Series of semi-synthetic polyene macrolide antibiotics (PMAs) that were prepared by chemical modification in original research by the authors are reviewed. Chemical modification, in particular phosphorylation, was shown to produce highly efficacious PMAs with low toxicities and extended spectra of biological activity. The prospects of using liposomal and nano-derivatives of these antifungal antibiotics are discussed. Crucial issues related to the resistance of pathogenic fungi and the expanding distribution of invasive mycoses are identified. Semi-synthetic PMAs are shown to be highly effective at preventing and treating invasive mycoses and opportunistic fungal infections occurring in AIDS patients. Special attention is paid to structure—activity relationships for the semi-synthetic PMAs. Possible mechanisms of action of these compounds on pathogenic fungi are discussed. An automated intellectual information system was developed for selecting the optimal conditions for development, synthesis, and application in medical practice of new PMAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Fungal infections continue to rise because of environmental pollution, increased radiation background, irrational use of broad-spectrum antibiotics, extensive use of cytostatics and immunosuppressants, and several other factors [1,2,3]. Invasive mycoses are becoming more problematical for medical practice because of the rising population of immunocompromised patient populations [4,5,6]. The number of available and approved systemic antifungal antibiotics is known to be inadequate today [7,8,9]. However, progress in the design of new antifungal drugs is not keeping pace with the increase of mycological diseases, in particular, invasive fungal infections that are an existential and growing problem for modern medicine [10,11,12].
Polyene macrolide antibiotics (PMAs) such as amphotericin B, levorin, nystatin, pimaricin, candicidin, and others are widely used in medical practice to treat both surface and deep mycoses [4,5,6]. PMAs include a numerous group of natural compounds with over 200 drugs that are active against yeast, yeast-like, and saprophytic and pathogenic species of mycelial fungi [13,14,15,16,17,18]. Also, PMAs used in medical practice do not fully meet the needs of physicians and clinicians because of their limited efficacy due to their low water solubility [4,5,6], high toxicity (mainly nephrotoxicity) [7,8,9], and emergence of resistant species of pathogenic fungi [19, 20]. Therefore, the search for new PMAs with improved medical and biological properties is critical.
Preparation of new semi-synthetic PMAs by chemical modification
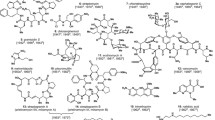
PMAs are known to be polyfunctional compounds with large lactone rings (from 26 to 33 atoms) and conjugated double bonds (from 4 to 7) [10,11,12,13,14]. PMAs are subdivided according to number of double bonds as tetraenes, pentaenes, hexaenes, and heptaenes. PMA molecules are usually separated into two parts, i.e., the rigid unsaturated part of the macrolactone ring with hydrophobic properties and the flexible polyol part that is responsible for the hydrophilicity. Other important structural components of PMAs are the carboxylic acid and amine in the carbohydrate moiety (Fig. 1).
Most studied PMAs contain the amino sugar mycosamine (3-amino-3,6-dideoxy-D-mannose). The overwhelming majority of PMAs are prepared by modifying these antifungal drugs at the carboxylic acid or amine.
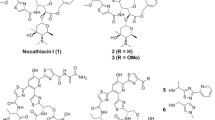
Currently, various semi-synthetic PMA derivatives prepared by chemical modification have been reported [11, 12, 21,22,23,24,25]. However, information on the preparation of organophosphorus derivatives of these antifungal drugs is missing. Organophosphorus compounds are widely employed in various industrial sectors. Their industrial output is constantly expanding [26,27,28,29,30]. An especially valuable property of organophosphorus compounds is their biological activity that enables them to be used in medical practice [31,32,33,34,35,36,37]. In this respect, the use of synthetic methods developed for organophosphorus chemistry for chemical modification of biologically active compounds and especially antibiotics is a promising direction for discovering highly efficacious drugs. Hydrophosphorylation using hypophosphorous acid and aromatic aldehydes was proposed by us as a method for chemical modification of PMAs. This reaction can be considered a variation of the Kabachnik—Fields reaction [26, 38, 39]. The first reaction step consists of addition of the primary amine of the PMA carbohydrate moiety to the aromatic aldehyde carbonyl to form an azomethine intermediate. The second step involves the reaction of hypophosphorous acid with the C=N bond of the azomethine intermediate to give hydrophosphoryl PMAs. Hydrophosphoryl derivatives of levorin [40], nystatin [41], amphotericin B [42], mycoheptin [43], pimaricin [44], and lucensomycin [45] were synthesized using the proposed method. Use of various dialkylphosphites and 4-bromobenzaldehyde for chemical modification of pimaricin also under Kabachnik—Fields reaction conditions was shown by us to produce 3′-N-α-dialkoxy(diphenoxy)phosphonate derivatives of this tetraene macrolide antibiotic [46]. The possibility of using an Atherton – Todd reaction for chemical modification of amphotericin B and lucensomycin was studied by us [47,48,49,50,51]. Thus, dialkyl(aryl)amidophosphate derivatives were formed by the reaction of these PMAs with various dialkyl(aryl)phosphites in the presence of an organic base. Pimaricin was chemically modified by diethyl chloroacetylenephosphonate with high selectivity to form its phosphorylated aldoketenimine derivative, i.e., the organophosphorus reagent reacted with the mycosamine primary amine [52, 53].
Fluorinated amphotericin B [54] and nystatin [55] derivatives were prepared via reactions with anhydrides of perfluorocarboxylic acids. Fluorinated levorin esters resulted from esterification of this heptaene macrolide antibiotic by organic fluoroalcohols [56]. Nystatin reacted with trialkylchlorosilanes to synthesize the N-trialkylsilyl derivatives [57, 58]. The N-benzyl derivatives of amphotericin B [59], pimaricin [60, 61], and lucensomycin [62] were prepared by reductive amination. Nucleophilic aromatic substitution was used to synthesize N-aryl-substituted pimaricin derivatives [63]. Biological tests showed that semi-synthetic PMAs exhibited pronounced antifungal activity against a large group of pathogenic fungi, primarily yeast-like fungi of the genus Candida. Also, they were 3 – 5 times less toxic than the starting antibiotics. Furthermore, chemical modification of PMAs can introduce various functional groups, e.g., hydrophosphoryl, which increased considerably the water solubility of levorin, nystatin, amphotericin B, mycoheptin, pimaricin, and lucensomycin derivatives [40,41,42,43,44,45, 64, 65]. This improved the biopharmaceutical properties of the antifungal preparations.
Chemical modification of biologically active compounds in several instances altered the spectrum of biological activity of the derivatives and reduced their toxicity [21, 22, 31, 32]. Previously, various researchers found that PMAs had nonspecific antiviral [66,67,68,69,70] and antitumor activity [68,69,70]. The mechanism of action of these antifungal antibiotics involved reorientation of the lipid (sterol) component of the virion shell surface or virus-specific receptors of cellular cytoplasmic membrane leading to inactivation of the virus or prevention of its penetration into a sensitive cell [71,72,73,74,75,76]. Chemical modification of PMAs by us changed their spectra of biological activity. Thus, additional virology testing found that several semi-synthetic derivatives of levorin [40], nystatin [41], amphotericin B [42], mycoheptin [43], and lucensomycin [45] that were prepared by us exhibited high antiviral activity against DNA-containing Vaccinia virus and RNA-containing oncogenic Rous sarcoma and type A and B influenza viruses. The results obtained for hydrophosphoryl derivatives of these PMAs in the RNA-containing Rous sarcoma retrovirus model were especially interesting because this model was proposed as a retrovirus model suitable for screening and studying anti-AIDS drugs [66, 67].
Search for liposomal and nanoscale PMAs
Biopharmaceutical approaches to reducing toxicity and improving PMA (mainly amphotericin B and nystatin) pharmacokinetics have recently become well known. Therefore, various amphotericin B derivatives were prepared as liposomal preparations (AmBisome®) [77,78,79,80,81,82,83,84,85,86,87], lipid complexes (Abelcet®) [88,89,90,91,92], colloidal dispersions (Amphocil) [93,94,95], and liposomal nystatin preparation (Nyotran®) [96,97,98,99,100,101,102,103,104]. Liposomal dosage forms of amphotericin B (AmBisome) and nystatin (Nyotran) are complete spherical vesicles formed by aqueous dispersions of certain polar lipids, e.g., phospholipids and cholesterol. Homogenization of phospholipids in aqueous solution forms single or multiple concentric bilayer membranes [105,106,107]. The lipophilic groups in amphotericin B enable it to be incorporated into liposome lipid bilayers. The preparation distributes as intact liposomes in tissues with fungal infections. The active ingredient is released only after touching cells of pathogenic fungi and not those of normal tissues. Other advantages of PMA liposomal preparations are lower toxicity, prolonged pharmacokinetics, and better tolerability [108, 109]. The lipid complex of amphotericin B (Abelcet) is a combination of the antibiotic and two lipids, i.e., dimyristoylphosphatidylcholine and dimyristoylphosphatidylglycerol with a 1:1 ratio of drug to lipids [88,89,90,91,92]. Clinical trials of this preparation are currently modest although it is used for candidiasis, aspergillosis, cryptococcosis, and other serious fungal diseases [7,8,9]. Amphocil preparation is a colloidal suspension of amphotericin B consisting of equimolar amounts of antibiotic and cholesterol sulfate [93,94,95]. Use of Amphocil preparation is considered efficacious for treating serious deep mycoses, in particular lung aspergillosis. Abelcet, Amphocil, and AmBisome preparations reduced substantially the nephrotoxicity of amphotericin B.
