Purpose
To study the fundamental effects of the spinning capsule on the overall performance of a dry powder inhaler (Aerolizer®).
Methods
The capsule motion was visualized using high-speed photography. Computational fluid dynamics (CFD) analysis was performed to determine the flowfield generated in the device with and without the presence of different sized capsules at 60 l min−1. The inhaler dispersion performance was measured with mannitol powder using a multistage liquid impinger at the same flowrate.
Results
The capsule size (3, 4, and 5) was found to make no significant difference to the device flowfield, the particle-device impaction frequency, or the dispersion performance of the inhaler. Reducing the capsule size reduced only the capsule retention by 4%. In contrast, without the presence of the spinning capsule, turbulence levels were increased by 65%, FPFEm (wt% particles ≤6.8 μm in the aerosol referenced against the amount of powder emitted from the device) increased from 59% to 65%, while particle-mouthpiece impaction decreased by 2.5 times. When the powder was dispersed from within compared to from outside the spinning capsule containing four 0.6 mm holes at each end, the FPFEm was increased significantly from 59% to 76%, and the throat retention was dropped from 14% to 6%.
Conclusions
The presence, but not the size, of a capsule has significant effects on the inhaler performance. The results suggested that impaction between the particles and the spinning capsule does not play a major role in powder dispersion. However, the capsule can provide additional strong mechanisms of deagglomeration dependent on the size of the capsule hole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many attempts have been made in the past decade to optimize the delivery of drug particles to the lung (1–5). This has led to a rapid development with many inventions of dry powder aerosol delivery systems (6). Although it is well-known that the performance of a powder aerosol delivery system depends not only on the powder formulation but also the inhaler device (7), fundamental studies on how the powder interacts with the device during dispersion are scarce (8,9). Recently, we have demonstrated that small variations in the device design can produce significant performance variations in a dry powder inhaler such as the Aerolizer® (10).
There are currently a large number of dry powder inhalers that use a capsule to store and dispense the drug formulation. However there is little published data examining the fundamental mechanisms of deagglomeration involved when powder is dispersed from within a capsule. Upon inhalation, the flowfield generated within a dry powder inhaler such as the Aerolizer® acts to rotate the capsule at high speed. This ejects powder contained in the capsule through the capsule holes into the surrounding flowfield. It is believed that break-up could occur through a number of capsule induced deagglomeration mechanisms: 1) Powder agglomerates could impact with the internal walls of the capsule, prior to ejection, as it rotates; 2) Forcing powder agglomerates through the small holes in the capsule could cause large agglomerates to break-up, preventing slugs of powder from exiting the capsule; 3) High speed impactions with the surrounding walls of the device could occur as the particles are ejected from the capsule, and 4) The spinning capsule could act as a rotor to deagglomerate ejected particles through mechanical impaction with the external walls of the capsule. Furthermore, it is believed that the flowfield generated in the device with different capsule sizes could vary significantly, potentially affecting the powder dispersion.
The aim of this study is to examine the role the capsule has on the overall performance of the inhaler. Specifically, how the size and the presence of the spinning capsule affects the flowfield generated in the device and how the flowfield variations affect the powder dispersion. Furthermore, the performance difference between loading powder inside compared with outside the capsule is examined.
Materials and Methods
This work consists of three individual studies, each performed to provide a better understanding of how the spinning capsule affects the overall performance of an Aerolizer® (Plastiape S.p.A.). High-speed photography analysis was carried out to visualize the motion of different sized capsules as they rotated within the device. Once the motion of the capsule was known, computational fluid dynamics (CFD) analysis, using ANSYS CFX5.7 (11), was performed to determine the nature of the flowfield generated in the device at a flow rate of 60 l min−1. For each case, the performance of the inhaler was determined experimentally using a multistage liquid impinger (see “Dispersion Methodology”).
