound is around 100% in the absence of BSA and the compound is highly protein bound (99.9%; f u = 0.1%), then the expected maximal permeation rate on the addition of extracellular BSA, based on this equation, would be approximately twice the baseline rate, e.g., a P app of 1.5 × 10−6 cm s−1 in the absence of BSA might increase to 3 × 10−6 cm s−1 in the presence of BSA. Importantly, such differences in P app values could alter the permeability ranking of compounds in the screening setting. If the compound is poorly recovered in the absence of BSA, the impact of BSA will be different. Using Eq. (4), the permeability can be reestimated, allowing for the presence of BSA, by using the fraction of drug that is not bound to BSA. This theoretical permeability value would reflect the rate of transport of the compound in the absence of BSA, with a theoretical recovery of 100%. If the bound fraction is used to correct the concentration to calculate the permeation of the compound in the absence of BSA, both sets of data (with and without BSA) can be used similarly.
The addition of BSA and the process of correcting for the unbound fraction also provide further information about the active component. For example, at a donor concentration of 10 μM, the transport rate for quinidine in the absence of BSA was equal in both directions (Fig. 5D), which has commonly been interpreted as meaning that quinidine is not actively transported and/or that the transporter was saturated. In the presence of BSA, the transport rate increased in the absorptive direction and decreased in the secretory direction. However, for an apically effluxed compound, if PB is accounted for, the lowering of the initial donor concentration by PB will lead to an increase in secretory transport and a decrease in absorptive transport to anextent related to the permeability-concentration dependency for the compound, as seen in this study for quinidine (Fig. 7). Thus, after correction for the unbound fraction, the data for quinidine can now be interpreted as showing an efflux component because, at a concentration of 6 μM, which is the unbound concentration after correction for f u, the transporter was not saturated. Furthermore, at a concentration of 10 μM, the transporter may also not be saturated, if it is assumed that a concentration of five times the K m value is required for saturation [K m(a-to-b) = 6.8 ± 1.1 μM and K m(b-to-a) = 2.2 ± 0.2 μM (21)]. Thus, equal bidirectional transport rates may not necessarily indicate passive diffusion or saturation of the involved transporters.
P-gp is expressed almost entirely throughout the gastrointestinal (GI) tract (expression is highest in the lower small intestine) (22,23) and may limit f a (because of efficient intestinal secretion). At higher oral doses, this secretory process becomes saturated (24), resulting in an increased f a and consequently in potential for increased oral bioavailability. Our data suggest that, in vivo, the impact of intestinal efflux may be even less important for highly protein-bound compounds because the sink conditions in the plasma will reduce the intracellular concentration and thereby the concentration at the binding site of the efflux transporter, ashas been shown for doxorubicin (Fig. 6). This finding is supported by a study investigating the metabolism of midazolam [a compound that is 95% bound to plasma proteins (8)]. Extracellular BSA reduced the intracellular midazolam concentration and changed the transport direction of the metabolite from excretion to absorption (8). Our data further suggest that a compound, such as quinidine, which interacts with apical efflux transporters, has high PB, and easily permeates the GI membrane, will probably not be associated with absorption problems after oral administration, although clinically relevant interactions might occur (25,26). However, a substrate for the efflux transporter with low PB, such as digoxin, may be poorly absorbed in vivo because of efflux, although the drug permeates the GI membrane easily. This is especially important for digoxin, a compound with a narrow therapeutic window.
According to our data, obtained in the presence of relevant plasma concentrations of BSA, the efflux ratio obtained in the absence of BSA may overestimate the importance of transport in the secretory direction. For example, in this study, the efflux ratio of quinidine was reduced from 3 to 1.3 and that of doxorubicin from 8.5 to 2.5 in the presence of basolaterally applied BSA (Figs. 4 and 5, Table III). We therefore suggest that efflux ratios obtained in the presence of basolaterally applied BSA will better reflect the in vivo relevance of transport in the secretory direction due to apical efflux or basolateral uptake transporters.
Conclusion
We conclude that the basolateral application of BSA, with the aim of mimicking in vivo protein binding, modifies both passive and active drug transport in the Caco-2 cell model. The measurement of C u provides the opportunity to elucidate the different mechanisms behind these alterations. In summary, our results indicate that BSA in the basolateral compartment will more closely reflect the effect of PB in the blood circulation in vivo. Thus, a system has been proposed that successfully refines a traditional cell culture permeability model by including more aspects of the in vivo situation and that can be used as a tool for secondary screening in drug discovery.