The safety, biodegradability, and accessibility of liposomes are attractive to researchers [110,111,112]. Furthermore, the high variability of liposomes is a big advantage. For example, the characteristics of liposomal carriers of biologically active compounds can be changed over wide limits by changing the methods for forming lipid vesicles and including various molecules in them. The physical and chemical instability typical of phospholipid membranes is one of the problems with using liposomes as carriers because it destabilizes the lipid bilayer and destroys the liposomes [110, 112, 113]. Polymers such as polyethylene glycol and its synthetic derivatives that protect liposomes from premature destruction are used to produce more stable liposomes and prolong their circulation in the blood pool [110, 113, 114].
Nanotechnology has recently played a significant role in the search for innovative PMAs because medical nanotechnological studies are focused on producing a new generation of preparations with more effective drug delivery methods and improved stability [115,116,117,118,119,120].
Nanoscale structures such as fullerenes and carbon nanotubes were proposed in several studies to be used as containers for medicinal preparations [121, 122]. Fullerenes have unique properties because of their high reactivity resulting from the large number of free carbon valences. Encapsulation in lipid vesicles is one method for administering them [123]. However, pure fullerenes are little suited for biomedical applications because of their insolubility in aqueous solutions. Therefore, functionalization of fullerenes was demonstrated to increase the bioavailability of these compounds and; therefore, make them more efficacious for biosystem research [124]. Carbon nanotubes have increased affinity for lipid structures. They can form stable supramolecular complexes (ensembles) with peptides and nucleic acids [125, 126] and encapsulate these molecules [127, 128]. Nanocomposite polyelectrolyte capsules were used as effective drug delivery vehicles. They were prepared by poly-ion assembly, which consisted of sequential adsorption of oppositely charged polyelectrolytes on the surface of colloidal particles followed by dissolution and removal of the starting template [105, 130]. Capsules of widely varying sizes (from 50 nm to 50 μm) can be prepared using this method. Practically any synthetic and natural polyelectrolytes [130, 131], lipid bilayers, inorganic nanoparticles [e.g., Ag, Au, or Fe(II)-oxide nanoparticles], and multivalent metal ions can be chosen as the shell material with controlled thickness and multifunctional walls [132, 133]. The researchers emphasized that the studied capsules had controlled permeability for any low- and high-molecular-mass compounds [134]. Inorganic nanoparticles of magnetite Fe3O4 with highly pronounced magnetic properties were added to the shell composition of such microcapsules during their synthesis so that their movement could be controlled by applying an external magnetic field [129]. However, reports of the application of nanostructures as containers for drug delivery to target cells are still scarce (except for liposomes). Biological safety issues are still insufficiently studied for nanomaterials [135,136,137]. Toxicity data for fullerenes [137,138,139] and carbon nanotubes [131,132,133, 140] from pharmacological tests in animal models are now available.
The principal research results and recommendations for the production of various nanoparticles, primarily medicines, were reported [121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140] and used further to prepare nanoscale derivatives of drugs, including PMAs. Thus, reviews have emphasized that targeted screening of PMA nanoparticles is a promising direction for producing safe derivatives with pronounced antifungal activity [141,142,143,144]. A series of nanoscale derivatives of the heptaene macrolide antibiotic amphotericin B were recently prepared by modern nanotechnology methods [145,146,147,148,149,150,151,152,153,154,155,156]. Nanoscale amphotericin B derivatives possessed pronounced antifungal activity and were less toxic and more stable than the starting antibiotics [157,158,159,160,161,162,163,164]. Nanoscale nystatin and natamycin (pimaricin) derivatives were investigated [165,166,167,168,169,170,171,172,173] and also exhibited better bioavailability and less toxicity than the starting antibiotics. They were considered promising antifungal drugs for treating candidiasis of various etiologies. Nanoscale derivatives of the tetraene macrolide antibiotics pimaricin (natamycin), nystatin A1, lucensomycin, and tetramycin B that were coated with the surfactant Tween-80 were prepared by us [174]. Production of the nanoscale tetraene macrolide antibiotic derivatives included two steps of 1) synthesis of the copolymer of D, L-lactide (LD) and polyethylene glycol (PEG) and 2) use of the LD—PEG copolymer to produce nanoscale tetraene macrolide antibiotic derivatives using Tween-80 surfactant. The research found that the nanoscale tetraene macrolide antibiotic derivatives prepared using the developed method could improve considerably the biopharmaceutical properties of these antifungal antibiotics.
Drug resistance of pathogenic fungi and overcoming it using new PMAs
Systemic administration of antifungal drugs, including PMAs, to treat mycoses led to the development of resistance to them in a series of pathogenic fungi [175,176,177,178]. Resistance of fungi is known to be natural and acquired. Natural (primary) resistance is characterized by a lack of antimycotic targets in fungal species and is encountered extremely rarely [179,180,181]. In practice, natural resistance is understood to mean that a fungus species remains vital in the presence of an antimycotic at concentrations actually achievable in humans. Acquired (secondary) resistance is understood to mean that separate fungal strains remain vital at those drug concentrations that suppress the main population of pathogenic fungi [179, 181, 182]. Acquired resistance in most instances results from the acquisition of new genetic information or a change of the expression level of autologous genes [183,184,185]. An increase of the minimal inhibitory concentration of a drug and a structural change of the target of antimycotic action as a result of spontaneous mutations in genes coding it are important parameters indicating the development of secondary resistance. This decreases (or eliminates) the ability of the target to bind to the antimycotics [186,187,188].
PMAs are known to exhibit both fungistatic and fungicidal activity due to binding of these antimycotics to ergosterol in the fungal membrane, which leads to destruction of the membrane, loss of the cytoplasm contents, and cell death [4,5,6, 13, 14]. Development of resistance could result from complicated genetic processes leading to changes in membrane component biosynthesis because fungal cell structural elements and not enzymes are the targets of PMA action [6,7,8,9, 189]. The probability of such events is relatively low and related to the comparatively low incidence of resistance to PMAs in pathogenic fungi. The biochemical and genetic aspects of resistance to PMAs have been insufficiently studied. However, several researchers support the hypothesis that the ergosterol content in the cytoplasmic membrane is reduced and that of its structural analogs is increased in resistant strains [190,191,192,193,194]. Experimental results indicate that new sterols observed in cells of resistant strains are frequently intermediates from ergosterol [191]. Furthermore, new sterols can also be products of circuitous pathways to synthesize the sterol component. Biochemical tests found that lanosterol transmethylation was disturbed in resistant fungal strains. Sterols were synthesized further by a different pathway that did not require methyl transfer [190, 191]. Additional research showed that fungal resistance was a consequence of shielding or reorientation of membrane sites that interact with PMAs [195, 196]. Such changes could result from mutations or phenotypic modifications affecting not the sterols themselves but other membrane components. Some data indicate that phospholipids may be directly involved in the action of PMAs on fungal cells. The magnitude of the damage from PMAs was observed to depend on the membrane phase state, which was determined by the presence of unsaturated bonds in the phospholipid fatty acids [190, 191, 197, 198].
Several researchers noted that resistance to PMAs varied significantly as a function of medium composition and cultivation conditions for yeast and yeast-like fungi [196, 197, 199]. Resistance to PMAs increased as the culture passed from the logarithmic growth phase to steady-state.
One of the fungal resistance mechanisms to the action of PMAs was active elimination (efflux, ejection) of drugs from the fungal cell [197, 200, 201].
Our previous results on chemical transformation of PMAs and literature data from the latest research on the resistance mechanisms of pathogenic fungi to the action of PMAs [6, 10, 13, 15,16,17,18, 183, 187, 198, 201] showed that the obtained semi-synthetic PMA derivatives were effective against many resistant strains of pathogenic fungi and, primarily, the yeast-like genus Candida [45, 47, 49, 202,203,204,205,206,207,208,209,210].
The importance of the search for new antimycotics, including semi-synthetic PMAs that exhibit pronounced fungicidal activity against resistant pathogenic fungi, should be emphasized because the problem of their resistance to the arsenal of antimycotics available to physicians and clinicians has reached threatening levels. This is an important problem for treating mycoses of various etiologies in both developed and developing countries. Therefore, the World Health Organization (WHO) developed an international program called Global Strategy of WHO for Delaying Resistance to Antimicrobial Drugs that is directed at preventing the constantly growing number of pathogenic microorganisms (including fungi) exhibiting resistance to drugs used systemically for many years and those recently implemented into medical practice [211].
Prospects for medical application of new PMAs for treating invasive mycoses
The wide availability of new medical technologies, diagnostic and therapeutic procedures, cytostatic and immunosuppressive therapies, transplantation, pandemic HIV infections, and progress in treating bacterial infections has increased the immunocompromised patient population with a high risk of invasive mycoses [7, 9, 212, 213]. Invasive mycoses form a group of infection complications due to invasion (penetration) of fungi into various human tissues. According to many researchers, the number of invasive mycoses is continuously increasing. The lethal outcomes associated with them remain very high at 40 – 90% [214,215,216]. The main etiological agents of these diseases are Candida spp., Aspergillus fumigatus, and Cryptococcus neoformans. The main outcomes of resistance problems are the clinical inefficacy of many antifungal preparations used to treat mycoses and a change in the fungal pathogen flora, in particular, dominance of yeast-like Candida spp. other than albicans, e.g., C. glabrata and C. krusei [1, 9, 217, 218]. Furthermore, a rise in the incidence of invasive mycoses caused by various strains of Fusarium spp., Scedosporium spp., Rhizopus spp., Mucor spp., and others was reported. These species are becoming resistant to the most widely used antifungals [7,8,9, 219,220,221,222,223,224,225,226].