The first study was carried out to determine the effect of the capsule size on the overall performance of the inhaler. CFD analysis was performed to simulate the flowfield generated in the device with the presence of a standard size three, size four and size five spinning capsule. The dimensions of the different size capsules are summarized in Table I. The performance of the device was determined by loading powder directly into the capsules, followed by dispersion. The 4-pin piercing mechanism currently employed in the Aerolizer® is unable to pierce the size 5 capsule. Therefore, a drill press was used to produce a 1.0 mm hole in each capsule prior to powder loading. To aid future discussion of the results, this dispersion method will be referred to as PIC1.0 (powder loaded inside the capsule with a single 1.0-mm hole).
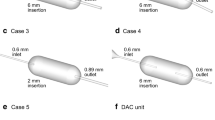
The second study was performed to determine the effect the presence of the capsule had on the overall inhaler performance. Additional CFD analysis was performed to determine the nature of the flowfield generated in the device without the presence of a spinning capsule. In order to determine the performance of the inhaler without a capsule, dispersion analysis was undertaken by loading powder directly into the device onto the surface adjacent to the spinning capsule, shown in Fig. 1. Dispersions were then carried out with an empty size three, size four and size five capsule present in the device and also without the presence of a capsule. To aid future discussion of the results, these dispersion methods will be referred to as POC (powder loaded outside the capsule with an empty capsule present) and PONC (powder loaded outside the capsule with no capsule present), respectively. This study could also determine the effect of the capsule acting as a rotor to cause deagglomeration by mechanical impaction.
The third study involved comparing the performance of the device in dispersion method POC with that of dispersion method PIC1.0 for the size three capsule. Loading powder outside the capsule eliminates the possibility of particle break-up caused by deagglomeration mechanisms (1) to (3) in Introduction (collectively referred to as initial capsule deagglomeration). Therefore comparing dispersion method POC with dispersion method PIC1.0 allows a determination of how particle deagglomeration and subsequent inhaler performance are affected by initial capsule deagglomeration. To determine whether the size of the capsule hole affects the device performance, additional dispersion analysis was performed with powder loaded directly into a size three capsule that had been pierced using the 4-pin piercing mechanism currently employed in the Aerolizer®. This loading method will be referred to as PIC40.6 (powder loaded into the capsule with four 0.6 mm holes). A summary of the nomenclature used for the dispersion methods is shown in Table II.
High-Speed Photography
The motion of the capsule as it spun within the device was visualized using a high-speed Phantom v4.0, CMOS video camera (Vision Solutions, Wayne, NJ, USA), capable of capturing 1000 frames per second. The visualization techniques were used to gain a better understanding of the general motion of the rotating capsule and also to determine the capsule angular velocity. The motion of a size three, size four and size five capsule was studied at a device flow rate of 60 l min−1.
In each case, the motion of the spinning capsule within the device was found to be largely regular. However, occasional fluctuations in the motion of the capsule were observed, leading to large variations in the axis of rotation (Fig. 2a), as well as variations to the position of the spinning capsule, with the centre point of rotation continually changing (Fig. 2b).
The angular velocity of the capsule was determined by averaging the time taken for each complete capsule revolution over 1000 frames of data. Two sets of data were used to determine the average angular velocity for each capsule size (Table I). Observations throughout the high-speed photography showed that the capsule frequently collides with the walls of the device as it rotates. This increases the time period between each complete revolution, reducing the average rotational velocity. A greater number of collisions was observed with the size five capsule, giving rise to the reduced angular velocity.
Computational Methodology
The flowfield generated in the device with and without the presence of the capsule was obtained by solving the Reynolds Averaged Navier Stokes equations together with the SST (Shear Stress Transport) turbulence model (12) and scalable wall functions using the commercial CFD code ANSYS CFX 5.7 (11), as described in Coates et al. (10). Due to the difficulty in modeling the irregular motion of the spinning capsule, a simplified model using a fixed axis of rotation, fixed centre point of rotation and a constant angular velocity was employed. The motion of the capsule was modeled using a cylindrical rotating domain having a computational mesh size of 0.4 mm, in which the different sized capsules were housed. The capsule rotation was simulated in a rotating reference frame with the flow in the main body of the device being solved in a stationary frame. The two flow domains were linked via a transient rotor stator model that maps quantities across the unmatched grid interface in a consistent manner (11).