References
S. Yamashita T. Furubayashi M. Kataoka T. Sakane H. Sezaki H. Tokuda (2000) ArticleTitleOptimized conditions for prediction of intestinal drug permeability using Caco-2 cells Eur. J. Pharm. Sci. 10 195–204 Occurrence Handle1:CAS:528:DC%2BD3cXitlOksL8%3D Occurrence Handle10767597
P. Saha J. H. Kou (2002) ArticleTitleEffect of bovine serum albumin on drug permeability estimation across Caco-2 monolayers Eur. J. Pharm. Biopharm. 54 319–324 Occurrence Handle1:CAS:528:DC%2BD38XoslGgurw%3D Occurrence Handle12445562
P. Artursson (1990) ArticleTitleEpithelial transport of drugs in cell culture. I: a model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells J. Pharm. Sci. 79 476–482 Occurrence Handle1:CAS:528:DyaK3cXkvFSru74%3D Occurrence Handle1975619
G. Wilson I. F. Hassan C. J. Dix I. Williamson R. Shah M. Mackay P. Artursson (1990) ArticleTitleTransport and permeability properties of human Caco-2 cells: an in vitro model of the intestinal epithelial cell barrier J. Control. Release 11 25–40 Occurrence Handle10.1016/0168-3659(90)90118-D Occurrence Handle1:CAS:528:DyaK3cXit12hu78%3D
G. Krishna K.-j. Chen C.-c. Lin A. A. Nomeir (2001) ArticleTitlePermeability of lipophilic compounds in drug discovery using in-vitro human absorption model, Caco-2 Int. J. Pharm. 222 77–89 Occurrence Handle10.1016/S0378-5173(01)00698-6 Occurrence Handle1:CAS:528:DC%2BD3MXkt1Kmsb0%3D Occurrence Handle11404034
M. E. Taub L. Kristensen S. Frokjaer (2002) ArticleTitleOptimized conditions for MDCK permeability and turbidimetric solubility studies using compounds representative of BCS classes I–IV Eur. J. Pharm. Sci. 15 331–340 Occurrence Handle1:CAS:528:DC%2BD38Xjt1Crtb4%3D Occurrence Handle11988394 Occurrence Handle10.1016/S0928-0987(02)00015-5
R. A. Walgren T. Walle (1999) ArticleTitleThe influence of plasma binding on absorption/exsorption in the Caco-2 model of human intestinal absorption J. Pharm. Pharmacol. 51 1037–1040 Occurrence Handle10.1211/0022357991773366 Occurrence Handle1:CAS:528:DyaK1MXmslKhurs%3D Occurrence Handle10528987
J. M. Fisher S. A. Wrighton J. C. Calamia D. D. Shen K. L. Kunze K. E. Thummel (1999) ArticleTitleMidazolam metabolism by modified Caco-2 monolayers: effects of extracellular protein binding J. Pharmacol. Exp. Ther. 289 1143–1150 Occurrence Handle1:CAS:528:DyaK1MXislGisbY%3D Occurrence Handle10215698
G. Colmenarejo (2003) ArticleTitleIn silico prediction of drug-binding strengths to human serum albumin Med. Res. Rev. 23 275–301 Occurrence Handle10.1002/med.10039 Occurrence Handle1:CAS:528:DC%2BD3sXjsVWitLg%3D Occurrence Handle12647311
S. Neuhoff A.-L. Ungell I. Zamora P. Artursson (2003) ArticleTitlepH-Dependent bidirectional transport of weakly basic drugs across Caco-2 monolayers: implications for drug–drug interactions Pharm. Res. 20 1141–1148 Occurrence Handle10.1023/A:1025032511040 Occurrence Handle1:CAS:528:DC%2BD3sXmtVGrtL4%3D Occurrence Handle12948010
A. L. Ungell J. Karlsson (2003) Cell cultures in drug discovery: an industrial perspective H. Waterbeemd Particlevan De H. Lennernäs P. Artursson (Eds) Drug Bioavailability. Estimation of Solubility, Permeability, Absorption and Bioavailability Wiley-VCH Verlag GmbH & Co. KGaA Weinheim 90– 131
L. Z. Benet S. Oie J. B. Schwartz (1996) Goodman & Gilman's: The Pharmacological Basis of Therapeutics McGraw-Hill New York
P. Artursson J. Karlsson (1991) ArticleTitleCorrelation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells Biochem. Biophys. Res. Commun. 175 880–885 Occurrence Handle10.1016/0006-291X(91)91647-U Occurrence Handle1:CAS:528:DyaK3MXhslOrt7c%3D Occurrence Handle1673839
M. Rowland T. N. Tozer (1989) Clinical Pharmacokinetics. Concepts and Applications Lea and Febiger Philadelphia
Food and Drug Administration, U.S. Department of Health and Human Services, CDER. Guidance for industry: waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. http://www.fda.gov/cder/guidance/3618fnl.pdf (2000).