Opportunistic fungal infections in AIDS patients are another important problem in clinical medicine. Human immunodeficiency virus (HIV) infection is a slowly progressing infectious disease resulting from infection by HIV, which affects primarily the immune system [227, 228]. HIV belongs to the family of RNA-containing retroviruses and is classified in the subfamily of lentiviruses, i.e., viruses of slow infections. HIV has selective tropism for T4-lymphocytes (T-helpers-inductors), which seriously disturbs immune reactions and results in the organism becoming highly susceptible to opportunistic infections and tumors that eventually lead to a lethal outcome [229,230,231,232,233]. Cases of acquired immunodeficiency syndrome (AIDS), which is the final stage of HIV-infection progression, have now been recorded globally in most countries. Illnesses and infections to an even larger extent have risen consistently over the past 20 years and reached gigantic numbers. This led to the conclusion that HIV-infection could be characterized as a pandemic. The WHO designated the battle against AIDS as a high priority problem with global significance [4,5,6, 10,11,12,13,14,15, 234, 235].
Multi-year studies indicated that AIDS enabled protozoal, fungal, bacterial, and viral opportunistic infections that were the main cause of lethal outcomes [7,8,9, 16,17,18,19,20]. Yeast-like Candida fungi should be identified first among fungal infections causing opportunistic illnesses because they are commonly distributed in HIV patients and can cause clinical forms of surface and deep mycoses (candidiasis) [4,5,6, 10, 11, 179]. According to the latest data, there are ~200 Candida species, only some of which are pathogenic. Four species dominate in humans, i.e., C. albicans, C. glabrata, C. krusei, and C. tropicalis [236,237,238,239]. These yeastlike fungi produce several toxic substances and other aggressive factors such as endotoxins, enzymes (phospholipase, proteinase, collagenase), and cell parts [179, 237, 238]. The interactions of fungi with cells take various forms from surface candidiasis not associated with host-cell (carrier) destruction to penetration of fungi into the bloodstream with development of candidemia and formation of multiple infections in internal organs.
Other important opportunistic fungi include 1) Cryptococcus neoformans, which causes cryptococcosis that leads to infections of the lungs, bone marrow, lymph nodes, liver, and joints, and 2) Aspergillus fumigatus, which is responsible for aspergillosis of the lungs, brain, thyroid, spleen, and kidneys [240,241,242,243,244,245,246]. Furthermore, cases of other opportunistic fungal infections in AIDS patients such as histoplasmosis, blastomycosis, zygomycosis, and paracoccidioidomycosis have been reported [247,248,249,250,251,252,253].
In this respect, use of PMAs with broad spectra of antifungal activity for prevention and therapy of invasive mycoses and opportunistic fungal infections in AIDS patients is very significant. However, as already noted above, the therapeutic efficacy of PMAs used currently in mycological practice is limited for several reasons. The obtained semi-synthetic amphotericin B derivatives were shown by us to exhibit pronounced antifungal activity against several resistant strains of pathogenic fungi such as C. albicans, A. fumigatus, and C. neoformans, which cause opportunistic fungal infections [40,41,42,43,44,45,46, 49,50,51, 60,61,62,63, 254,255,256,257,258,259].
Mechanism of action of semi-synthetic PMAs
PMAs are membranotropic agents according to available information because they interact with sterols localized primarily in the hydrophobic parts of cellular and model membranes to induce irreversible changes of ion and nonelectrolyte permeability [4,5,6, 260]. Fungal cells treated with PMAs lose ions and low-molecular-mass compounds (K+,i-PO43–, carboxylic acids, amino acids, etc.), change respiration rate, and slow protein synthesis. Various research groups found that more significant changes in the membrane structure led to greater growth in the number and size of various components diffusing into the cell and out of it. The process was regulated only by their concentration gradients [1,2,3, 12,13,14,15,16, 20, 261, 262]. Nucleotides and proteins were observed leaving cells at high PMA concentrations. Molecular dynamics of the mechanism of action of PMAs (mainly using amphotericin B as an example) were studied with great interest [263,264,265]. The change of ion permeability of fungal cell membranes after interaction with PMAs was determined by the number and position of hydroxyls on these antifungal drugs, which were concentrated in the channel inner cavity [266, 267]. Modern approaches for studying the mechanism of action of PMAs using Langmuir—Blodgett films were reported [268, 269]. The method is based on the formation on a hydrophilic surface of a monolayer of an amphiphilic compound (amphotericin B) and its subsequent transfer to a solid substrate. The polyol fragment was confirmed to affect the PMA mechanism of action using targeted synthesis of amphotericin B derivatives substituted in the 7-position on the hydrophilic part of the molecule [270]. The mechanism of action of amphotericin B was studied using electron transfer, auto-oxidation, oxidative stress, determination of the electron affinity of an atom, and used of reactive oxygen species [271]. A complex of amphotericin B containing 19F in the 14- and 32-positions and ergosterol containing 13C in the 4-, 26-, and 27-positions was synthesized for a more detailed study of the interaction of amphotericin B and ergosterol [272].
The following basic proposals can be made based on existing literature data on the mechanism of action of PMAs [1,2,3, 12,13,14,15,16, 20,21,22, 259, 261,262,263,264,265,266,267,268,269,270,271,272] and considering structural analyses of semi-synthetic PMAs synthesized by us from amphotericin B, nystatin, levorin, pimaricin, and lucensomycin [40,41,42,43,44,45,46,47,48,49,50, 53,54,55,56,57,58,59,60,61,62,63]: 1) chemical modification of PMAs resulting in the formation of their semi-synthetic derivatives with a larger number of hydroxyls than the starting drugs obviously facilitates faster and more widespread formation of channels through which fungal cell structural components diffuse; 2) the presence in modified PMAs of new functional groups such as phosphate, phosphonate, and fluorinated organic and organosilicon probably leads to many interactions with fungal cell membrane sterol sensitive sites. This increases their reorientation and, as a result, the additional permeability for structural components. However, special biological studies including the use of modern methods of molecular biology, medical microbiology, and pharmacology are required to confirm these proposals.
Structure – activity relationship for semisynthetic PMAs
Statistically significant correlations between structural features and biological properties were found for various classes of drugs, including for PMAs [3, 13, 189, 193]. Relationships between the structures of various PMAs and their antifungal activity were recently studied by several research groups [23,24,25, 273,274,275].
The structure – activity relationship of the semi-synthetic PMA antifungals prepared by us was also studied. Thus, a study of the relationship between the chemical structures of N-benzyl amphotericin B derivatives and their antifungal activity found that compounds with halide atoms in the phenyl para-position were most active [59]. Derivatives containing alkyl or no substituents in the para-position were least active. An analogous trend was noted for N-benzyl pimaricin derivatives [61]. An analysis of the structure—activity relationship of antifungal hydrophosphoryl derivatives of levorin [40], nystatin [41], amphotericin B [42], mycoheptin [43], pimaricin [44], and lucensomycin [45] containing various substituents in the phenyl para-position found the same trend, i.e., compounds with halides on the phenyl ring, primarily Br or F, had the highest antifungal activity. Organophosphorus derivatives (phosphates or phosphonates) of PMAs [40,41,42,43,44,45,46,47,48,49,50] were shown by us to have the greatest antifungal activity of the functionally substituted PMAs such as fluorinated organic [54,55,56], organosilicon [57, 58], N-benzyl [59,60,61,62], and N-aryl derivatives [63] against both test cultures and clinical strains of various classes of pathogenic fungi and yeast-like fungi of the genus Candida.
Development of an automated intellectual information system
An analysis of medical information systems and bioinformatics development pathways showed that various types of information systems are used to find new directions in highly efficient synthesis and manufacturing of drugs, including for treating various diseases including fungal infections [276,277,278,279,280,281,282,283,284].
The synthesis of new efficacious PMAs and the study of their medical and biological properties constitute a long and costly process requiring input from chemists, biologists, and computer modeling and experimental and theoretical data processing experts [285,286,287,288,289,290]. The architecture of an automated intellectual information system (AIIS) aimed at various users, i.e., synthetic chemists, biotechnology engineers, physicians and clinicians, and medical information experts, and systems programmers was developed by us to shorten the times for research and analysis of existing practical and theoretical data [50, 210, 291, 292]. The AIIS recommends to synthetic chemists a selected direction for chemical modification of PMAs; to the physician and clinician, a selection of PMAs and their semi-synthetic derivatives with given characteristics considering the health of the patient. The developed interfaces enable information experts and engineers to supplement the system with data about newly synthesized and modified antibiotics. The AIIS architecture is a machine software set including PMAderivative synthesis apparatuses, instruments for establishing their structures, a laboratory for conducting biological tests, and a computer client—server system loaded with applied software developed by us [293,294,295]. The software structure includes a subsystem for developing the documentation for laboratory regulation of PMA derivatives and a subsystem for intellectual data analysis to select highly efficacious PMAs based on neuronal networks. The information module includes a database of rules for evidence-based selection of antifungal drugs and patient and user databases. Therefore, the proposed AIIS architecture and its software enable the synthesis parameters for new PMA derivatives with improved medical and biological properties and great potential for applications in medical mycology to be rationally selected from an intellectual analysis.