The flowfield generated in the device with and without the presence of the capsule was found to exhibit a small degree of unsteadiness in all cases. Therefore a steady state simulation was run first in order to obtain the basic flowfield, and then a transient simulation was restarted from this initial solution, and run for a period of around 0.1 s, in order to resolve the unsteady motion. Typically a timestep of 0.0005 s was used and the equations were solved to a normalized residual of 1 × 10−5 at each timestep. It was observed that the transient motion resulted from a slight precession of the flow around the axis of the device. The results at the end of the simulation were taken as representing a typical snapshot of the flowfield.
Lagrangian particle tracking was performed as a post-processing operation, in which the fate of 1000 particles with a density of 1520 kg m−3 (13) and particle diameter of 3.2 μm were tracked through the fluid after release from the capsule region and subjected to drag and turbulent dispersion forces. Realistic requirements limit the number of particles that can be simulated to less than 10,000. Previous studies have shown that no significant difference in the frequency of impactions was observed with a ten-fold increase in the number of simulated particles (10), giving confidence in the particle impaction data obtained for the dispersion of 1000 particles. By separately releasing particles from the capsule and the base of the inhaler and by setting the different walls within the device to have a zero coefficient of restitution, it was possible to determine the frequency and location of wall impactions for the different dispersion methods. The scope of this current study does not allow a determination of the number of particle-capsule impactions as the capsule rotates.
As a simplified model has been employed to simulate the rotation of the capsule within the device, the computational results provided in this study are not intended to be treated purely quantitatively, but rather to illustrate the significant trends in the nature of the flowfield observed with and without the presence of different sized capsules.
Dispersion Methodology
The dispersion performance of the inhaler was determined using a 4-stage (plus filter) liquid impinger (Copley, Nottinghamshire, UK) setup as described in the BP (14). For dispersion method PIC1.0 and PIC40.6, spray-dried mannitol (particle size d50 = 3.2 μm, span 1.3) was filled into the different sized hard gelatin capsules and dispersed into the impinger. For capsule sizes three and four, three capsules were filled with approximately 20 mg of mannitol and for the size five capsule, four capsules were filled with approximately 15 mg, to prevent overfilling. For dispersion methods POC and PONC, approximately 20 mg of the same mannitol powder was loaded onto the appropriate region of the device (Fig. 1) using a spatula. Care was taken when placing the empty capsule into the device in dispersion method POC to prevent powder from falling into the capsule reservoir.
For each case, the impinger was run at 60 l min−1 for a total of 4 seconds using a timed valve. The runs were performed in triplicate to obtain mean values. Mannitol was assayed by high performance liquid chromatography (HPLC) (Waters, Milford, USA) using refractive index detection. Centrifuged samples (100 μl) were injected into a C18 radial-pak column with de-ionized water as the mobile phase running at a flow rate of 1 ml min−1 for 10 min. A calibration curve was constructed using standard solutions of mannitol which allowed the mass of powder deposited on each stage of the impinger and the fine particle fraction to be determined.
In this study, the fine particle fraction was defined as the mass fraction of particles smaller than 6.8 μm, as this was the cut off for stage 2 at 60 l min−1. Numerically, the fine particle fraction was expressed as the percentage of powder collected on stages 3, 4 and the filter, referenced against either the total mass of powder loaded into (FPFLoaded), or the total mass of powder emitted from (FPFEm), the device. The percentage recovery throughout the dispersion analysis was 100 ± 4%. Analysis of variance (ANOVA) tests were carried out with a probability of less than 0.05 considered statistically significant (Minitab 13). Throughout the dispersion analysis, the temperature and relative humidity of the laboratory were maintained at 22 ± 2°C and 25 ± 10%, respectively.
