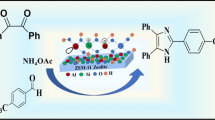
Azeolite ZSM-5 catalyzed simple, one-pot solvent-free, cost-effective and environmental benign protocol for the synthesis of dihydropyrimidines (DHPMs) have been developed. Aseries of substituted 1,4-DHPM derivatives have been synthesized by one-pot Biginelli type cyclocondensation of ethylacetoacetate, aromatic/heteroaromatic aldehydes, and urea/thiourea with a catalytic amount of zeolite under solvent-free protocol in a sufficiently high yield. The catalyst is recyclable and can be used repeatedly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyrimidine, being an important part of DNA and RNA, leads to a huge area of biologically active compounds, and their potent inhibitory properties regarding the tyrosine kinase domain of epidermal growth factor receptor [1], 5-phosphoribosyl-1-pyrophosphate synthetase [2], and dihydrofolate reductase [3] have been fully demonstrated. Vast research has been made to investigate the antitumor [4], antiviral [5], antioxidant [6], antifungal [7], and hepatoprotective [8] activities of these compounds. As a result, a number of reports have appeared in the literature that usually describe forcing conditions, long reaction times, and complex reaction pathways [9]. Thus, new routes for the synthesis of these molecules have attracted considerable attention by allowing for a rapid entry to these heterocycles.
Multi-component reactions (MCRs) [10] are economically and environmentally very advantageous because multi-step syntheses produce considerable amounts of waste mainly due to complex isolation procedures often involving costly, toxic, and harmful solvents after each step. The development of one-pot reaction has been of great interest in organic synthesis because this methodology provides easy access to highly complex molecules from relatively simple reagents under economically favorable reaction conditions. Thus, one-pot strategy with the use of environmentally friendly zeolite catalysts becomes a powerful means of preparation of specific target compounds to minimize pollutants and to reduce production cost [11]. Zeolites, used as an important class of catalysts for various organic synthesis processes, have received considerable attention in the past decade due to their characteristic properties such as acidity, thermal stability, and environmentally friendly work-up. Zeolites are widely used as heterogeneous catalysts in the petroleum refining and chemical industries [12] as well as in the preparation of fine chemicals [13]. Main advantages of acidic zeolites over homogeneous acid catalysts are that they are noncorrosive and nonpolluting. The dihydropyrimidinone moiety and its derivatives are an important class of heterocyclic compounds, as they are present in a variety of natural products and have a broad range of biological activities. They have attractive therapeutic and pharmacological properties [14, 15]. Synthesis of these moieties by the Biginelli reaction over various homogeneous catalysts such as polyphosphate ester [16], LaCl3 · 7H2O [17], and LiClO4 [18] were reported. A heterogeneous catalyst such as KSF (montmorillonite) [19, 20] can be used, but its reusability is limited to few runs only. A single-step liquid phase cyclocondensation reaction with aromatic aldehydes, β-ketoesters, and urea in toluene over zeolites (HY, HZSM-5 and MCM-41) with high yields and selectivity under reflux conditions has been reported [21]. The HY catalyst furnished higher yield for aliphatic aldehydes, but normally shows extremely poor yields in the Biginelli reaction [22].
As a part of work aimed to develop simple and environmentally compatible synthetic methodologies, we have explored the catalytic activity of zeolite ZSM-5 for organic synthesis. Herein we report the synthetic utility of zeolite as a catalyst for a one-pot Biginelli reaction of the aromatic aldehydes, ethylacetoacetate/methylacetoacetate, and urea/thiourea to give the corresponding dihydropyrimidines (DHPMs) (Schemes 1 and 2).
To separate the catalyst from the product, the mixture was treated with hot ethanol and filtered. The residue, being a catalyst, was dried and reused.
Experimental Chemical Part
Melting points of all the synthesized compounds were recorded by using digital melting point apparatus (Model EQ-730). Purity was checked by thin layer chromatography (TLC) using silica gel 60 F 254, ethyl acetate – petroleum ether mixture as a mobile phase, and UV cabinet (Model BTI-49) for spot visualization. 1H NMR spectra were recorded by CDRI Lucknow on Bruker DRX 300 in CDCl3 at 300 MHz.