Chemical modification of PMAs was used to prepare a series of semi-synthetic derivatives with improved pharma-cological and biopharmaceutical properties and pronounced activity against various fungal infections.
An analysis of the reasons for fungal pathogen resistance led to the conclusion that studies of the structure and functions of the cellular membranes of these microorganisms are very important for developing measures to overcome the drug resistance of pathogenic fungi and for a deeper understanding of the mechanism of action of these antifungal antibiotics.
References
A. V. Katlinskii, Yu. O. Sazykin, M. V. Bibikova, and S. N. Orekhov, Antibiot. Khimioter., 48(9), 20 – 27 (2003).
J. D. Nosanchuk, Recent Pat. Anti-Infect. Drug Discovery, 1(1), 75 – 84 (2006).
E. Jucker (ed.), Antifungal Agents: Advances and Problems, Special Topic: Progress in Drug Research, Basel, Birkhaeuser Verlag (2003).
A. Yu. Sergeev and Yu. V. Sergeev, Candidiasis. Nature of Infection, Mechanism of Aggression and Protection, Laboratory Diagnosis, Clinic and Treatment [in Russian], Triada-X, Moscow (2001), pp. 187 – 188.
A. Yu. Sergeev and Yu. V. Sergeev, Fungal Infections. Handbook for Physicians [in Russian], BINOM, Moscow (2008), pp. 142 – 145.
S. N. Kozlov and L. S. Strachunskii, Modern Antimicrobial Chemotherapy [in Russian], OOO Meditsinskoe Informatsionnoe Agentstvo, Moscow (2009), pp. 19 – 23.
N. N. Klimko and A. V. Veselov, Klin. Mikrobiol. Antimikrob. Khimioter., 5(4), 342 – 353 (2003).
N. N. Klimko and A. S. Kolbin, Probl. Med. Mikol., 7(3), 3 – 11 (2005).
A. V. Veselov, Klin. Mikrobiol. Antimicrob. Khimioter., 9(1), 73 – 80 (2007).
Yu. V. Sergeev, B. I. Shpigel’, and A. Yu. Sergeev, Pharmacotherapy of Mycoses [in Russian], Meditsina dlya Vsekh, Moscow (2003).
R. A. Aravinskii, N. N. Klimko, and N. V. Vasil’eva, Diagnosis of Mycoses [in Russian], Izdatel’skii Dom SPbMAPO, St. Petersburg (2004).
S. B. Zotchev, Curr. Med. Chem., 10(3), 211 – 223 (2003).
A. T. Coste and P. Vandeputte (eds.), Antifungals: From Genomics to Resistance and the Development of Novel Agents, Caister Academic Press, Norfolk, UK (2015).
G. San-Blas and R. A. Calderone (eds.), Pathogenic Fungi: Insights in Molecular Biology, Caister Academic Press, Norfolk, UK (2008).
E. Reiss, H. J. Shadomy, and G. M. Lyon, Fundamental Medical Mycology, Wiley-Blackwell, Hoboken, NJ, USA (2011).
D. J. Sillivan and G. P. Morgan (eds.), Human Pathogenic Fungi: Molecular Biology and Pathogenic Mechanisms, Caister Academic Press, Norfolk, UK (2014).
S. Omura (ed.), Macrolide Antibiotics: Chemistry, Biology and Practice, Academic Press, New York (2002).
M. Masayuki and K. Gomi (eds.), Aspergillus: Molecular Biology and Genomics, Caister Academic Press, Norfolk, UK (2010).
R. Grillot and B. Lebeau, in: Antimicrobial Agents, A. Bryskier (ed.), American Society for Microbiology, Washington (2005), pp. 1260 – 1287.
T. C. White, J. Harry, and B. G. Oliver, in: Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research, K. Esser and J. W. Bennet (eds.), Springer-Verlag, Berlin (2004), pp. 319 – 337.
Yu. D. Shenin, V. V. Belakhov, and R. A. Araviiskii, Khim.- farm. Zh., 27(2), 14 – 21 (1993).
Yu. D. Shenin and V. V. Belakhov, Antibiot. Khimioter., 42(4), 34 – 46 (1997).
A. A. Volmer, A. M. Szpilman, and E. M. Carreira, Nat. Prod. Rep., 27(9), 1329 – 1349 (2010).
M. Sedlak, Mini-Rev. Med. Chem., 9(11), 1306 – 1316 (2009).
S. E. Solov’eva, E. N. Olsuf’eva, and M. N. Preobrazhenskaya, Usp. Khim., 80(20), 115 – 138 (2011).
R. G. Hall, Chimia, 64(1–2), 34 – 36 (2010).
M. Jokanovic, Curr. Top. Med. Chem. (Sharjah, United Arab Emirates), 12(16), 1775 – 1789 (2012).
E. Balint, E. Fazekas, and J. Takacs, Phosphorus Sulfur Silicon Relat. Elem., 188(1–3), 48 – 50 (2013).
S. S. Le Corre, M. Berchel, H. Couthon-Gourves, et al., Beilstein J. Org. Chem., 10, 1166 – 1196 (2014).
D. E. C. Corbridge, Phosphorus: Chemistry, Biochemistry and Technology, CRC Press (Taylor & Francis Group), Boca Raton, FL, USA (2013).
M. Dziegielewski, J. Pieta, E. Kaminska, and L. Albrecht, Eur. J. Org. Chem., 2015(4), 677 – 702 (2015).
H. R. Hudson, N. J. Wardle, S. W. A. Bligh, et al., Mini-Rev. Med. Chem., 12(4), 313 – 325 (2012).
L. Albrecht, A. Albrecht, H. Krawczyk, and K. A. Jorgensen, Chem. Eur. J., 16(1), 28 – 48 (2010).
Q. Xi, Y-B. Zhou, C.-Q. Zhao, et al., Mini-Rev. Med. Chem., 13(6), 824 – 835 (2013).
B. Lejczak and P. Kafarski, in: Topics in Heterocyclic Chemistry, Vol. 20, Phosphorous Heterocycles I, R. K. Bansal (ed.), Springer, (2009), pp. 31 – 63.
A. Mucha, P. Kafarski, and L. Berliki, J. Med. Chem., 54(17), 5955 – 5980 (2011).
V. I. Krutikov, A. V. Erkin, and V. V. Krutikova, Zh. Obshch. Khim., 82(5), 713 – 718 (2012).
S. Demkowicz, J. Rachon, M. Dawsco, and W. Kozak, RSC Adv., 6(9), 7101 – 7112 (2016).
G. Keglevich and E. Balint, Molecules, 17(11), 12821 – 12835 (2012).
V. V. Belakhov, Yu. D. Shenin, B. I. Ionin, et al., Antibiot. Khimioter., 35(8), 31 – 35 (1990).
V. V. Belakhov, Yu. D. Shenin, B. I. Ionin, et al., Khim.-farm. Zh., 25(11), 45 – 48 (1991).
V. V. Belakhov, Yu. D. Shenin, R. A. Araviiskii, and E. B. Shtil’bans, Antibiot. Khimioter., 41(7/8), 4 – 8 (1996).
V. V. Belakhov and Yu. D. Shenin, Khim.-farm. Zh., 41(6), 26 – 30 (2007).
V. V. Belakhov, Yu. D. Shenin, and B. I. Ionin, Russ. J. Gen. Chem., 78(2), 305 – 312 (2008).
V. V. Belakhov, V. A. Kolodyaznaya, and B. I. Ionin, Khim. Prom-st., 89(2), 64 – 76 (2012).
V. V. Belakhov and A. V. Garabadzhiu, Zh. Obshch. Khim., 85(2), 236 – 244 (2015).
V. V. Belakhov, A. V. Garabadzhiu, and B. I. Ionin, in: Proceedings of the VIIIth International Scientific and Practical Conference “Perspective Directions of World’s Science” Byal-GRAD OOD, Sofia, Bulgaria, 34, 80 – 84 (2012).
V. V. Belakhov and B. I. Ionin, Izv. SPbGTI(TU), No. 17, 51 – 52 (2012).
V. V. Belakhov, V. A. Kolodyaznaya, and A. V. Garabadzhiu, Zh. Obshch. Khim., 84(10), 1676 – 1684 (2014).
V. V. Belakhov, A. V. Garabadzhiu, T. B. Chistyakova, et al., Zh. Obshch. Khim., 86(3), 427 – 436 (2016).
V. V. Belakhov, V. A. Kolodyaznaya, A. V. Garabadzhiu, et al., in: Progress in Medical Mycology [in Russian], XVI, National Academy of Mycology, Moscow (2016), pp. 114 – 119.
A. V. Dogadina, V. V. Belakhov, B. I. Ionin, et al., in: Proceedings of the First Russian Conference on Medicinal Chemistry (MedChem Russia – 2013) [in Russian], RBR Print, Moscow (2013), p. 55.
V. V. Belakhov, A. V. Dogadina, and B. I. Ionin, Izv. SPbGTI(TU), No. 19, 67 – 70 (2013).
Yu. D. Shenin, V. V. Belakhov, L. I. Shatik, and R. A. Araviiskii, Antibiot. Khimioter., 43(12), 8 – 11 (1998).
Yu. D. Shenin, V. V. Belakhov, and R. A. Araviiskii, Khim.-farm. Zh., 32(2), 52 – 53 (1998).
Yu. D. Shenin, V. V. Belakhov, and R. A. Araviiskii, Khim.-farm. Zh., 41, No. 9, 26 – 28 (2007).