Results
Aerosol Characterization Results
Capsule Size Effects
The experimental powder dispersions showed that no statistically significant difference in both the values of the FPFLoaded and the FPFEm was observed as the size of the capsule was reduced. Reducing the size of the capsule led to a slight increase in the FPFLoaded, from 43.7% for the size three capsule to 46.6% and 47.3% for the size four and size five capsules, respectively (Fig. 3a). No trend in the values of the FPFEm was observed for the different capsule sizes. Reducing the size of the capsule also reduced the amount of powder retained in the capsule after dispersion (Fig. 3b).
Capsule Presence Effects
When powder was loaded directly into the device with an empty capsule present (POC), no significant difference in the performance of the inhaler was observed as the size of the capsule was reduced. Values of the FPFLoaded and the FPFEm were found to be 46.0 ± 1.8% and 58.3 ± 1.3%, respectively, for the three capsule sizes (Fig. 4a). Without the presence of a capsule (PONC) a slight but statistically significant difference in the values of both the FPFLoaded and the FPFEm was observed, with the FPFLoaded and the FPFEm increasing to 50.5% and 64.9%, respectively (Fig. 4a). No significant difference in the amount of capsule, device and throat retention was observed between dispersion methods POC and PONC (Fig. 4b).
(a) Effect of the presence of the spinning capsule on %FPF loaded and emitted. **For both the %FPF loaded and emitted, the differences between no capsule and all other cases were statistically significant (p < 0.05). (b) Effect of the presence of the spinning capsule on %mass deposited on individual locations.
Initial Capsule Deagglomeration Effects
The experimental dispersions performed to determine the effect of initial capsule deagglomeration showed no significant difference in the FPFLoaded between dispersion methods POC and PIC1.0. A significant difference in the FPFEm was observed, increasing from 58.5% for POC to 64.5% for PIC1.0 (Fig. 5a). For dispersion method PIC40.6, the FPFLoaded and FPFEm were significantly increased to 54.9% and 76.3%, respectively. The amount of powder retained in the throat of the impinger was found to reduce from 13.9% for dispersion method POC, to 10.4% and 5.7% for dispersion methods PIC1.0 and PIC40.6, respectively (Fig. 5b).
CFD Results
Capsule Size Effects
Figure 6 shows that although small differences in the flowfield generated in the region adjacent to the spinning capsule were observed, the inherent nature of the flowfield generated in the device was the same for all three capsule sizes. Reducing the capsule size slightly increased the overall levels of turbulence generated in the device, with values of the volume averaged turbulence kinetic energy increasing from 5.9 J kg−1 for the size three capsule to 6.4 J kg−1 and 6.6 J kg−1 for the size four and five capsules, respectively (Fig. 7). No significant difference in the integral scale strain rate profiles at a position of 2 mm upstream of the grid was observed as the size of the capsule was reduced (Fig. 8) and no difference in the integral length scale of the turbulence was found. The particle impaction data showed no significant difference in the number of particle-grid, particle-mouthpiece and particle-inhaler base impactions as the size of the capsule was reduced (Table III).
Capsule Presence Effects
Figure 6d shows that the flowfield generated in the device changes significantly when no capsule is present. A highly turbulent region is generated in the base of the inhaler with the volume averaged turbulence kinetic energy increasing to 11.1 J kg−1 (Fig. 7d). No significant difference in the integral length scale and the integral scale strain rate profiles 2 mm upstream from the grid were observed without a capsule present (Fig. 8d).
No difference in the number of particle-grid and particle-inhaler base impactions was observed with and without the capsule present (Table III). However, with no capsule present, a significant decrease in the number of particle-mouthpiece impactions was observed, indicating that the difference in the flowfield caused by the presence of the capsule affects the amount of particle-mouthpiece contact.
