A quantitative determination method for total flavonoids in blood-red hawthorn leaves recalculated as vitexin 2″-O-rhamnoside using difference spectrophotometry at 392 nm was developed. The error of a single determination of total flavonoids in blood-red hawthorn leaves was ± 1.57% with confidence probability 95%. The contents of total flavonoids in blood-red hawthorn leaves varied from 2.65 ± 0.05 to 3.53 ± 0.06%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Blood-red hawthorn (Crataegus sanguinea Pall., Rosaceae) is broadly distributed in the Russian Federation [1, 2]. Only hawthorn flowers and fruit are currently used in medical practice in Russia as cardiotonic drugs [3]. Hawthorn leaves are also a promising raw material source [4, 5]. Leaves of blood-red hawthorn had a complicated chemical composition comprising flavonoids (hyperoside, vitexin, vitexin rhamnoside, etc.), ascorbic acid, tanning agents, etc. [6]. It is noteworthy that hawthorn leaves together with flowers are used in foreign medicine [7], which is of interest from the viewpoint of comprehensive utilization of this plant.
Hawthorn leaves with flowers are described in the US Pharmacopeia (USP), European Pharmacopoeia (EP), and Pharmacopoeia of the Republic of Belarus (PRB) [7,8,9]. Hawthorn leaves with flowers are determined qualitatively in the USP using TLC. The standard is a solution containing rutin, chlorogenic acid, hyperoside, and vitexin [7]. Furthermore, the USP provides for qualitative analysis of hawthorn leaves with flowers using HPLC [7]. Quantitative determination of hawthorn leaves with flowers according to the USP includes HPLC determination of C-glycosylated flavones recalculated as vitexin, the content of which should be at least 0.6%, and O-glycosylated flavonoids recalculated as hyperoside, the content of which should be at least 0.45%.
Quantitative determination of target compounds in leaves with flowers according to the EP and PRB includes spectrophotometric determination of total flavonoids recalculated as hyperoside, which should be at least 1.5% in raw material at 405 (EP) and 410 nm (PRB) [7, 9].
Leaves are considered separately as a type of medicinal plant raw material in the PRB, where the identity is determined using reactions with ammonium chloride (greenish-yellow color after heating) and Fe(III) ammonium sulfate solution in BuOH in the presence of HCl (red color after heating) [8]. Active compounds in hawthorn leaves are quantitatively determined in the PRB using spectrophotometric determination of total flavonoids recalculated as rutin (at least 0.25%) at 409 nm and spectrophotometric determination of total procyanidins recalculated as cyanidin chloride (at least 5.0%) at 550 nm [8]. Recalling that the characteristic flavonoids of blood-red hawthorn leaves are hyperoside and vitexin, which are a flavonol and flavone, the experience with determining the total flavonoid content recalculated as rutin in this plant raw material has been challenging. Furthermore, the flavonoid group, i.e., flavonol or flavone, that should be used to standardize this plant raw material must be justified. This question can be solved only by studying the constituent composition of flavonoids from blood-red hawthorn leaves. The possibility of using spectrophotometry to standardize this plant raw material remains critical despite the success achieved in standardizing hawthorn leaves with flowers and fruit [7, 9, 10].
The goal of the present investigation was to develop a quantitative determination method for total flavonoids in blood-red hawthorn leaves.
Experimental Part
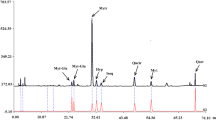
Blood-red hawthorn leaves were collected in Samara Oblast in May 2016 and used in the work. Spectra were recorded in the range 190 – 700 nm using 10-mm cuvettes on a Specord 40 spectrophotometer (Analytik Jena). The constituent composition of flavonoids from blood-red hawthorn leaves was evaluated preliminarily using TLC on Sorbfil PTSKh-AF-A-UF plates, CHCl3—EtOH–H2O (26:16:3), and authentic samples of rutin, hyperoside, and vitexin. Hyperoside and vitexin were detected in the aqueous EtOH extract although rutin was not. The dominant compound detected on the chromatogram with Rf 0.60 was especially interesting.
Preparative column chromatography was used to isolate the dominant flavonoid from blood-red hawthorn leaves. For this, blood-red hawthorn leaves were extracted with EtOH (70%) in a 1:10 ratio. The extract was evaporated in vacuo, placed on silica gel L 40/100, and dried. Compounds were separated by column chromatography over silica gel L 40/100 with elution by CHCl3, CHCl3–EtOH mixtures of various proportions, and EtOH. Eluates were divided into fractions of approximately identical volume (200 mL) and evaporated in vacuo.