S. Neuhoff A.-L. Ungell I. Zamora P. Artursson (2005) ArticleTitlepH-Dependent passive and active transport of acidic drugs across Caco-2 cell monolayers Eur. J. Pharm. Sci. 25 211–220 Occurrence Handle1:CAS:528:DC%2BD2MXks1Gitr4%3D Occurrence Handle15911216
J. Hunter B. H. Hirst N. L. Simmons (1991) ArticleTitleEpithelial secretion of vinblastine by human intestinal adenocarcinoma cell (HCT-8 and T84) layers expressing P-glycoprotein Br. J. Cancer 64 437–444 Occurrence Handle1:CAS:528:DyaK3MXmslSisbY%3D Occurrence Handle1680366
Å. Frostell-Karlsson H. Widegren C. E. Green M. D. Hämäläinen L. Westerlund R. Karlsson K. Fenner H. Waterbeemd Particlevan de (2005) ArticleTitleBiosensor analysis of the interaction between drug compounds and liposomes of different properties; a two-dimensional characterization tool for estimation of membrane absorption J. Pharm. Sci. 94 25–37 Occurrence Handle10.1002/jps.20215 Occurrence Handle1:CAS:528:DC%2BD2MXjslyktg%3D%3D Occurrence Handle15761927
R. L. Lalonde D. M. Tenero B. S. Burlew V. L. Herring M. B. Bottorff (1990) ArticleTitleEffects of age on the protein binding and disposition of propranolol stereoisomers Clin. Pharmacol. Ther. 47 447–455 Occurrence Handle1:CAS:528:DyaK3cXktFOnsbc%3D Occurrence Handle2328552 Occurrence Handle10.1038/clpt.1990.56
T. J. Raub C. L. Barsuhn L. R. Williams D. E. Decker G. A. Sawada N. F. Ho (1993) ArticleTitleUse of a biophysical-kinetic model to understand the roles of protein binding and membrane partitioning on passive diffusion of highly lipophilic molecules across cellular barriers J. Drug Target. 1 269–286 Occurrence Handle1:CAS:528:DyaK2cXjtVOhtL0%3D Occurrence Handle8069569
M. D. Troutman D. R. Thakker (2003) ArticleTitleEfflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers Pharm. Res. 20 1200–1209 Occurrence Handle1:CAS:528:DC%2BD3sXmtVGrtLY%3D Occurrence Handle12948018
A. Seithel J. Karlsson C. Hilgendorf A. Björquist A. L. Ungell (2004) Variability in Gene Expression of Drug Transporters by Age and Passage in Caco-2 Cells EUFEPS Copenhagen
G. Englund, F. Rorsman, A. Rönnblom, L. Lazorova, A. Kindmark, and P. Artursson. Regional RNA expression of drug transporting proteins in the human gut. In 3rd World Conference on Drug Absorption, Transport and Delivery. Clinical Significance and Regulatory Impact. Barcelona, Spain (2005).
K. Westphal A. Weinbrenner T. Giessmann M. Stuhr G. Franke M. Zschiesche R. Oertel B. Terhaag H. K. Kroemer W. Siegmund (2000) ArticleTitleOral bioavailability of digoxin is enhanced by talinolol: evidence for involvement of intestinal P-glycoprotein Clin. Pharmacol. Ther. 68 6–12 Occurrence Handle1:CAS:528:DC%2BD3cXlvVOrs7o%3D Occurrence Handle10945310
M. Verschraagen C. H. Koks J. H. Schellens J. H. Beijnen (1999) ArticleTitleP-glycoprotein system as a determinant of drug interactions: the case of digoxin-verapamil Pharm. Res. 40 301–306 Occurrence Handle10.1006/phrs.1999.0535 Occurrence Handle1:CAS:528:DyaK1MXms1ygt74%3D
B. Angelin A. Arvidsson R. Dahlqvist A. Hedman K. Schenck-Gustafsson (1987) ArticleTitleQuinidine reduces biliary clearance of digoxin in man Eur. J. Clin. Investig. 17 262–265 Occurrence Handle1:STN:280:DyaL2szgsVChsQ%3D%3D Occurrence Handle10.1111/j.1365-2362.1987.tb01246.x
Acknowledgments
This work was supported by AstraZeneca R&D (Mölndal, Sweden), the Swedish Animal Welfare Agency, and the Swedish Medical Research Council (grant 9478). The authors wouldlike to thank Dr. Carl Gunnar Regårdh for his curiosity about the effect of protein binding in cell culture experiments, which initiated this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neuhoff, S., Artursson, P., Zamora, I. et al. Impact of Extracellular Protein Binding on Passive and Active Drug Transport Across Caco-2 Cells. Pharm Res 23, 350–359 (2006). https://doi.org/10.1007/s11095-005-9304-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-9304-3