Purpose
These studies evaluated the ability of common household food and drink products to mask the bitter taste of three selected anti-terrorism drugs.
Methods
Three anti-terrorism drugs (doxycycline, ciprofloxacin hydrochloride, and potassium iodide) were mixed with a variety of common household food and drinks, and healthy adult volunteers evaluated the resulting taste and aftertaste. In parallel, the ASTREE Electronic Tongue was used to evaluate taste combinations. Stability of the mixtures over time was monitored, as was the dosage uniformity across preparations.
Results
Foods and drinks were identified that satisfactorily masked the bitter flavor of each drug. Dose uniformity and stability were also acceptable over the range studied, although some combinations were significantly less stable than others. The electronic tongue was able to differentiate between tastes, but ranked masking agents in a different order than human volunteers.
Conclusions
Doxycycline, potassium iodide, and ciprofloxacin, which are stockpiled in solid tablet form, can conveniently be prepared into more palatable formulations, using common household foods and drinks. The electronic tongue can be used to perform an initial screening for palatability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of the terrorist attacks on the USA on September 11, 2001 and the subsequent anthrax incidents across the USA, the need for enhanced national security was reinforced. The US Food and Drug Administration (FDA) is actively involved in this effort and has taken a number of actions to help ensure adequate response to future incidents of both bio- and nuclear terrorism. One such action involves working with other federal agencies to make sure sufficient supplies of medicine and vaccines will be available in a timely manner.
When preparing for disasters and terrorist events, it is essential to ensure that the needs of children are met. Pediatric treatment needs are unique in a number of ways. Children require different dosage forms and dosing charts, and they can have higher susceptibility than adults to the effects of radiation or infectious agents. However, many of the drugs that are stockpiled by the government are only available in solid dosage forms, which are not readily swallowed by infants and children. Additionally, these drugs may be either too salty or bitter to be acceptable for oral delivery to a child. Because many dosage regimens may need to be followed for several weeks, it is essential that children and infants be dosed with palatable preparations of the medicines to ensure patient compliance. For these reasons, the FDA has undertaken the task of developing appropriate procedures so that parents or other caregivers can convert the solid tablets into a form that can be administered to infants and small children (1). As a result of these studies, the FDA currently provides parents with instructions on how to mix several counterterrorism drugs with common household foods and drinks, using ordinary kitchen utensils, to disguise the unpleasant taste of the medicine.
Three drugs that are currently stockpiled for response to specific terrorist actions were chosen for this study—doxycycline, ciprofloxacin hydrochloride (cipro), and potassium iodide (KI). Doxycycline is a broad-spectrum antibiotic drug used to treat several types of infections, including Bacillus anthracis (anthrax). It is synthetically derived from oxytetracycline and is available commercially in tablet, capsule, and suspension dosage forms. Ciprofloxacin hydrochloride is a drug used to treat infections caused by susceptible strains of several microorganisms, including B. anthracis. It is currently indicated to reduce the incidence or progression of disease following exposure to aerosolized B. anthracis, which occurs during inhalational anthrax (postexposure). It is commercially available as tablets and as an oral suspension. Potassium iodide is a drug used to prevent the uptake of radioactive iodine by the thyroid gland and is also used as an expectorant and in the treatment of dermatoses. It is only available in tablet form.
All three products are stockpiled in the form of solid tablets and thus, from considerations of stability and bulk volume, are not suitable for administration to infants and small children. Furthermore, when drugs are scored for division into smaller doses, they may crumble. This makes accurate dosing difficult, as the powder must be divided manually. Finally, not all food and drinks have equal ability to mask the bitter taste of the drug. Therefore, studies were conducted to evaluate the hypothesis that drugs, once ground and mixed with appropriate food or drinks, would remain stable for an acceptable period of time and could be taken orally with reasonable palatability and with uniform dosage. The results of the palatability studies in human subjects were compared with those obtained with the ASTREE electronic tongue. The current paper describes these studies.
Materials and Methods
Materials
Doxycycline
United States Pharmacopeia (USP) Reference Standard Doxycycline was used as a reference material. Generic doxycycline hyclate tablets (Watson Laboratories, Phoenix, AZ, USA) with a label value of 100 mg were used for stability and dosage studies.
Ciprofloxacin Hydrochloride
Ciprofloxacin hydrochloride tablets from Bayer, Inc., Shawnee Mission, KS, USA weighing on average 722.3 mg and containing a label claim of 500 mg of cipro were used in this study.
Potassium Iodide
Potassium iodide tablets (Iostat) from Anbex, Inc., Palm Harbor, FL, USA containing 130 mg of KI were used.
All chemicals were commonly available analytical reagents and were used without further purification. Solutions were prepared with deionized water. Food and drink were obtained from local stores.
Methods
Dosage Uniformity
Doxycycline
Before combining with food or drink, tablets were analyzed directly by dissolving an amount equivalent to one tablet in 1,000 mL of 0.01 N HCl. The resulting solution was filtered through a high-volume, low pressure-type (HVLP-type) 0.45-μm, 25-mm filter. The filtered solution was directly injected onto the high-performance liquid chromatograph (HPLC) following the assay procedure from the Doxycycline Hyclate Tablet USP monograph (2). An initial study was conducted to ensure complete and linear recovery of doxycycline from low-fat chocolate milk over a wide dosage range. Thirty tablets (100 mg) were crushed and combined. A series of solutions from 10 to 230 mg of this tablet composite equivalent to 4–100 mg of doxycycline was mixed with 5 mL of low-fat chocolate milk. Each mixture was quantitatively transferred to a 250-mL volumetric flask and diluted to volume with 0.01 N HCl. Solutions were then filtered and analyzed by HPLC.