V. V. Belakhov, A. A. Levina, Yu. D. Shenin, and B. I. Ionin, Khim.-farm. Zh., 25(3), 86 – 87 (1991).
V. V. Belakhov and Yu. D. Shenin, Khim.-farm. Zh., 42(7), 15 – 18 (2008).
V. V. Belakhov and Yu. D. Shenin, Khim.-farm. Zh., 41(7), 20 – 24 (2007).
V. V. Belakhov and V. A. Kolodyaznaya, in: Progress in Medical Mycology [in Russian], XII, National Academy of Mycology, Moscow (2014), pp. 377 – 379.
V. V. Belakhov, Yu. D. Shenin, and V. A. Kolodyaznaya, Izv. SPbGTI(TU), No. 23, 34 – 38 (2014).
V. V. Belakhov, A. V. Garabadzhiu, V. A. Kolodyaznaya, and O. V. Topkova, Khim-farm. Zh., 50(3), 7 – 15 (2016).
V. V. Belakhov, Yu. D. Shenin, and B. I. Ionin, Khim.-farm. Zh., 44(9), 19 – 25 (2010).
V. V. Belakhov and B. I. Ionin, in: Proceedings of the Xth International Scientific-Practical Conference “Innovation in Science” [in Russian], Part 1, Sibirskaya Assotsiatsiya Konsul’tantov, Novosibirsk (2012), pp. 20 – 24.
V. V. Belakhov, B. I. Ionin, and V. A. Kolodyaznaya, in: Progress in Medical Mycology [in Russian], XI, National Academy of Mycology, Moscow (2013), pp. 302 – 304.
M. A. Shneider, Mol. Genet. Mikrobiol. Virusol., No. 5, 41 – 46 (1984).
M. A. Shneider and N. P. Chizhov, Vopr. Virusol., 31(1), 18 – 31 (1986).
W. Wang, et al., US Pat. 8,217,013, Jul. 10, 2012; Chem. Abstr., 150, 206299v (2009).
J. Lamontagne, C. Mills, R. Mao, et al., Antiviral Res., 98(1), 19 – 26 (2013).
J. Feng, M. Weitner, W. Shi, et al., Antibiotics (Basel, Switz.), 4(3), 397 – 410 (2015).
P. Vaishnav and A. L. Demain, Biotechnol. Adv., 29(2), 223 – 229 (2011).
Y. Chen, S. Wang, and X. Lu, Blood, 117(23), 6392 – 6403 (2011).
M. Altendorfer, R. Mario, F. Sasse, et al., Org. Biomol. Chem., 11(13), 2116 – 2139 (2013).
S. Sheikh, A. Sturzu, H. Kalbacher, et al., Med. Chem. (Sharjah, United Arab Emirates), 10(4), 348 – 354 (2014).
S. Sarkar, A. Doering, F. J. Zemp, et al., Nat. Neurosci., 17(1), 46 – 55 (2014).
T. Meszaros, A. I. Csincsi, B. Uzonyi, et al., Nanomedicine (N. Y., NY, U. S.), 12(4), 1023 – 1031 (2016).
Y. Kaneo, K. Taguchi, T. Tanaka, and S. Yamamoto, J. Drug Delivery Sci. Technol., 24(4), 344 – 351 (2014).
V. Strenger, A. Meinitzer, J. Donnerer, et al., J. Antimicrob. Chemother, 69(9), 2522 – 2526 (2014).
T. Meszaros, G. Szenasi, L. Rosivall, et al., Eur. J. Nanomed., 7(3), 257 – 262 (2015).
V. Leonard, R. V. Alasino, I. D. Bianco, et al., Curr. Drug Delivery, 12(4), 406 – 414 (2015).
K. M. Wasan, O. Sivak, K. Bartlett, et al., Drug Dev. Ind. Pharm., 41(9), 1425 – 1430 (2015).
Y. Ohata, Y. Tomita, K. Suzuki, et al., Drug Metab. Pharmacokinet., 30(6), 400 – 409 (2015).
M. Hagihara, Y. Yamagishi, J. Hirai, et al., BMC Res. Notes, 8, 510/1 – 510/4 (2015).
N. Itoh, E. Yamamoto, T. Santa, et al., Pharm. Res., 33(6), 1440 – 1446 (2016).
V. Colapicchioni, M. Tilo, L. Digiacomo, et al., Int. J. Biochem. Cell Biol., 75, 180 – 187 (2016).
J. A. Jackman, T. Meszaros, T. Fulop, et al., Nanomedicine (N. Y., NY, U. S.), 12(4), 933 – 943 (2016).
N. R. Stone, T. Bicanic, R. Salim, and W. Hope, Drugs, 76(4), 485 – 500 (2016).
F. Saliba, V. Delvart, P. Ichai, et al., Med. Mycol., 51, No. 2, 155 – 163 (2013).
S. Mignani, S. El. Kazzouli, M. Bousmina, and J. P. Majoral, Adv. Drug Delivery Rev., 65(10), 1316 – 1330 (2013).
D. R. Serrano, M. P. Ballesteros, A. G. Schatzlein, et al., Pharm. Nanotechnol., 1(4), 250 – 258 (2013).
D. M. Casa, T. C. M. M. Carraro, L. E. Alves de Camargo, et al., J. Nanosci. Nanotechnol., 15(1), 848 – 854 (2015).
G.-L. M. Chong, W. W. J. van de Sande, G. J. H. Dingemans, et al., J. Clin. Microbiol., 53(3), 868 – 874 (2015).
Y. M. Brustoloni, R. V. Cunha, L. Z. Consolo, et al., Infection (Munich, Ger.), 38(4), 261 – 267 (2010).
C. Cifani, S. Constantino, M. Massi, and L. Berrino, Acta Bio Med. Atenei Parmensis, 83(2), 154 – 163 (2012).
C. M. Santos, R. Barbosa de Oliveira, V. T. Arantes, et al., J. Biomed. Nanotechnol., 8(2), 322 – 329 (2012).
C. C. Pupe, M. Villardi, C. R. Rodriges, et al., Int. J. Nanomed., 6, 2581 – 2590 (2011).
F. F. Campos, A. C. Calpena-Campmany, G. R. Deldago, et al., J. Pharm. Sci., 101(10), 3739 – 3752 (2012).
M. A. Khan, A. Aljarbou, A. Khan, and M. Owais, FEMS Immunol. Med. Microbiol., 66(1), 88 – 97 (2012).
D. Marin-Quintero, F. Fernandez-Campos, A. C. Calpena-Campmany, et al., J. Pharm. Sci., 102(11), 4015 – 4023 (2013).
F. Fernandes-Campos, B. C. Naveros, O. L. Serano, et al., Mycoses, 56(1), 70 – 81 (2013).
C. Martin, W. L. Low, A. Gupta, et al., in: Advances in Liposomes Research, Nova Science Publishers, Inc., New York (2014), pp. 27 – 61.
L. H. Samein, Int. J. Pharm. Pharm. Sci., 6(2), 592 – 597 (2014).
H. C. Nwuke, I. T. Nzekwe, C. O. Agubata, et al., Int. J. Pharm. Sci. Res., 6(2), 624 – 629 (2015).
O. Dumitriu-Buzia, N. Mardare, and C. Diaconu, Rev. Chim. (Bucharest, Rom.), 67(2), 232 – 235 (2016).
Z. Drulis-Kawa and A. Dorotkiewicz-Jach, Int. J. Pharm., 387(1–2), 187 – 198 (2010).
S. R. Naik, S. K. Desai, P. D. Shah, and S. M. Wala, Recent Pat. Inflammation Allergy Drug Discovery, 7(3), 202 – 214 (2013).
I. P. Kaur and S. Kakkar, Expert Opin. Drug Delivery, 7(11), 1303 – 1327 (2010).
J. P. Barrett, K. A. Vardulaki, C. Conlon, et al., Clin. Ther., 25(5), 1295 – 1320 (2003).
J. H. Rex and S. Arikan, Expert Opin. Emerging Drugs, 7(1), 3 – 32 (2002).
V. P. Torchilin, Nat. Rev. Drug Discovery, 4(2), 142 – 160 (2005).
I. A. Yamskov, A. N. Kuskov, K. K. Babievskii, et al., Prikl. Biokhim. Mikrobiol., 44(6), 688 – 693 (2008).
R. D. Seifulla, Pharmacology of Liposomal Preparations [in Russian], Globus Kontinental’, Moscow (2010).
V. Torchilin and V.Weissig, Liposomes: A Practical Approach, 2nd Ed., Oxford University Press, Oxford (2003).
T. A. ElBayoumi and V. P. Torchilin, in: Methods in Molecular Biology, Vol. 605, V. Weissig (ed.), Humana Press Inc., Totowa, NJ, USA [Liposomes, 1, 1 – 27 (2010)].
J. J. Torrado, R. Espada, M. P. Ballesteros, and S. Torrado-Santiago, J. Pharm. Sci., 97(7), 2405 – 2425 (2008).
C. P. Poole and F. J. Owens, Introduction to Nanotechnology, J. Wiley, Hoboken, NJ (2003) [Russian translation, Tekhnosfera, Moscow (2007), pp. 271 – 290].
E. Gazit, Nanobiotechnology: Unlimited Prospects for Development [in Russian], Nauchnyi Mir, Moscow (2011), pp. 83 – 91.
M. H. Fulekar, Nanotechnology: Importance and Applications, I. K. International Publishing House Pvt. Ltd., New Delhi (2010), pp. 175 – 182.