Initial Capsule Deagglomeration Effects
The computational model used throughout this study is unable to determine if any difference in the flowfield generated in the device containing a filled and an empty capsule occurs. However, as no difference in the flow development profiles in the inhaler (i.e., change in flow rate with time) was observed (Fig. 9), it can be reasonably assumed that any variations in the device flowfield will be minor and are unlikely to have a significant effect on the performance of the inhaler between POC and PIC. With a capsule present, no significant difference in the number of particle-grid, particle-mouthpiece and particle-inhaler base impactions was observed when the particles were dispersed from the capsule (PIC) compared with dispersion from the base of the inhaler (POC) (Table III).
Discussion
Capsule Size Effects
This study showed that reducing the size of the capsule had an insignificant effect on the overall performance of the inhaler. Reducing the size of the capsule slightly increased the overall levels of turbulence generated in the device. When estimating the potential of a turbulent flow to break-up powder agglomerates, the integral length scales of the turbulence also needs to be studied as these energetic eddies have a strong potential to break-up agglomerates (15). No significant difference in the integral scale strain rate at a position 2 mm above the grid (Fig. 8) or the integral length scale of the turbulence was observed as size of the capsule was reduced.
Since no significant trend in the number of particle impactions was observed as the size of the capsule was reduced (Table III), the similarity in the inhaler performance observed experimentally suggests that the small increase in the deagglomeration potential of the flowfield caused by the generation of slightly greater levels of turbulence was not sufficient to have a significant effect on the overall performance of the inhaler. The reduction in the amount of powder retained in the capsule after dispersion accounted for the slight increase in the FPFLoaded.
Capsule Presence Effects
The presence of the spinning capsule had a greater effect on the overall performance of the Aerolizer® than the size of the capsule. Higher levels of turbulence were generated without the capsule present, increasing the deagglomeration potential of the flowfield. Varying the size of the capsule varied the overall levels of turbulence generated in the device by less than 15%. In contrast, without the presence of the capsule, the overall levels of turbulence were increased by 65% which was shown as sufficient to have a significant effect on the overall performance of the inhaler. The increase in the performance of the inhaler without the presence of the capsule also suggests that the small difference in the number of particle-mouthpiece impactions (Table III) played an insignificant role on the overall performance of the inhaler.
The results further showed that the break-up of powder caused by particle contact with the high speed rotating capsule is a weak mechanism for deagglomeration. The present study is not capable of showing the frequency of particle-capsule impactions as the capsule rotates, therefore no quantitative analysis can be made. However, the significant increase in the performance of the inhaler without the presence of the capsule indicates that particle-capsule impactions have an insignificant effect on the overall performance of the inhaler relative to the increased turbulence levels.
Initial Capsule Deagglomeration Effects
This study demonstrated that the initial capsule deagglomeration can strongly affect the overall performance of the inhaler, with the effects being dependent on the size of the capsule hole. The latter was also found by Chew and Chan (16), but the current study provides further insights into the mechanism.
The similarity in the flow development profiles (Fig. 9) and the number of particle-device impactions (Table III) suggests that the increase in the FPFEm observed experimentally in dispersion methods PIC1.0 and PIC40.6 compared with dispersion method POC was not caused by particle interaction with the flowfield or by particle-device impactions, but rather by the initial capsule deagglomeration produced by dispersing powder from within the capsule. The reduced amount of capsule retention for dispersion method POC (Fig. 5b) prevents any difference in the FPFLoaded being observed between dispersion methods POC and PIC1.0. The reducing trend in the throat retention for dispersion methods PIC1.0 and PIC40.6 compared to POC (Fig. 5b) also reflects the increasing performance of the inhaler which resulted in the release of a lesser amount of large particles to impact on the impinger throat.