To investigate dose uniformity, utensils and conditions were used to simulate preparation of dosages in a kitchen. A single tablet (100 mg) was placed in a piece of aluminum foil (4 × 4 in.), and the foil was folded in half enclosing the tablet. The back of a spoon was used to crush the tablet. The foil was then opened, and the tablet was further crushed with the spoon into a fine powder. The resulting powder was used in its entirety or split visually into either two or four equal portions using a knife or razor blade. One or more portions were scraped onto a second piece of foil. This powder was then transferred onto a tablespoon. To the tablespoon was added a teaspoon (∼5 mL) of low-fat chocolate milk, resulting in ∼5- to ∼20-mg/mL mixtures. The mixtures were stirred for 30 s with a toothpick. Each milk and drug mixture was quantitatively transferred with 0.01 N HCl to a 1,000-mL volumetric flask for 50- to 100-mg dosages or to a 250-mL volumetric flask for 25-mg dosages. Samples were diluted to mark with 0.01 N HCl and analyzed by HPLC. Multiple dosages were prepared in low-fat chocolate milk and analyzed to determine dosage uniformity.
To measure the homogeneity of the drug in chocolate milk preparations, one tablet was crushed and mixed with 20 mL of low-fat chocolate milk. Five 1-mL aliquots were taken, diluted to 50 mL with 0.01 N HCl, filtered, and analyzed by HPLC.
Ciprofloxacin Hydrochloride
Ciprofloxacin hydrochloride is not soluble in water. To determine analytical recovery of a suspension of the drug, ten tablets (500 mg) were crushed and mixed into a composite. Five portions of the composite sample representing the approximate weight of 1/16, 1/8, 1/4, 1/2, and 1 tablet were each shaken with 5 mL of water to form a suspension, an aliquot removed and analyzed by the HPLC method. The assay procedure in the USP, Cipro Tablet Monograph (3), was followed to quantify the cipro for recovery, dose uniformity, and stability testing. The HPLC system used for analysis employed a Waters 2690 separation module and 2487 detector, Waters Symmetry C18 column (25 cm). The HPLC system met the USP system suitability requirements of resolution, theoretical plates, tailing factor, and relative standard deviation. The method was found to be linear from 0.07 to 0.566 mg/mL, which encompasses the range of samples analyzed.
To determine how uniform a dose could be administered using household utensils, four whole tablets (500 mg) were shaken with 16 teaspoons (equivalent to 80 mL) of water. Two separate medicinal dispensing cups commonly supplied with over-the-counter pediatric medications were used. Cup 1 had a total volume of 3 teaspoons and cup 2 had a total volume of 4 teaspoons. One teaspoon of the suspension would be equal to a dose of 125 mg. The individual dose of ∼125 mg was transferred into a 250-mL volumetric flask, diluted with the mobile phase to volume, and analyzed by HPLC.
Potassium Iodide
To determine how uniform a dose could be administered using household utensils, ten tablets (130 mg) were shaken with 20 teaspoons of water. Two separate analysts were asked to take 1 teaspoon of the prepared stock solution and mix with 1 teaspoon of water using either a common household teaspoon or a medicinal dispensing cup commonly supplied with over-the-counter pediatric medications. One teaspoon of this mixture would be equivalent to a dose of 32.5 mg. This procedure was repeated five times by each analyst. These individual doses were quantitatively diluted to 5 mg/L and analyzed by HPLC. Ion chromatography with electrochemical detection was performed according to Dionex Application Note 37, “The Determination of Iodide in Milk Products”. The ion chromatography system (Dionex DX-500) consisted of an AS3500 Autosampler, ED40 Electrochemical Detector, pulsed amperometry, silver working electrode, and an IonPac AS11 (anion exchange) column (250 × 4 mm). The mobile phase was 0.05 M nitric acid, with a flow rate of 1.5 mL/min. This method showed good linearity from 1.64 to 13.08 mg/mL (R2 = 0.9998) and acceptable reproducibility [3.2% relative standard deviation (RSD) at 6.5 mg/mL, n = 5]. One-day recovery ranged from 103% from water to 106% from low-fat milk.
Stability
Doxycycline
To determine stability in food and drinks, a composite of 20 crushed tablets was made and the equivalent of one tablet (230.9 mg) was mixed with 20 mL of drink or 20 g of food. The foods and drinks used were water, apple juice with table sugar (183 g of sugar in 100 mL of apple juice), low-fat milk, low-fat chocolate milk, regular chocolate milk, chocolate pudding, grape jelly, strawberry jelly, yogurt with cherry flavor, and simple syrup with sour apple flavor (FlavorRx®). The drink samples (milks, juice, water, and syrup) were shaken for approximately 5 min. The food samples (pudding, yogurt, jellies) were mixed thoroughly with a spatula until visually uniform. A 1-mL aliquot from the drink or 1-g aliquot from the food was analyzed by diluting with 0.01 N HCl to 50 mL, filtering and injecting into the HPLC system. The remaining materials were covered, half was allowed to remain on the counter (22–26°C) and half was refrigerated (2–8°C). After 24 h, both the refrigerated and nonrefrigerated materials were resampled and analyzed by HPLC. The doxycycline in low-fat chocolate milk was also tested after 1 week in the refrigerator.