V. I. Balabanov, Nanotechnology. Science of the Future [in Russian], Eksmo, Moscow (2009).
A. Lamprekht (ed.), Nanodrugs. Drug Delivery Concepts in Nanoscience [in Russian], Nauchnyi Mir, Moscow (2010).
C.-M. Lin and T.-Y. Lu, Recent Pat. Nanotechnol., 6(2), 105 – 113 (2012).
S. Jayronia, A. Hardenia, and S. Jain, World J. Pharm. Res., 3(1), 295 – 310 (2014).
T. da Ros and F. Cataldo, Medicinal Chemistry and Pharmacological Potential of Fullerenes and Carbon Nanotubes, Springer, Amsterdam (Netherlands) (2013).
S. Kwatra, Int. J. Drug Dev. Res., 5(1), 1 – 10 (2013).
V. Rani, J. Chem. Pharm. Res., 7(7), 216 – 227 (2015).
X. Zhu, M. Sollogoub, and Y. Zhang, Eur. J. Med. Chem., 115, 438 – 452 (2016).
J.-F. Nierengarten and F. Langa, Fullerenes: Principles and Applications, Royal Society of Chemistry, Cambridge, UK (2011).
D. Iglesias, S. Bosi, M. Melchionna, et al., Curr. Topics Med. Chem. (Sharjah, United Arab Emirates), 16(18), 1976 – 1989 (2016).
T. A. Kolesnikova, B. N. Khlebtsov, D. G. Shchukin, and D. A. Gorin, Ross. Nanotekhnol., 3(9), 74 – 83 (2008).
M. A. Petrukhina and L. T. Scott (eds.), Fragments of Fullerenes and Carbon Nanotubes: Designed Synthesis, Unusual Reactions, and Coordination Chemistry, John Wiley & Sons, New York (2011).
E. Sheka, Fullerenes: Nanochemistry, Nanomagnetism, Nanomedicine, Nanophotonics, CRC Press (Taylor & Francis Group), Boca Raton, FL, USA (2011).
K. V. Koltover, in: Advances in Materials Science Research, 1, M. C. Wythers (ed.), Nova Science Publishers, New York (2012), pp. 259 – 275.
S. Chakrabarty, S. Choudhary, A. Doshi, et al., Adv. Synth. Catal., 356(10), 2135 – 2196 (2014).
A. A. Popov, S. Yang, and L. Dunsch, Chem. Rev., 113(8), 5989 – 6113 (2013).
W. Luther and A. Zweck (eds.), Safety Aspects of Engineered Nanomaterials, CRC Press (Taylor & Francis Group), Boca Raton, FL, USA (2013), p. 385.
V. Rao, Appl. Biosaf., 19(1), 11 – 19 (2014).
B. Fadeel (ed.), Handbook of Safety Assessment of Nanomaterials: From Toxicological Testing to Personalized Medicine, CRC Press (Taylor & Francis Group), Boca Raton, FL, USA (2015).
V. Srivastava, D. Gusain, and Y. C. Sharma, Ind. Eng. Chem. Res., 54(24), 6209 – 6233 (2015).
K. Bhattacharya, S. P. Mukherjee, A. Gallud, et al., Nanomedicine (N. Y., NY, U. S.), 12(2), 333 – 351 (2016).
Z. Li, Z. Liu, H. Sun, and C. Gao, Chem. Rev., 115, No. 15, 7046 – 7117 (2015).
A. Bhandari, A. N. Naik, and S. Lewis, Syst. Rev. Pharm., 4(1), 20 – 25 (2013).
I. F. Uchegbu and A. Siew, J. Pharm. Sci., 102(2), 305 – 310 (2013).
J. J. Torrado, D. R. Serrano, and I. F. Uchegbu, Ther. Delivery, 4(1), 9 – 12 (2013).
D. R. Serrano, M. P. Ballesteros, A. G. Schatzlein, et al., Pharm. Nanotechnol., 1(4), 250 – 258 (2013).
M. A. Bianco, M. Gallarate, M. Trotta, and L. Battaglia, J. Drug Delivery Sci. Technol., 20(3), 187 – 191 (2010).
N. Xu, J. Gu, Y. Zhu, et al., Int. J. Nanomed., 6, 905 – 913 (2011).
C. D. Rodrigues, D. M. Casa, L. F. Dalmolin, et al., Curr. Nanosci., 29(5), 594 – 598 (2013).
N. Pippa, M. Mariaki, S. Pispas, and C. Demetzos, Int. J. Pharm., 473(1–2), 80 – 86 (2014).
K. L. Nagarsekar, C. N. Galdhar, R. V. Gaikwad, et al., Drug Delivery Lett., 4, No. 3 208 – 220 (2014).
D. M. Casa, T. C. M. M. Carraro, L. E. Alves de Camargo, et al., J. Nanosci. Nanotechnol., 15(1), 848 – 854 (2015).
A. C. O. Souza, A. L. Nascimento, N. M. Vasconcelos, et al., Eur. J. Med. Chem., 95, 267 – 276 (2015).
X. Tang, R. Jiao, C. Xie, et al., Int. J. Clin. Exp. Med., 8(4), 5150 – 5162 (2015).
A. Ahmad, Y. Wei, F. Syed, et al., Microb. Pathog., 99, 271 – 281 (2016).
T. C. M. M. Carraro, N. M. Khalil, and R. M. Mainardes, Pharm. Dev. Technol., 21(2), 140 – 146 (2016).
D. Butani, C. Yewale, and A. Misra, Colloids Surf., B, 139, 17 – 24 (2016).
K. Tutai, R. Szlazak, K. Szalapata, et al., Nanomedicine (N. Y., NY, U. S.) (Nanotechnol. Biol. Med.), 12(4), 1095 – 1103 (2016).
M. Karimi, N. Solati, A. Ghasemi, et al., Expert Opin. Drug Delivery, 12(7), 1089 – 1105 (2015).
X. Tang, Y. Liang, Y. Zhu, et al., Int. J. Nanomed., 10, 6227 – 6241 (2015).
D. M. Casa, T. K. Karam, A. C. S. Alves, et al., J. Nanosci. Nanotechnol., 15(2), 10183 – 10188 (2015).
I. Javed, S. Z. Hussein, I. Ullah, et al., J. Mater. Chem. B, 3(42), 8359 – 8365 (2015).
X. Tang, J. Dai, J. Xie, et al., Nanoscale Res. Lett., 10(1), 1 – 11 (2015).
Q. Zia, A. A. Khan, Z. Swaleha, and M. Owais, Int. J. Nanomed., 10, 1769 – 1790 (2015).
K. Shirkhani, I. Teo, D. Armstrong-James, and S. Shaunak, Nanomedicine (N. Y., NY, U. S.), 11(5), 1217 – 1226 (2015).
D. R. Serrano, A. Lalatsa, M. A. Dea-Ayuela, et al., Mol. Pharm., 12(2), 420 – 431 (2015).
R. Khalil, M. Kassem, A. A. Elbary, et al., Int. J. Pharm. Sci. Res., 4(6), 2292 – 2300 (2013).
A. Melkoumov, M. Goupil, F. Louhichi, et al., J. Antimicrob. Chemother, 68(9), 2099 – 2105 (2013).
G. Badea, A. G. Bors, I. Lacatusu, et al., C. R. Chim., 18(6), 668 – 677 (2015).
C. P. Reis, L. V. Roque, M. Babtista, and P. Rijo, Pharm. Dev. Technol., 21(3), 282 – 287 (2016).
M. Mobasheri, H. Attar, A. M. R. Sorkhabadi, et al., Molecules, 21(1), 1 – 26 (2016).
K. Niemirowicz, B. Durnas, G. Tokajur, et al., Nanomedicine (N. Y., NY, U. S.) (Nanotechnol., Biol. Med.), 12(4), 2395 – 2404 (2016).
A. A. Kassem, A. M. Mohsen, R. S. Ahmed, and T. M. Essam, J. Mol. Liq., 218, 219 – 232 (2016).
C. Bouaoud, S. Xu, E. Mendes, et al., J. Appl. Polym. Sci., 133(31), 1 – 10 (2016).
H. Chandasana, Y. D. Prasad, Y. S. Chhonker, et al., Int. J. Pharm., 477(1–2), 317 – 325 (2014).
V. V. Belakhov, A. V. Garabadzhiu, and V. A. Kolodyaznaya, in: Progress in Medical Mycology [in Russian], Proceedings of the Third International Mycological Forum, 14, Izd. National Academy of Mycology, Moscow (2015), pp. 334 – 337.
N. Y. Villa, P. Moussatche, S. G. Chamberlin, et al., J. Mol. Evol., 73(3–4), 134 – 152 (2011).
M. S. A. Khan and I. Ahmad, Appl. Microbiol. Biotechnol. 90(3), 1083 – 1094 (2011).
A. Devprakash, P. Singh, K. K. Srinvasan, et al., J. Pharm. Res. Opin., 1(3), 85 – 88 (2011).
I. V. Ene, C. J. Heilmann, J. Clemens, et al., Proteomics, 12(21), 3164 – 3179 (2012).
N. P. Elinov, Probl. Med. Mikol., 6(4), 3 – 8 (2004).
N. V. Beloborodova and T. Yu. Vostrikova, Klin. Mikrobiol. Antimikrob. Khimioter., 11(1), 22 – 30 (2009).
L. V. Ivanova, E. P. Barantsevich, and E. V. Shlyakhto, Probl. Med. Mikol., 13(1), 14 – 17 (2011).
A. B. Yakovlev, Mycosporia trichophytia Favus, OOO Novik, Moscow (2013).
D. Sanglard and T. C. White, in: Molecular Principles of Fungal Pathogenesis, Chap, 14, J. Heitman (ed.), American Society for Microbiology, Washington (2006), pp. 197 – 212.