The scope of this study does not allow us to determine exactly which mechanism of the initial capsule deagglomeration has the strongest affect on the overall performance of the inhaler. However since there was no difference in the number of particle-inhaler base impactions between dispersion methods PIC1.0 and PIC40.6 (Table III) and assuming that particle impaction with the internal walls of the capsule is independent of the capsule hole size, the significant increase in the inhaler performance using the smaller capsule hole size in dispersion method PIC40.6 compared with PIC1.0 indicates that the break-up mechanism produced by forcing powder agglomerates through the capsule holes plays an important role on the powder dispersion.
The total area of the capsule hole(s) for dispersion methods PIC40.6 and PIC1.0 were calculated to be 0.36π and 0.25π mm2, respectively. Additional dispersion analysis performed using a size 3 capsule with a 1.5 mm hole (area 0.56π mm2) produced an FPFLoaded of 40.0% and an FPFEm of 59.5% (compared with 43.7% and 64.5% for PIC1.0 and 54.9% and 76.3% for PIC40.6), indicating that the initial capsule deagglomeration effects are not correlated with the total area of the capsule hole(s). Examining the time taken for powder to empty out of the capsule, measured using a laser photometer as described in Chew et al. (16) and Clark and Bailey (17), showed that increasing the size of the capsule hole significantly reduced the capsule emptying time. For PIC40.6, the capsule emptying time was 2.9 ± 0.1 s, compared with 1.3 ± 0.2 s and 0.8 ± 0.05 s for PIC1.0 and PIC1.5, respectively (n = 3). The capsule emptying time is an important factor to consider if it is less than or comparable with the time taken to reach the full flow rate in the device, as this could cause a large fraction of powder to exit the device before the maximum levels of turbulence are generated. However, as the capsule emptying times were significantly greater than the flow development time (approximately 0.06 s) (Fig. 9), the difference in the emptying times is unlikely to affect the overall performance of the inhaler.
Conclusions
This study showed that the size of the capsule used to disperse powder in an Aerolizer® has an insignificant effect on the overall performance of the inhaler. Reducing the size of the capsule led to a small increase in the overall levels of turbulence generated in the device, but this was not sufficient to affect the powder dispersion. A smaller amount of capsule retention was observed as the size of the capsule was reduced.
When powder was loaded directly into the device onto the surface adjacent to the spinning capsule, the presence of the capsule was found to actually reduce the overall performance of the inhaler by reducing the overall turbulence levels. However, when powder was loaded inside the capsule, additional strong initial capsule deagglomeration was provided by the capsule that could far outweigh the performance reduction due to reduced turbulence levels. The initial capsule deagglomeration depends on the effects of shearing agglomerates through the capsule holes and preventing large powder agglomerates from exiting the capsule. These effects were found to be strongly determined by the size of the capsule hole, with the four 0.6 mm holes significantly increasing the performance of the inhaler.
This study also showed that capsule-particle impaction is a weak mechanism for deagglomeration. The potential role of the capsule acting as a rotor to cause deagglomeration by mechanical impaction is insignificant on the overall performance of the inhaler.