To determine the stability of the crushed tablets, 30 tablets were crushed and mixed into a composite. Six hundred milligrams of this composite was wrapped in aluminum foil and placed on the counter. Once a day, for 6 days, the foil was opened and a 58-mg portion of this composite (equivalent to 25 mg of doxycycline) was removed, dissolved in 250 mL of 0.01 N HCl, filtered, and assayed by the HPLC method.
Ciprofloxacin Hydrochloride
The stability of cipro in water, apple juice, chocolate milk, imitation maple syrup, strawberry jelly, chocolate syrup, and premixed infant formula over 7 days was determined. Infant formula was only used for the stability studies, and not the palatability studies, because cipro dispersed in formula would not be palatable to adult subjects. The stability of cipro in infant formula was studied because in the event of an emergency, formula could be used to mask the taste of cipro for infants that may not be able to eat or drink any of the other foods or drinks used in this study. Because cipro is insoluble in water, one tablet was added to 5 mL of water and shaken to dissolve the film coating and disperse the tablet contents. The suspension was then mixed with foods or drinks. For the drinks (water, apple juice, chocolate milk, and premixed infant formula), 15 mL of the drink (3 teaspoons) was mixed with 5 mL of water containing the drug. For the foods (syrups and jelly), 30 g (3–4 teaspoons) of food was mixed with 5 mL of water containing the drug. The samples were covered with a sheet of aluminum foil and kept in the refrigerator for the duration of the stability study. Upon storage, the drug would settle to the bottom of the container, so samples were stirred with a toothpick before each use. Samples were taken on Days 1, 2 (24 h), 4, and 7 and analyzed by HPLC. A cipro standard solution was also analyzed along with the samples. Blank solutions of media were prepared and analyzed by the HPLC method. No significant interference peaks were found in foods or drinks.
Potassium Iodide
The stability of KI in water, orange juice, soda, raspberry syrup, low-fat milk, low-fat chocolate milk, and baby formula (Similac) over 7 days was determined. Two stock solutions were prepared. The first stock solution consisted of ten tablets shaken with 100.0 mL water. The second stock solution included five tablets shaken with 100.0 mL water. To make a 32.5-mg dose, 5.0 mL of the first stock solution was mixed with 5.0 mL of each food or drink. On the day of analysis, 0.5 mL of each mixture was diluted to 500.0 mL with water and analyzed by the HPLC method. To make a 16.25-mg dose, 5.0 mL of the second stock solution was mixed with 5.0 mL of each food or drink. On the day of analysis, 0.5 mL of each mixture was diluted to 250 mL with water and analyzed by the HPLC method. Blank solutions of each food and drink were also prepared and analyzed by HPLC. Only a trace of iodide was found in the low-fat chocolate milk. Samples were taken on Days 1, 2, 4, and 7. On each day of analysis, the first stock solution was analyzed along with the other samples to check its stability.
Palatability Studies
Human Panel
The FDA Research Involving Human Subjects Committee (RIHSC) approved the palatability study protocol and volunteer consent form for each of the three studies involving human subjects. Thirty healthy adult volunteers participated in each study (20 in the case of doxycycline). The number of volunteers was selected based on literature recommendations (4,5) and experimental design considerations to enable adequate power to detect a significant difference among preparations. Approximately 90% of volunteers were in the age group from 20 to 29; none were under the age of 20 or over the age of 50. Age was not studied as a covariate. Gender was of interest as a covariate. Half of the volunteers in each case were males and half were females. Racial and/or ethnic minorities were not excluded. Volunteers were excluded for any of the following reasons: an existing medical condition that might interfere with the ability to discriminate taste such as common cold, sinus infection, upper respiratory infection; a history of allergic reaction to doxycycline, food, and drinks used in this study; ingestion of any prescription medication (other than birth control pills) or over-the-counter medication for 14 days prior to the study; or pregnancy. To assure balance, volunteers were tested in five separate groups. Each volunteer was randomly assigned a specific sequence of the preparations, and each volunteer received the samples in the specified order.
Volunteers were asked to refrain from eating and drinking for 1 h before the study. Before the first tasting and between tastings, the volunteer was asked to cleanse his/her mouth by eating an unsalted soda cracker and to take several swallows of bottled water. The preparations were administered approximately 5 min apart to allow time for taking and rating the preparation and cleaning the mouth. Each preparation was evaluated for responses on both taste and aftertaste. After the testing phase of the study, each volunteer was asked to remain at the testing site for approximately 1 h for observation.
A scoring system on a scale of 1–5, with 1 indicating the lowest score and 5 the highest, was employed. A score of 1 indicated that the preparation was not palatable, whereas a score of 2 only indicated slightly palatable. A score of 3 was considered average, and a score of 4 indicated that the preparation is palatable. Finally, a score of 5 showed that the preparation was highly palatable. The score was communicated by holding up fingers instead of pointing to smile-faces so that the volunteers could be blindfolded, and the effect of appearance and texture could be minimized or eliminated. Any aspects of texture that might be detected visually (for example, “bumpy” vs. “smooth”) are aspects of appearance. This scoring system has been used by other investigators (6–9). Steele et al. (10) used a system with 0 indicating the least palatability and 5 the highest. Powers et al. (11) and Toscani et al. (12) employed a gender-specific smile-face scale depicting various degrees of pleasure.