I. Leven-Reisman, I. Ronin, O. Gefen, et al., Science, 355(6327), 826 – 830 (2017).
Z. A. Kanafani and J. R. Perfect, Clin. Infect. Dis., 46, 120 – 128 (2008).
K. W. Gammelsrud, B. L. Lindstad, and P. Gaustad, Med. Mycol., 50(6), 619 – 625 (2012).
D. Sanglard, A. Coste, and S. Ferrari, FEMS Yeast Res., 9(7), 1029 – 1050 (2009).
K. A. Vinogradova, V. G. Bulgakova, A. N. Polin, and P. A. Kozhevin, Antibiot. Khimioter., 58(5–6), 38 – 48 (2013).
M. Razzaghi-Abyaneh, M. Shams-Ghahfarokhi, and M. Rai (eds.), Medical Mycology: Current Trends and Future Prospects, CRC Press, Boca Raton, FL, USA (2015).
C. M. Hull, N. J. Purdy, and S. C. Moody, Future Microbiol., 9(3), 307 – 325 (2014).
A. M. Borman, R. Petch, C. J. Linton, M. D. Palmer, et al., J. Clin. Microbiol., 46(3), 933 – 938 (2008).
A. Espinel-Ingroff, E. Jonhson, H. Hockey, and P. Troke, J. Antimicrob. Chemother, 61(3), 616 – 620 (2008).
S. Sanchez and A. L. Demain (eds.), Antibiotics: Current Innovations and Future Trends, Caister Academic Press, Poole, UK (2015).
J. H. Shin, M.-N. Kim, S. J. Sook, et al., J. Clin. Microbiol., 50(6), 1852 – 1855 (2012).
A. Vartak, V. Mutalik, R. R. Parab, et al., Lett. Appl. Microbiol., 58(6), 591 – 596 (2014).
M. M. Tawfick and A. S. Gad, Am. J. Drug Discovery Dev., 4(1), 32 – 40 (2014).
D. W. Denning and M. J. Bromley, Science, 347(6229), 1414 – 1416 (2015).
I. P. Kaur and S. Kakkar, Expert Opin. Drug Delivery, 7(11), 1303 – 1327 (2010).
A. M. S. Al-Hatmi, M. Mirabolfathy, F. Hagen, et al., Fungal Biol., 120(2), 265 – 278 (2016).
S. S. Goncalves, A. C. R. Souza, and A. Chowdhary, Mycoses, 59(4), 198 – 219 (2016).
M. Slisz, B. Cybulska, J. Grzybowska, et al., J. Antibiot., 60(7), 436 – 446 (2007).
Yu. D. Shenin, V. V. Belakhov, and R. A. Araviiskii, in: Pharmacy from Century to Century. Proceedings of a Scientific-Practical Conference, Part IV, Izd. SPGKhFA, St. Petersburg (2004), pp. 104 – 109.
Yu. D. Shenin and V. V. Belakhov, in: Proceedings of an International Scientific-Practical Conference Dedicated to the 85 th Birthday of the Academy [in Russian], Izd. SPGKhFA, St. Petersburg (2004), pp. 322 – 324.
Yu. D. Shenin, V. V. Belakhov, and R. A. Araviiskii, in: Current State and Optimization Pathway for Public Drug Supply. Proceedings of a Russian Scientific-Practical Conference [in Russian], Izd. Perm State Pharmaceutical Academy, Perm (2008), pp. 395 – 397.
V. V. Belakhov, Y. D. Shenin, R. A. Araviisky, and B. I. Ionin, in: Strategic Problems of World’s Science, Proceeding of V International Scientific and Practical Conference [in Russian], Nauka i Studia, Przemysl, Poland (2009), pp. 7 – 10.
V. V. Belakhov and B. I. Ionin, in: Scientific Search in the Modern World, Proceedings of the IInd International Scientific-Practical Conference [in Russian], Pero, Moscow (2012), pp. 45 – 50.
V. V. Belakhov, Y. D. Shenin, A. V. Garabadzhiu, and B. I. Ionin, in: Modern Scientific Achievements, Proceedings of IX International Scientific and Practical Conference, Education and Science, Prague, Czech Republic (2013), pp. 94 – 101.
V. A. Kolodyaznaya, Yu. D. Shenin, V. V. Belakhov, and B. I. Ionin, in: Proceedings of 17th European Carbohydrate Symposium, Tel-Aviv, Israel (2013), p. 107.
V. V. Belakhov, A. V. Garabadzhiu, and V. A. Kolodyaznaya, in: Proceedings of Annual Meeting of the Israel Society for Microbiology, Ramat-Gan, Israel (2015), p. 45.
V. V. Belakhov, T. B. Chistyakova, I. A. Smirnov, and A. V. Garabadzhiu, in: Proceedings of 81st Annual Meeting of the Israel Chemical Society, Tel Aviv, Israel (2016), p. 86.
WHO Global Strategy for Containment of Antimicrobial Resistance, World Health Organization (WHO), Geneva, Switzerland (2001).
N. V. Vasil’eva, N. N. Klimko, and V. A. Tsinzerling, Vestn. Sankt-Peterburg. Med. Akad. Poslediplom. Obraz., 2(4), 5 – 18 (2010).
A. Perrella, C. Esposito, O. Perrella, et al., Infect. Dis., 48(2), 161 – 166 (2016).
O. A. Cornely, S. Arkan-Akdagli, E. Dannaoui, et al., Clin. Microbiol. Infect., 20(3), 5 – 26 (2014).
F. Fernandez-Silva, J. Capilla, E. Mayayo, et al., Int. J. Antimicrob. Agents, 44(2), 136 – 139 (2014).
G. Maschmeyer, T. Calandria, N. Singh, et al., Med. Mycol., 47, No. 6, 571 – 583 (2009).
M. Nucci, K. A. Marr, M. J. G. T. Vehreschild, et al., Clin. Microbiol. Infect., 20(6), 580 – 585 (2014).
A. Kumar, R. Babu, S. Bijulal, et al., J. Clin. Microbiol., 52(11), 4094 – 4099 (2014).
A. H. Groll and T. J. Walsh, in: Aspergillus fumigatus and Aspergillosis, Chap. 30, W. J. Steibach (ed.), American Society for Microbiology, Washington (2009), pp. 391 – 415.
D. Andes, A. Pascual, and O. Marchetti, Antimicrob. Agents Chemother., 53(1), 24 – 34 (2009).
D. P. Kontoyiannis, Am. J. Med., 12(1), S25 – S38 (2012).
C. Kobyashi, T. Hanadate, T. Niwa, et al., J. Infect. Chemother., 21(6), 438 – 443 (2015).
M. Blatzer, E. Jukic, W. Posch, et al., Antioxid. Redox Signaling, 23(18), 1424 – 1438 (2015).
S. Cordoba, M. G. Isla, W. Szusz, et al., Mycoses, 59(6), 351 – 356 (2016).
C. Coelho and A. Casadevall, Cell. Microbiol., 18(6), 792 – 799 (2016).
T. N. Doan, C. M. Kirkpatrick, P. Walker, et al., J. Antimicrob. Chemother., 71(2), 497 – 505 (2016).
A. L. Leal, J. Faganello, A. M. Fuentefria, et al., Mycopathologia, 166(2), 71 – 75 (2008).
A. Chakrabarti, S. S. Chatterjee, and M. R. Shivaprakash, Jpn. J. Med. Mycol., 49(3), 165 – 172 (2008).
B. P. Mathew and M. Nath, ChemMedChem., 4(3), 310 – 323 (2009).
M. S. Ferreira and A. S. Borges, Rev. Soc. Bras. Med. Trop., 42(2), 192 – 198 (2009).
D. F. S. Freitas, H. B. de Siqueira, A. S. F. do Valle, et al., Med. Mycol., 50(2), 170 – 178 (2012).
G. P. Bisson, M. Molefi, S. Bellamy, et al., Clin. Infect. Dis., 56(8), 1165 – 1173 (2013).
J. Manoj, J. Priyanka, V. Shinde, et al., Clin. Pharmacol. Drug Dev., 2(1), 48 – 52 (2013).
R. K. Vadlapatla, M. Patel, D. K. Paturi, et al., Expert Opin. Drug Metab. Toxicol., 10(4), 561 – 580 (2014).
UNAIDS, WHO, AIDS Epidemic Update: December 2000, Joint United Nations Program on HIV / AIDS, Geneva (2000).
N. N. Klimko, Mycoses: Diagnosis and Treatment: Guide for Physicians [in Russian], Vi Dzhi Group, Moscow (2008).
N. P. Elinov, N. V. Vasil’eva, A. A. Stepanova, and G. A. Chilina, Candida. Candidiasis. Laboratory Diagnosis [in Russian], KOSTA, St. Petersburg (2010).