Fractions eluted by CHCl3–EtOH (85:15) afforded the dominant compound with Rf 0.60 that was identified using UV, PMR, and 13C NMR spectroscopy, mass spectrometry, and various chemical transformations (acid hydrolysis).
Vitexin 2″-O-rhamnoside (Fig. 1) was a light-yellow crystalline powder of composition C27H30O14, mp 213 – 215° C; UV spectrum: λmaxEtOH — 272 and 334 nm. PMR spectrum (300 MHz, DMSO-d6, δ, ppm, J/Hz): 1.70 (3H, d, 2 Hz, CH3 rhamnose), 3.2 – 5.25 (10H sugars), 5.22 (1H, d, 7 Hz, H-111 glucopyranose), 5.33 (1H, br.s, H-1111 rhamnopyranose), 6.25 (1H, s, H-6), 6.83 (1H, s, H-3), 6.92 (2H, d, 9 Hz, H-31 and H-51), 8.21 (2H, d, 9 Hz, H-21 and H-61), 10.37 (1H, br.s, C-41 OH1), 11.03 (1H, br.s, C-7 OH), 13.21 (1H, c, 5-OH).
13C NMR spectrum(50 MHz, DMSO-d6, δC, ppm): C-4 (182.05), C-2 (169.22), C-7 (164.05), C-41 (162.08, C-5 (161.23), C-9 (156.44), C-21 (129.04), C-61 (129.04), C-11 (121.58), C-31 (116.21), C-51 (115.80), C-1111 rhamnose (103.98), C-111 glucose (102.47), C-511 (97.81), C-3111 (82.02), C-311 (82.02), C-211 (75.68), C-2111 (72.52), C-411 (70.93), C-5111 (70.46), C-611 (60.99), CH3 (20.44).
Mass spectrum (ESI-MS, 180 °C, m/z): M+ 579 (578 + H), M+ 601 (578 + Na), M+ 617 (578 + K).
Results and Discussion
Vitexin 2″-O-rhamnoside was mainly responsible for the absorption spectrum of the aqueous EtOH extract of blood-red hawthorn leaves (Figs. 2–4), especially the difference spectrum (Figs. 5 and 6). This meant it was a diagnostically significant compound for this type of raw material. Recalling that absorption maxima of a solution of vitexin 2″-O-rhamnoside and the aqueous EtOH extract of blood-red hawthorn leaves were located near 392 nm (Figs. 5 and 6), the content of total flavonoids recalculated as vitexin 2″-O-rhamnoside should be determined at 392 nm.
Studies of the dependence of various extraction parameters on the yield of active compounds from the raw material showed that the optimal extraction parameters were raw material particle size 2 mm with a single extraction by EtOH (70%) on a boiling-water bath for 60 min with a raw-material–extractant ratio of 1:50 (Table 1).
Quantitative determination method for total flavonoids in blood-red hawthorn leaves. An analytical sample of blood-red hawthorn raw material is milled to particle size 2 mm. A portion (1 g, accurate weight) of the milled raw material is placed into a 100-mL flask with a ground-glass joint and treated with EtOH (70%, 50 mL). The flask is closed with a stopper and weighed on a tared balance to ± 0.01 g. The flask is connected to a reflux condenser, heated on a boiling-water bath (moderate boiling) for 60 min, closed with the same stopper, weighed again, and supplemented with extractant to the initial mass. The extract is filtered through a loose wad of cotton or a red-band filter and cooled for 30 min (leaf extract).
The test solution for total flavonoid analysis was prepared by placing the obtained extract (1 mL) into a 25-mL volumetric flask, adding AlCl3 solution (3%) in EtOH (1 mL), and adjusting to the mark with EtOH (95%) (test solution).
The reference solution was prepared by placing the obtained extract (1 mL) into a 25-mL volumetric flask and adjusting to the mark using EtOH (95%) (reference solution).
Total flavonoid contents were calculated by preparing a solution of standard vitexin 2″-O-rhamnoside, treating it with AlCl3 (3%) in EtOH, and measuring the optical density of the colored complex at the analytical wavelength of 392 nm. The determined optical density was used in the calculations.
The solution of vitexin 2″-O-rhamnoside standard was prepared by placing a portion (0.02 g, accurate weight) into a 50-mL volumetric flask, dissolving in EtOH (96%), and adjusting to the mark with EtOH (96%) (solution A, vitexin 2″-O-rhamnoside). Then, solution A (1 mL) was placed into a 25-mL volumetric flask, treated with AlCl3 (3%) in EtOH (1 mL), and adjusted to the mark with EtOH (95%). The reference solution was prepared by placing the obtained solution (1 mL) into a 25-mL volumetric flask and adjusting to the mark with EtOH (95%).