The average scores of taste and aftertaste for each preparation were calculated and compared using an analysis of variance for repeated measures with the general linear models (GLM) procedure (SAS® Version 6.12). The simplifying assumption was made that the scores from 1 to 5 could be treated as a continuous variable. An analysis using a simple proportional odds model for ordered categorical data confirmed the results. However, because of the flexibility of modeling with PROC GLM and ease of explanation of results, only the GLM findings are reported.
The goal was to order the preparations in terms of taste and aftertaste, and to identify significant differences among the preparations, using the Duncan's multiple range test (13). The factors included in the model were group, sex, preparation, and position of each preparation within the randomization order.
Doxycycline
For each of the test matrices, a 100-mg doxycycline tablet was ground using household utensils and mixed with one of the ten foods used for stability testing. The preparations of ground doxycycline in water and simple syrup with sour apple flavor served as negative and positive controls. The other eight preparations were chosen based on extensive internal discussion, including input from the FDA Pediatric Working Group. The masking agents selected were water, apple juice with table sugar (183 g of sugar in 100 mL of apple juice), low-fat milk, low-fat chocolate milk, regular chocolate milk, chocolate pudding, grape jelly, strawberry jelly, yogurt with cherry flavor, and simple syrup with sour apple flavor (FlavorRx®). The final concentration of each preparation for evaluation of palatability was 5 mg/mL. All test preparations were prepared on the day of the study, stored in a refrigerator, and warmed to room temperature for at least half an hour before use. Number assignments instead of the food or drinks' name were used to identify each preparation to test subjects to ensure the blind nature of the study. The study was conducted at an FDA facility. Each volunteer was asked to taste ten 2.5 mL of preparations of doxycycline. Each preparation contained 12.5 mg doxycycline, and the total doxycycline consumed by each volunteer was 125 mg, which is much less than the 200-mg first dose of doxycycline, as recommended in the package insert.
Ciprofloxacin hydrochloride
Approximately 24 h prior to administration, six cipro tablets (500 mg) were weighed together. The total weight was divided by 24 to obtain the powder paperweight equivalent to 125 mg of cipro. The six tablets were ground to a fine powder in a glass mortar and pestle, and the equivalent weight of 125 mg cipro was made for each powder paper. Excess cipro from each lot was discarded.
Individual doses for administration were prepared approximately 9 min before administration to the subject by taking the contents of a single powder paper and placing in a metal tablespoon (serving spoon). Five milliliters of masking agent was slowly added using a 10-mL plastic syringe. The masking agents used were water, apple juice, low-fat chocolate milk, imitation maple syrup, strawberry jam, and chocolate syrup. In the case of jam, 5 mL was estimated using a 5-mL plastic teaspoon. The cipro and masking agent were mixed until visually uniform. Thirty seconds prior to dosing, a final mixing was conducted. At dosing, subjects were asked to open their mouth widely and the spoon was placed in their mouth, with the subjects immediately swallowing the mixture. Ratings were made within 10 s of subject swallowing and again 1 min after swallowing for aftertaste.
The study was conducted at the University of Tennessee. Study volunteers ingested 125 mg of cipro in each of the six foods/drinks, totaling 750 mg of cipro for each subject. This dose was less than the recommended daily dose, as listed in the package insert (500 mg, twice a day for up to 28 days).
Potassium Iodide
A single (130 mg) KI tablet was ground using household utensils, and the resulting powder was mixed with 10 mL of water to yield a 13-mg/mL solution. An aliquot of 5 mL was further diluted with 5 mL of each of the six masking agents—water, low-fat milk, low-fat chocolate milk, orange juice, (flat) soda, and raspberry syrup. One teaspoon (5 mL) of this final dilution (6.5-mg/mL final concentration) was given to the subjects. The preparations of ground KI in water and raspberry syrup served as negative and reference controls. Each preparation was evaluated in two categories: taste and aftertaste.
This study was conducted at the University of Tennessee. Total KI consumption was 195 mg (32.5 mg per dose for six samples) per subject. This dose was less than the recommended daily dose for an oral suspension product (SSKI, Upsher-Smith, Maple Grove, MN, USA), which is listed in the package insert as 300–600 mg, three to four times a day for as long as necessary, for the indication of use as an expectorant.
Electronic Tongue
In addition to the human panel, the palatability of various food and drink matrices was evaluated using an electronic tongue. The ASTREE™ Electronic Tongue is a liquid and taste analyzer already used in the food industry (14) and is gaining popularity in the pharmaceutical industry (15). It is composed of several different coated sensors based on CHEMFet technology, which transforms chemical information into electrical signals.
To predict the effectiveness of the food or drink matrix for taste masking, each matrix is evaluated both with and without drug. The placebo sample (matrix without drug) is considered the “best” tasting formulation because the bitter drug is not present. The measured output represents the aggregate potential difference for each of the sensors vs. the reference electrode. Using principal component analysis (PCA), these measured potentials for the placebo and active (matrix with drug) samples are compared to determine their similarity. This difference is related to the bitterness masking efficiency of the matrix (i.e., the closer the potentials, the better the masking) and is described mathematically by the discrimination index (DI), which accounts for the spread of the data as well as the distance between groups.
The ASTREE™ pharmaceutical sensors set, Sensor array No. 2 (ZZ05, AB21, BA23, BB20, CA22, DA10, and JE20), was used. The sample volume was 100 mL, with an analysis time of 180 s and an acquisition time of 120 s.
Prior to application of the sensor array for taste evaluation, replicate measurements are carried out for each individual sensor to assess its stability in the test matrix. Only those sensors that exhibit good stability (small RSD) in the target matrix are included in the sensor array used for taste evaluation employing that matrix.