References
A. L. Adjei P. K. Gupta (1997) ArticleTitleDry-powder inhalation aerosols Lung Biol. Health Dis. 107 625–655 Occurrence Handle1:CAS:528:DyaK1cXmsFajsg%3D%3D
T. Hino Y. Kawashima (1998) ArticleTitleParticle design of dry powder inhalers Pharm. Technol. Japan 14 1555–1563 Occurrence Handle1:CAS:528:DyaK1cXms12rur0%3D
C. A Dunbar A. J. Hickey P. Holzer (1998) ArticleTitleDispersion and characterisation of pharmaceutical dry powder aerosols Kona 16 7–45 Occurrence Handle1:CAS:528:DC%2BD3MXjtlKjtLg%3D
N. Y. K. Chew H.-K. Chan. (2002) Dry powder aerosols: emerging technologies J. Swarbrick J. C. Boylan (Eds) In Online Version of the Encyclopedia of Pharmaceutical Technology EditionNumber2nd ed. Marcel Dekker, Inc. New York 1–7
N. Y. K. Chew H.-K. Chan (2002) ArticleTitleThe role of particle properties in pharmaceutical powder inhalation formulation J. Aerosol Med. 15 325–330 Occurrence Handle1:CAS:528:DC%2BD38XnsVSltLo%3D Occurrence Handle12396421
H.-K. Chan (2003) ArticleTitleInhalation drug delivery devices and emerging technologies Expert Opin. Ther. Pat. 13 1333–1343 Occurrence Handle1:CAS:528:DC%2BD3sXmslSiurY%3D
A. R. Clark (1995) ArticleTitleMedical aerosol inhalers: past, present, and future Aerosol Sci. Tech. 22 374–391 Occurrence Handle1:CAS:528:DyaK2MXlsVyitrg%3D
Z. Wang C. F. Lange W. H. Finlay (2004) ArticleTitleUse of an impinging jet for dispersion of dry powder inhalation aerosols Int. J. Pharm. 275 123–131 Occurrence Handle1:CAS:528:DC%2BD2cXivFGktLg%3D Occurrence Handle15081143
A. Voss W. H. Finlay (2002) ArticleTitleDeagglomeration of dry powder pharmaceutical aerosols Int. J. Pharm. 248 39–50 Occurrence Handle1:CAS:528:DC%2BD38XosFags7g%3D Occurrence Handle12429458
M. S. Coates D. F. Fletcher H.-K. Chan J. A. Raper (2004) ArticleTitleEffect of design on the performance of a dry powder inhaler using computational fluid dynamics. Part 1: grid structure and mouthpiece length J. Pharm. Sci. 93 2863–2876 Occurrence Handle1:CAS:528:DC%2BD2cXpvV2itrs%3D Occurrence Handle15389665
ANSYS CFX, (2003) http://www.ansys.com/cfx (accessed 08/01/04).
F. R. Menter (1994) ArticleTitleTwo-equation eddy-viscosity models for engineering applications AIAA J 32 269–289
P. G. Stecher M. Windholz D. S. Leahy (Eds) (1968) The Merck Index: An Encyclopedia of Chemicals and Drugs EditionNumber8th ed. Merck & Co., Inc. Rahway
British Pharmacopoeia, Appendix XII, Aerodynamic Assessment of Fine Particles-Fine Particle Dose and Particle Size Distribution, Apparatus C (2001).
W.H. Finlay (2001) The Mechanics of Inhaled Pharmaceutical Aerosols Academic Press Inc., Ltd. London
N. Y. K. Chew H.-K. Chan D. F. Bagster J. Mukhraiya (2002) ArticleTitleCharacterization of pharmaceutical powder inhalers: estimation of energy input for powder dispersion and effect of capsule device configuration J. Aerosol Sci. 33 999–1008 Occurrence Handle1:CAS:528:DC%2BD38XktFOgurc%3D
A. R. Clark and R. Bailey. Inspiratory flow profiles in disease and their effects on the delivery characteristics of dry powder inhalers. In R. D. Dalby, P. N. Byron, and S. J. Farr (eds.), Respiratory Drug Delivery V, Interpharm Press, Buffalo Grove, IL, 1996, pp. 221–230.
Acknowledgments
This work is funded by a grant from the Australian Research Council. Matthew S. Coates is a recipient of an International Postgraduate Research Scholarship. The authors would like to thank Assoc. Prof. L. Bilston and M. Yuen of the Prince of Wales Medical Research Institute for their invaluable assistance throughout the high-speed photography analysis and Plastiape S.p.A. for the supply of the inhalers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coates, M.S., Fletcher, D.F., Chan, HK. et al. The Role of Capsule on the Performance of a Dry Powder Inhaler Using Computational and Experimental Analyses. Pharm Res 22, 923–932 (2005). https://doi.org/10.1007/s11095-005-4587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-4587-y