Optical density of all solutions was measured at 392 nm 40 min after their preparation.
The contents of total flavonoids (X, %) recalculated as vitexin 2″-O-rhamnoside and absolute dry raw material were calculated using the formula:
where D is the optical density of the test solution; D0, optical density of the vitexin 2″-O-rhamnoside standard solution; m, mass of raw material (g); m0, mass of vitexin 2″-O-rhamnoside standard (g); and W, mass loss on drying (%).
The theoretical specific absorption coefficient of 232 should be used if vitexin 2″-O-rhamnoside standard is not available:
where D is the optical density of the text solution; m, mass of raw material (g); m0, mass of vitexin 2″-O-rhamnoside standard (g); 232, specific absorption coefficient \( \left({E}_{1\mathrm{cm}}^{1\%}\right) \) of vitexin 2″-O-rhamnoside standard at 392 nm; and W, mass loss on drying (%).
Statistical processing of the test results showed that the error of a single determination of total flavonoids in blood-red hawthorn leaves was ± 1.57% at confidence probability 95%. The contents of total flavonoids varied from 2.65 ± 0.05 to 3.53 ± 0.06%. Therefore, a value of at least 2.5% recalculated for the dominant diagnostically significant flavonoid vitexin 2″-O-rhamnoside and not hyperoside or rutin as specified earlier should be recommended as the lower limit for total flavonoid content in blood-red hawthorn leaves [8, 11, 12].
Table 2 presents metrological characteristics of the quantitative determination method for total flavonoids in blood-red hawthorn leaves.
The developed method was validated for specificity, linearity, accuracy, and precision. Specificity was determined from the agreement of absorption maxima for the flavonoid complex from blood-red hawthorn leaves and for vitexin 2″-O-rhamnoside with AlCl3. Linearity was determined using a series of solutions of vitexin 2″-O-rhamnoside with concentrations in the range 0.00250 – 0.03904 mg/mL. The correlation coefficient was 0.99997.
Accuracy was determined by adding aliquots of a solution of vitexin 2″-O-rhamnoside of known concentration (25, 50, and 75%) to the test solution. The average percent recovery was 98.25%.
Thus, the research results indicated that blood-red hawthorn leaves should be standardized by determining total flavonoids using spectrophotometry at analytical wavelength 392 nm and recalculation as vitexin 2″-O-rhamnoside.
References
V. A. Kurkin, Pharmacognosy [in Russian], Samara (2016), pp. 812 – 816.
V. A. Kurkin, Principles of Phytotherapy [in Russian], Samara (2009), pp. 418 – 419.
State Pharmacopoeia of the USSR, XIth Ed., No. 2, Moscow (1990).
S. V. Khasanova, S. V. Trofimova, and N. V. Kudashkina, Modern Medicine and Pharmaceutics: Analysis and Development Prospects: Proceedings of the 8 th International Scientific-Practical Conference [in Russian], (2013), pp. 36 – 36.
S. V. Trofimova, S. V. Khasanova, and N. V. Kudashkina, Med. Vestn. Bashkortostana, 5(2), 299 – 302 (2011).
Plant Resources of the USSR: Flowering Plants, Their Chemical Composition, Use: Families Hydrangeaceae–Haloragaceae [in Russian], Leningrad (1987), pp. 34 – 42. 7. European Pharmacopoeia, 8th Ed., Strasbourg (2013).
32 United States Pharmacopeia; http: // www.uspbpep.com / usp32 / pub / data / v32270 / usp32nf27s0m36580.html
State Pharmacopoeia of the Republic of Belarus, 1st Ed., Vol. 2, Minsk (2007).
V. A. Sagaradze, E. V. Babaeva, and E. I. Kalenikova, Khim.-farm. Zh., 51(4), 30 – 33 (2017); Pharm. Chem. J., 51(4), 277 – 280 (2017).
A. S. Avrach, E. V. Sergunova, and I. A. Samylina, Farmatsiya, 3, 14 – 16 (2013).
S. R. Khasanova, Doctoral Dissertation in Pharmaceutical Sciences, Ufa (2016).
V. A. Kurkin, T. V. Morozova, and O. E. Pravdivtseva, Khim. Rastit. Syr’ya, 3, 169 – 173 (2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 10, pp. 34 – 38, October, 2018.
Rights and permissions
About this article
Cite this article
Kurkin, V.A., Morozova, T.V., Pravdivtseva, O.E. et al. Quantitative Determination of Total Flavonoids in Blood-Red Hawthorn Leaves. Pharm Chem J 52, 850–854 (2019). https://doi.org/10.1007/s11094-019-1913-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-1913-y