The matrices tested using the electronic tongue were water, low-fat milk, apple juice (100 mL of apple juice with 183 g of dissolved sugar), low-fat chocolate milk, orange juice, raspberry syrup, chocolate syrup, and maple syrup. Note that the food products tested by the sensory human panel and with the ASTREE™ system may be slightly different as the first were purchased in the USA and the second in France.
Samples for electronic analysis were prepared as follows. For doxycycline, 500 mg of the drug was mixed with 100 mL of each pure matrix. For KI, 325 mg of the drug was mixed with 50 mL of distilled water and 50 mL of pure matrix. For cipro, 750-mg tablets [average tablet weight (ATW), 1.15 g] or 500-mg tablets (ATW, 0.772 g) were ground to form composite samples. A weight of 3.83 g (750-mg tablets) or 3.81 g (500-mg tablets) of the tablet composite was mixed with 25 mL of distilled water and 75-mL drink or 150-g food. All the samples were prepared and analyzed at room temperature. The test samples were mixed well before use. It should be noted that the concentration used for testing KI was lower than that used with the human taste panel. The electronic tongue and stability testing were performed using the 3.25-mg/mL dose recommended for 1 month through 3 years, whereas the human taste panel used the 6.5-mg/mL dose recommended for 3–18 years.
Results
Dose Uniformity and Stability
Doxycycline
When generic tablets were assayed directly, they were found to weigh on average 230.9 mg and contain on average 97 mg of doxycycline. In subsequent calculations, these values were used to calculate 100% of a desired dose. Recoveries of doxycycline from chocolate milk were found to range from 95.1 to 100.7% with good linearity, R2 = 0.9997.
Dose variability results were assessed for low-fat chocolate milk with average dosages found to be good for administration of 1/4, 1/2, 3/4, or 1 tablet (93–99% of desired). The range of dosages was most variable for the 1/4 tablet (75–132% of desired). Variability in the desired dosage is caused by the accuracy of visibly dividing the powder into two or four fractions. Analysis of five aliquots of a doxycycline tablet dissolved in low-fat chocolate milk gave assays with a RSD of 0.93% (n = 5), which demonstrates a high degree of homogeneity of the doxycycline within the milk.
Good original recoveries (all >94%) and 24-h stability (all >95%) (refrigerated and room temperature) were seen for the doxycycline tablets crushed and mixed with all ten foods and drinks. In addition, doxycycline in low-fat chocolate milk was found to be stable when refrigerated for 1 week (>98% recovery).
For the crushed tablets wrapped in aluminum foil, no significant loss in drug concentration was seen with the activity remaining greater than 95% over all 6 days.
Ciprofloxacin Hydrochloride
Recovery up to the 1/2 tablet dispersed in 1 teaspoon of water was good—over 99.5% for 1/16, 1/8, and 1/4 tablet in 1 teaspoon water. However, higher tablet portions were more difficult to keep suspended, such that a decrease in percentage recovery was seen to 92.0% for 1/2 tablet and 87.8% for 1 tablet in 1 teaspoon of water. Subsequent dose uniformity and stability work was performed with one tablet dispersed in 4 teaspoons of water to ensure adequate suspension of the cipro.
In uniformity studies, the average dose was found to be about 134.1 mg or 7% above the target dose. The range of 121.6–147.0 mg, with RSD of 6.2%, shows adequate dose-to-dose consistency.
Stability data show that a cipro suspension prepared in water or chocolate syrup can be stored for up to 7 days in a refrigerator with good stability (>92% recovery, Table I). Stability of cipro in all other media is acceptable for 24 h (recovery >91%) but not beyond.
Potassium Iodide
The average dose uniformity was 32.0 mg with a RSD of 8.2%, for two analysts with five runs each. Stability testing of both 16- and 32-mg doses revealed greater than 90% recovery for all dosages in all foods for the entire 7 days.
Palatability Studies
Doxycycline
Table II shows the mean scores (Duncan group) of each preparation for taste and aftertaste. The analysis found that preparation effect was highly significant. For both taste and aftertaste, no significant difference was found between males and females. A slight position effect was noted for aftertaste, which means that the order in which the preparations were sampled made a slight difference. However, more complex modeling failed to reveal any consistent evidence of a carryover effect. Finally, there was no significant effect due to testing group.
In the category of taste, low-fat chocolate milk, regular chocolate milk, and chocolate pudding were not significantly different from one another. They are in the best Duncan grouping and significantly better than the rest of the preparations. Preparations assigned the same letter according to Duncan's test are not significantly different from each other. Simple syrup with sour apple flavor, the positive control in this study, received a mean score of 2.95, which is lower than the chocolate preparations, but significantly higher than yogurt, jellies, and water. Meanwhile, the apple juice with table sugar and low-fat milk were not significantly different from simple syrup with sour apple flavor. As expected, water, our negative control, received the lowest score of 1.85. There were no significant differences among grape jelly, strawberry jelly, yogurt, and water.
In the category of aftertaste, as with the taste, chocolate pudding, regular chocolate milk, and low-fat chocolate milk are in the first group. Simple syrup with sour apple flavor was not significantly different from chocolate milks. Strawberry jelly and water are in the worst group, whereas low-fat milk, yogurt with cherry flavor, and grape jelly were in the group next to the worst. Again, apple juice with sugar is worse than the chocolate milks and better than water.