N. P. Elinov, Probl. Med. Mikol., 12(3), 3 – 9 (2010).
C. d’Enfert and B. Hube (eds.), Candida: Comparative and Functional Genomics, Caister Academic Press, Poole, UK (2007).
M. Corti, M. Priarone, J. Castrelo, et al., Rev. Soc. Bras. Med. Trop., 47(4), 524 – 527 (2014).
J. L. A. Rabjohns, Y.-D. Park, J. Dehdashti, et al., J. Biomol. Screening, 19(2), 270 – 277 (2014).
D. R. Boulware, D. B. Meya, C. Muzoora, et al., N. Eng. J. Med., 370(26), 2487 – 2498 (2014).
S. Anil, M. Hashem, S. Vellappally, et al., Mycopathologia, 178(3–4), 207 – 215 (2014).
K. Kumari, A. Kumar, and P. C. Sharma, Int. J. Pharm. Sci. Res., 5(2), 532 – 547 (2014).
A. V. Veselov, Klin. Mikrobiol. Antimicrob. Khimioter., 10(4), 292 – 304 (2008).
B. L. Yesudhason and K. Mohanram, J. Clin. Diagn. Res., 9, No. 7, DC14-DC16 (2015).
T. K. Ngouana, D. Krasteva, P. Drakulovski, et al., Mycoses, 58(1), 33 – 39 (2015).
S. Cordoba, W. Vivot, W. Szusz, et al., Mycopathologia, 179(5–6), 359 – 371 (2015).
B. P. Morales, L. Trilles, A. L. Bertho, et al., Mycoses, 58, No. 5, 273 – 279 (2015).
D. J. Krysan, Fungal Genet. Biol., 78, 93 – 98 (2015).
G. L. Lee, K. L. Woods, L. Clark, et al., AIDS Res. Hum. Retroviruses, 31(9), 889 – 892 (2015).
T. R. Rogers, J. Antimicrob. Chemother., 61(1), 35 – 39 (2008).
R. D. Nenoff, C. Kruger, H. Grob, et al., Hautarzt: Zeitschrift fur Dematologie, Venerologie, Verwandte Gebiete, 66(7), 522 – 532 (2015).
V. V. Belakhov, A. V. Garabadzhiu, V. A. Kolodyaznaya, et al., in: Innovation from Discovery to Application, Proceeding of 250th National Meeting of American Chemical Society (ACS), MEDI 60, ACS, Boston, MA, USA (2015).
V. V. Belakhov, Yu. D. Shenin, and B. I. Ionin, in: Theoretical and Practical Problems in Development of Modern Science, Proceedings of the First International Scientific-Practical Conference [in Russian], Pero, Moscow (2013), pp. 12 – 16.
V. V. Belakhov, Yu. D. Shenin, and B. I. Ionin, Khim. Prom-st., 90(3), 128 – 132 (2013).
V. V. Belakhov, Yu. D. Shenin, and B. I. Ionin, in: Development Prospects for Scientific Research in the 21 st Century, Proceedings of the First International Scientific-Practical Conference [in Russian], Pero, Moscow (2013), pp. 33 – 38.
V. V. Belakhov, Khim. Prom-st., 91(2), 104 – 108 (2014).
V. V. Belakhov, A. V. Garabadzhiu, and V. A. Kolodyaznaya, Bull. S.-Petersb. Inst. Technol., No. 30, 31 – 41 (2015).
G. Medoff, J. Brajtburg, and G. S. Kobayashi, Annu. Rev. Pharmacol. Toxicol., 23, 303 – 330 (1983).
D. Ellis, J. Antimicrob. Chemother., 49(S1), 7 – 10 (2002).
F. C. Odds, A. J. P. Brown, and N. A. R. Gow, Trends Microbiol., 11(6), 272 – 279 (2003).
M. Baginski, K. Sternal, J. Czub, and E. Borowski, Acta Biochim. Pol., 52(3), 655 – 658 (2005).
J. Czub and M. Baginski, J. Phys. Chem., 110, No. 33, 16743 – 16753 (2006).
M. Baginski, B. Cybulska, and W. I. Gruszecki, in: Advances in Planar Lipid Bilayers and Liposomes, 3, Chap. 9, A. L. Liu (ed.), Elsevier, Oxford, UK (2006), pp. 269 – 329.
A. A. Samedova and Kh. M. Kasumov, Antibiot. Khimioter., 54(11–12), 44 – 52 (2009).
Kh. M. Kasumov, Structure and Membrane Function of Polyene Macrolide Antibiotics [in Russian], Nauka, Moscow (2009).
K. Hac-Wydro and P. Dynarowicz-Latka, Colloids Surf., B, 53(1), 64 – 71 (2006).
D. M. Kaminski, Eur. Biophys. J., 43(10–11), 453 – 467 (2014).
T. Yamamoto, Y. Umezawa, H. Tsuchikawa, et al., Bioorg. Med. Chem., 23(17), 5782 – 5788 (2015).
P. Kovacic and A. Cooksy, MedChemComm, 3(3), 274 – 280 (2012).
Y. Nakagawa, Y. Umegawa, N. Matsushita, et al., Biochemistry, 55(24), 3392 – 3402 (2016).
M. N. Preobrazhenskaya, E. N. Olsufyeva, S. E. Solovieva, et al., J. Med. Chem., 52(1), 189 – 196 (2009).
B. Trygve, S. Havard, K. F. Degnes, et al., Appl. Environ. Microbiol., 77(18), 6636 – 6643 (2011).
A. N. Tevyashova, E. N. Olsufyeva, S. E. Solovieva, et al., Antimicrob. Agents Chemother., 57(8), 3815 – 3822 (2013).
G. F. Luger, Artificial Intelligence: Structures and Strategies for Complex Problem Solving, 6th Ed., Pearson Education Inc., Boston, USA (2009).
D. L. Poole and A. K. Mackworth, Artificial Intelligence: Foundations of Computational Agents, Cambridge University Press, New York (2010).
P. Ponce, A. M. Gutierrez, and J. Rodriguez (eds.), New Applications of Artificial Intelligence, InTech, Rijeka, Croatia (2016).
S. Rassel and P. Norvig, Artificial Intelligence. Modern Approach [in Russian], Vil?yams, Moscow (2015).
I. G. Sidorkina, Artificial Intelligence Systems [in Russian], KnoRus, Moscow (2011).
V. K. Finn, Artificial Intelligence. Methodology of Application and Philosophy [in Russian], Krasand, Moscow (2011).
I. E. Bulakh, Yu. E. Lyakh, V. P. Martsenyuk, and I. I. Khaimzon, Medical Informatics [in Russian], Meditsina, Moscow (2012), 426 pp.
L. S. Bolotova, Artificial Intelligence Systems. Knowledge- Based Models and Technologies [in Russian], Finansy i Statistika, Moscow (2012).
I. P. Korolyuk, Medical Informatics, OOO Ofort, Samara (2012).
B. A. Kobrinskii and T. V. Zarubina, Medical Informatics, Akademiya, Moscow (2013).
V. P. Omel’chenko and A. A. Demidova, Medical Informatics [in Russian], GEOTAR-Media, Moscow (2016).
P. P. Zotov, I. S. Kritsul, and I. M. Mikhalevich, Vrach Inf. Tekhnol., No. 1, 48 – 56 (2014).
M. A. Taranik and G. D. Kopanitsa, Vrach Inf. Tekhnol., No. 3, 6 – 12 (2014).
I. P. Lukashevich, K. V. Stepanyan, A. K. Popov, and R. Sh. Balugyan, Vrach Inf. Tekhnol., No. 2, 6 – 11 (2015).
B. A. Korbinskii, in: Proceedings of the 15 th National Conference on Artificial Intelligence with International Participation [in Russian], Vol. 2, Universum, Smolensk (2016), pp. 259 – 264.
T. B. Chistyakova, Yu. I. Shlyago, I. V. Novozhilova, and N. V. Mal’tseva, Intelligent Systems for Technology Design, Control and Training in Multi-facetted Production of Granulated Porous Materials from Disperse Particles, Ser.: Information Technology in the Chemical Industry [in Russian], Izd. SPbGTI(TU), St. Petersburg (2012).
T. B. Chistyakova, I. A. Smirnov, and V. V. Belakhov, in: Mathematical Methods in Engineering and Technology (MMTT-29), Collection of Works of the XXIXth International Scientific Conference, Saratov State Technical University [in Russian], St. Petersburg (2016), pp. 173 – 176.
V. V. Belakhov, A. V. Garabadzhiu, T. B. Chistyakova, et al., in: Proceedings of the 82 nd Annual Meeting of the Israel Chemical Society, Tel-Aviv, Israel (2017), p. 107.
V. V. Belakhov, T. B. Chistyakova, A. V. Garabadzhiu, et al., in: Modern Mycology in Russia, Proceedings of the IVth Convention of Mycologists in Russia [in Russian], XVI, National Academy of Mycology, Moscow (2017), pp. 214 – 216.
T. B Chistyakova, R. V. Makaruk, E. E. Musayev, and V. V. Belakhov, in: Proceedings of the XXth International Conference on Soft Computing and Measurements, St. Petersburg Electrotechnical University, St. Petersburg (2017), pp. 516 – 518.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 11, pp. 14 – 26, November, 2018.
Rights and permissions
About this article
Cite this article
Belakhov, V.V., Garabadzhiu, A.V. & Chistyakova, T.B. Polyene Macrolide Antibotic Derivatives: Preparation, Overcoming Drug Resistance, and Prospects for Use in Medical Practice (Review). Pharm Chem J 52, 890–901 (2019). https://doi.org/10.1007/s11094-019-01922-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-01922-3