Ciprofloxacin Hydrochloride
The order of randomization of flavors was followed as specified in the protocol for 30 subjects. The six participants in a given group, however, were not studied on the same day, as planned. They were actually studied as follows: 1–3 on study Day 1, 4–6 on Day 2, 7–17 on Day 16, 18–27 on Day 20, and 28–30 on Day 21. Assessment of the relative palatability scores adjusted for intended and actual grouping found no influence of either factor. The number of females per group ranged from two to four (2, 2, 4, 3, and 4 for groups 1–5, respectively).
The mean scores (Duncan groups) of each preparation for taste and aftertaste are listed in Table III.
Preparation effect was highly significant for both taste and aftertaste (p value < 0.0001 for the F-test for homogeneity). As anticipated, the standard errors were close to 1.0 (0.852 for taste and 0.808 for aftertaste), supporting the assumptions made for the sample size calculation. Results shown are from the simple model that assesses the differences due to preparation among the taste and aftertaste scores, without adjustment for gender, grouping, or randomization order. There were no differences in results due to gender, grouping, or randomization order.
Although palatability preferences for taste and aftertaste did not depend significantly on gender, the aftertaste preference shown by females for strawberry jam over maple syrup was reversed for males.
The overall palatability results did not depend on group, date of testing, or the order in which the preparations were sampled, with one exception. For one group of five participants who tasted in the order chocolate syrup, maple syrup, apple juice, strawberry jam, low-fat chocolate milk, and water, the aftertaste scores for chocolate syrup and maple syrup were much lower than the corresponding scores for the other 25 participants with other orderings of flavors. There seems no explanation for this finding. Adjusting the analysis, however, by order of sampling did not alter the overall palatability ordering for aftertaste.
It is worth noting that none of the subjects upon completing the cipro taste test said they really liked any of the formulations. Thus, a rating of 4+ for cipro in chocolate syrup does not mean it tastes good, but just better than the other formulations.
Potassium Iodide
Table IV shows the mean scores of each preparation for taste and aftertaste. The analysis indicates that preparation effect was highly significant for both taste and aftertaste (p < 0.0001). As anticipated, the standard errors were close to 1.0 (0.965 for taste and 0.929 for aftertaste), supporting the assumptions made for the sample size calculation. Results did not depend on gender or on the order in which the preparations were sampled.
Values presented in Tables IV result from the simple model that assesses the differences due to preparation among the taste and aftertaste scores, without adjustment for gender, either grouping, or randomization order. For both taste and aftertaste, raspberry syrup was best at masking KI. Water was the worst for both, along with low-fat milk for aftertaste.
Electronic Tongue
Doxycycline
The analysis was performed using seven sensors, but to optimize results, only five sensors were included in the statistical analysis. To determine sensor repeatability, replicate measurements were made for each sensor. The sensors (with calculated RSDs) used were ZZ05 (0.62%), BB20 (2.08%), CA2407 (2.36%), DA18 (0.68%), and JE09 (1.18%).
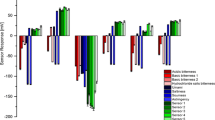
A principal component analysis was performed, which indicated that the electronic tongue was able to discriminate the different active samples (drug plus matrix) from each other as shown below. The DI evaluates the discrimination quality by measuring both the distance between groups and the size of each group. For all matrices studied for doxycycline, the DI was 96 out of 100.
To determine the best masking matrix, the group distance between each active sample and its corresponding placebo (matrix only) was calculated. The smallest group distance indicates that the two tastes are closest together thus identifying the best masking agent. It should be noted that not all the same matrices used in the palatability studies in human subjects were used for the studies using the ASTREE electronic tongue. Other than water, only three matrices were used for each drug (Tables V–VII).
According to electronic tongue assessment, the best masking food matrix for doxycycline is the low-fat chocolate milk, followed by the low-fat milk and then apple juice with sugar.
Ciprofloxacin Hydrochloride
The analysis was performed using seven sensors, but for optimal results, only six sensors were included in the statistical analysis. To determine sensor repeatability, several replicates of each sample were measured. The sensors (with calculated RSDs) used were ZZ21 (0.76%), BA15 (1.82%), CA18 (0.56%), DA18 (0.44%), and JE18 (1.10%).
A PCA was performed, which indicated that the electronic tongue was able to discriminate among the different active samples, with a discrimination index of 94 out of 100.
In order to determine the better masking matrix, the group distance between each active sample and its corresponding placebo was calculated.
According to electronic tongue assessment, the best masking food for cipro was apple juice, followed by chocolate syrup and finally maple syrup.
Potassium Iodide
The analysis was performed using seven sensors, but for optimal results, only six sensors were included in the statistical analysis. To determine the sensor repeatability, several replicates of each sample were measured. The sensors (with calculated RSDs) used were ZZ05 (0.56%), AB27 (0.46%), BA27 (0.48%), BB20 (0.77%), DA18 (0.20%), and JE09 (2.13%). A principal component analysis was performed which indicated that the electronic tongue was able to discriminate the different active samples from each other with a discrimination index of 94 out of 100.
In order to determine the better masking matrix, a group distance between each active sample and its corresponding placebo was calculated.
According to electronic tongue assessment, the best masking food matrix for KI is chocolate milk, following by raspberry syrup and orange juice.
Results from both human and ASTREE™ taste assessments, for all drugs, are compared in Table VIII.
Discussion
Of all the strategies that have been used to improve patient compliance, such as information handouts and self-monitoring calendars, taste perception is considered to be the single most important factor in achieving compliance in children (16). Indeed, few tasks are more overwhelming to a parent than trying to force a drug into their ill child's mouth with the child refusing. Thus, masking the unpleasant taste of a drug can improve patient compliance and therefore significantly impact treatment success. If the flavor is not acceptable for the first dose, administration of subsequent doses can become more difficult, risking noncompliance. For this reason, most antibiotic liquid dosage forms for pediatric use contain sweeteners and flavors.
As expected, the positive controls used in our studies (syrups with taste corresponding to that used in the marketed solution of the presently tested solid dosage form) received some of the highest scores in the studies with human subjects. However, an average household may not have available the types of flavors used by pharmaceutical companies to formulate the liquid dosage forms of those drugs used in the present study. Therefore, under the likely scenario where only solid tablets will be available fromgovernment stockpiles, parents should be provided with options for how to prepare palatable mixtures that can be administered to infants and children, for doxycycline, ciprofloxacin, and KI solid dosage forms. These instructions should bear in mind the types of ingredients that are likely to be available in American households and that might be readily used to effectively mask the bitterness of a drug that is to be administered to a child in the event of an emergency. All three of the drugs described in this study have important medicinal uses, and having a method to dose infants and small children is important for total preparedness.
In the present study, we make the assumption that palatability in adults and children is similar, and for practical reasons, we designed the study for adults. In fact, the effect of age on taste perception is debatable. There are reports indicating that children younger than 6 years of age are unable to discriminate taste among formulations, whereas others have concluded that the sense of taste is well developed in early infancy (17–19).
In general, we found that the preparations containing chocolate were among the highest ranking for taste. However, interestingly, in the case of ciprofloxacin, we did find that although chocolate syrup was the highest-ranking medium to mask the taste of the drug, low-fat chocolate milk was found to be rather unpalatable. Similarly, all our preparations were found to have good stability over a period of 7 days. However, in the case of ciprofloxacin, preparations other than in water and chocolate syrup were found to be stable for only 24 h.
These results show that suitable, readily available foods can be used to mask the bitter taste of each of the drugs under study. The doxycycline solid tablet, once ground and mixed with chocolate milks and pudding, can have acceptable taste, stability, and average dose uniformity. For home use, a tablet could be crushed and dissolved in 1/4 cup of chocolate milk. Each teaspoon would then be equivalent to ∼8 mg doxycycline, each tablespoon ∼25 mg of doxycycline. In the case of KI, it is very likely that maple syrup or other sweet syrups, which are most likely found in homes with children, will be able to mask the taste of the drug in such a way as to make a solution of KI palatable to children. For home use, a tablet of KI can be finely ground in a small bowl using the back of a metal teaspoon. Four teaspoons of water should be added and the solution stirred with the teaspoon until the powder dissolves completely. Four teaspoons of drink are then added which yields a dose of 16.25 mg per teaspoon. Dose amounts are age dependent (1). Ciprofloxacin solid tablets, once dispersed in water and mixed with chocolate syrup, can have acceptable taste, and the preparation is stable for up to 7 days. Ciprofloxacin in maple syrup may also have acceptable palatability; however, the stability of the drug in maple syrup does not extend beyond 24 h. For home use, a tablet of ciprofloxacin can be added to 4 teaspoons of water in a small cup and stirred with a toothpick or small spoon to disperse. Each teaspoon of this dispersion would then be equivalent to ∼125 mg of cipro. After stirring, the desired dose would be removed with a teaspoon (125 mg) and mixed with 3 teaspoons (1 tablespoon) of chocolate syrup. This entire mixture could then be given to a child who would be more likely to accept this and subsequent administrations of cipro.
The correlation between ASTREE™ and human rankings is encouraging. Although the order of the rankings may not have always been the same as that obtained in the human subject palatability studies, the electronic tongue suggests that this methodology may be useful in screening out unpalatable preparations of oral dosage forms. The small difference observed between the rankings obtained with the ASTREE™ electronic tongue and the ranking provided by the Human Sensory Panel may be due to the difference between food matrices used: the ones used by the FDA panel were bought in the USA, and those used for ASTREE™ measurement were purchased in France.
Conclusions
In an emergency situation, solid tablets will be the most probable source of counterterrorism drugs, and parents will wish to employ common food or drink matrices to mask the bitter taste of a drug to improve acceptance by a child. The current study investigated the ability of various foods and drinks to mask the bitter taste of three drugs, as well as the ability to prepare uniform and stable dosages.
Dose uniformity and stability were acceptable for most of the food and drug combinations studied. Moreover, the bitter taste of all three drugs can be successfully masked with appropriate drinks and food that are commonly found in households.
Although, for the drugs and matrices examined here, the electronic tongue yielded a different ranking than that obtained via the human taste panels (some variation is expected because of different sources for a given matrix), it should prove useful for the elimination of matrices that do little to improve the palatability of the drugs. This would serve to limit the number of matrices that need to be presented to a human taste panel.
Abbreviations
- ATW:
-
average tablet weight
- DI:
-
discrimination index
- Cipro:
-
ciprofloxacin hydrochloride
- FDA:
-
Food and Drug Administration
- GLM:
-
general linear models
- KI:
-
potassium iodide
- PCA:
-
principle component analysis
- RSD:
-
relative standard deviation
- USP:
-
United States Pharmacopeia
References
How to Prepare Emergency Dosages of Doxycycline at Home for Infants and Children, http://www.fda.gov/cder/drug/infopage/penG_doxy/doxycyclinePeds.htm (accessed 03/01/05), and Home Preparation Procedure for Emergency Administration of Potassium Iodide Tablets to Infants and Small Children, http://www.fda.gov/cder/drugprepare/kiprep.htm (accessed 03/01/05).
USP 24, United States Pharmacopeial Convention, 2000.
USP 25, United States Pharmacopeial Convention, 2002.
R. W. Steele M. P. Thomas R. E. Begue (2001) ArticleTitleCompliance issues related to the selection of antibiotic suspensions for children Pediatr. Infect. Dis. J. 20 1–5 Occurrence Handle11176558
J. Cohen (1988) Statistical Power Analysis for the Behavioral Sciences, Chapter 8 Lawrence Erlbaum Hillsdale, NJ 273–403
M. E. Ruff D. A. Schotik J. W. Bass J. M. Vincent (1991) ArticleTitleAntimicrobial drug suspensions: a blind comparison of taste of fourteen common pediatric drugs Pediatr. Infect. Dis. J. 10 30–33 Occurrence Handle2003053
D. M. Demers D. S. Chan J. W. Bass (1994) ArticleTitleAntimicrobial drug suspensions: a blind comparison of taste of twelve common pediatric drugs including cefixime, cefpofodime, cefprozil and loracarbef Pediatr. Infect. Dis. J. 13 87–89 Occurrence Handle8190556
K. M. Samulak G. M. El-chaar L. G. Rubin (1996) ArticleTitleRandomized, double-blind comparison of brand and generic antibiotic suspensions: I. A study of taste in adults Pediatr. Infect. Dis. J. 15 14–17 Occurrence Handle10.1097/00006454-199601000-00003 Occurrence Handle8684870
G. M. El-chaar G. Mardy K. Wehlou (1996) ArticleTitleRandomized, double-blind comparison of brand and generic antibiotic suspensions: II. A study of taste and compliance in children Pediatr. Infect. Dis. J. 15 18–22 Occurrence Handle10.1097/00006454-199601000-00004 Occurrence Handle8684871
R. W. Steele M. P. Thomas R. E. Begue (2001) ArticleTitleCompliance issues related to the selection of antibiotic suspensions for children Pediatr. Infect. Dis. J. 20 1–5 Occurrence Handle11176558
J. L. Powers W. M. Gooch L. P. Oddo (2000) ArticleTitleComparison of the palatability of the oral suspension of cefdinir vs. amoxicillin/clavulanate potassium, cefprozil and azithromycin in pediatric patients Pediatr. Infect. Dis. J. 19 S174–S180 Occurrence Handle11144401
M. Toscani M. Drehobl J. Freed S. Stool (2000) ArticleTitleA multicenter randomized, comparative assessment in healthy pediatric volunteers of the palatability of oral antibiotics effective in the therapy of otitis media Curr. Ther. Res. 61 278–285 Occurrence Handle10.1016/S0011-393X(00)80018-1
D. B. Duncan (1955) ArticleTitleMultiple range and multiple F-test Biometrics 11 1–42
R. N. Bleibaum H. Stone T. Tan S. Labreche E. Stain-Martin S. Isz (2002) ArticleTitleComparison of sensory and consumer results with electronic nose and tongue sensors for apple juices Food Qual. Prefer. 13 409–422 Occurrence Handle10.1016/S0950-3293(02)00017-4
A. Legin A. Rudnitskaya D. Clapham B. Seleznev K. Lord Y. Vlasov (2004) ArticleTitleElectronic tongue for pharmaceutical analytics: quantification of tastes and masking effects Anal. Bioanal. Chem. 380 36–45 Occurrence Handle10.1007/s00216-004-2738-3 Occurrence Handle15365669
J. E. Hoppe (1996) ArticleTitleRational prescribing of antibacterials in ambulatory children Pharmaco Economics 10 552–574
J. Sjofall A. Fogh B. Huifield et al. (1984) ArticleTitleMethods for evaluating the taste of pediatric formulations in children: a comparison between the facial hedinoc method and patient's own spontaneous verbal judgment Eur. J. Pediatr. 141 243–247 Occurrence Handle10.1007/BF00572770 Occurrence Handle6734676
G. M. El-Chaar G. Mardy K. Wehlou et al. (1996) ArticleTitleRandomized, double-blind comparison of brand and generic antibiotic suspensions: II. A study of taste and compliance in children Pediatr. Infect. Dis. J. 15 18–22 Occurrence Handle10.1097/00006454-199601000-00004 Occurrence Handle8684871
R. W. Steele M. P. Thomas R. E. Begue (2001) ArticleTitleCompliance issues related to the selection of antibiotic suspensions for children Pediatr. Infect. Dis. J. 20 1–5 Occurrence Handle11176558
Acknowledgments
The authors would like to thank Ajaz Hussain, Daniel Briggs, Alan Carlin, Christopher Ellison, Joseph Hanig, Robbe Lyon, Arzu Selen, John Strong, Chuck Anello, Donna Volpe, and Anna Wokovich. The authors also would like to thank the FDA RIHSC members for their timely action and many constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadrieh, N., Brower, J., Yu, L. et al. Stability, Dose Uniformity, and Palatability of Three Counterterrorism Drugs—Human Subject and Electronic Tongue Studies. Pharm Res 22, 1747–1756 (2005). https://doi.org/10.1007/s11095-005-6387-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-6387-x