Typical Procedure for the Synthesis of Substituted Pyrimidine Derivatives (4a – 4h)
A mixture of aromatic aldehydes (4.71 mmol), ethylacetoacetate/methylacetoacetate (4.71 mmol), urea/thiourea (7.07 mmol) and catalyst zeolite ZSM-5 (10 wt%) in relation to the amount of aldehydes used was heated at 50°C for 10 – 25 min. Completion of the reaction was determined on TLC plates using ethyl acetate – petroleum ether mixture as a mobile phase. The reaction mixture was cooled to room temperature, poured into crushed ice, and stirred for 5 – 10 min. The solid precipitate was filtered and washed with ice cold water. After separation of the catalyst from the product, it was purified by column chromatography using ethyl acetate and petroleum ether as eluent. The compound purified by column chromatography was sufficiently pure for recording spectra. Physical characteristics of compounds 4a – 4h are given in Table 1.
Spectroscopic characteristics of DHPMs are as follows:
Methyl 6-(4-chlorophenyl)-4-methyl-2-oxo-1,3,6-trihydropyrimidine-5-carboxylate (4a). IR (νmax, cm–1): 3368.16 (N–H); 3159.31 (N–H); 1699.77 (C=O); 1590.27 (v; =C–H and ring C=C str. vib.); 1466.22 (C=C str. vib.); 1406.45 (sym. CH3 def. vib. in O–CH2–CH3); 1204.28 (C–O–C str. vib.); 1H NMR (300 MHz; δ, ppm): 1.254 (t, CH3); 2.634 (s, CH3); 7.821 (s, N–H); 7.849 (s, N–H); 8.040 (d, Ar–H); 8.071(d, Ar–H).
Methyl 6-(4-chlorophenyl)-4-methyl-2-thioxo-1,3,6-trihydropyrimidine-5-carboxylate (4b). IR (νmax, cm–1): 3399.14 (N–H); 3149.31 (N–H); 1699.70 (C=O); 1580.27 (v; =C–H and ring C=C str. vib.); 1450.22 (C=C str. vib.); 1300.45 – 1214.38 (C–O–C str. vib.); 1H NMR (300 MHz; δ, ppm): 1.254 (s, CH3); 2.311 (s, CH3); 7.468 (d, N–H); 7.263 (s, N–H); 7.079 (s, CH); 7.850 (dd, Ar–CH); 8.045 (dd, Ar–CH).
Ethyl 4-methyl-6-[2-phenylvinyl]-2-oxo-1,6-dihydro-5-pyrimidinecarboxylate (4c). IR (νmax, cm–1): 3233.34 (N–H); 3100.10(N–H); 2975.59 (C–H) ;1697.89 (C=O); 1559.27 (v; =C–H and ring C=C str. vib.); 1444.88 (C=C str. vib.); 1366.93 – 1286.01 (C–O–C str. vib.); 1088.55 – 966.86 (C=C str. vib.); 1H NMR (300 MHz; δ, ppm): 1.414 (t, CH3); 2.232 (s, CH3); 4.176 (q, CH2); 5.007 (s, N–H); 5.664 (s, N–H); 6.164 (dd, CH=CH); 6.514 (d, CH); 7.18 – 572 (m, Ar–H).
Ethyl 2-hydroxy-6-(4-methoxyphenyl)-4-methyl-1,6-dihydropyrimidine-5-carboxylate (4d). IR (νmax, cm–1): 3336.16 (N–H); 3226.06 (N–H); 2966.97 (C–H); 1721.27 (C=O); 1596.93 (v; =C–H and ring C=C str. vib.); 1456.27 (C=C str. vib.); 1366.05 – 1275.28 (C–O–C str. vib.); 1117.01 – 1083.35 (–CH3 str. vib.); 1H NMR (300 MHz; δ, ppm): 1.318 (t, CH3); 1.507 (s, CH); 2.404 (s, CH3); 3.834 (s, O-CH3); 3.970 (q, CH2); 5.341 (s, N–H); 6.812 (s, N–H); 7.025 (dd, Ar–H); 7.861 (dd, Ar–H).
Ethyl 4-methyl-6-[2-phenylvinyl]-2-sulfanyl-1,6-dihydro-5-pyrimidinecarboxylate (4e). IR (νmax, cm–1): 3359 (N–H); 3254 (N–H); 3136 (C–H); 1457 (v; =C–H and ring C=C str.vib.); 1407 (C–O–C str. vib.); 1078 (C=C str. vib.).
Ethyl 4-(4-methoxyphenyl)-6-methyl-2-sulfanyl-1,4-dihydropyrimidine-5-carboxylate(4f). IR (νmax, cm–1): 3444 (N–H); 3227 (N–H); 1721 (C=O); 1511 (v; =C–H and ring C=C str. vib.).
Ethyl 2-hydroxy-6-methyl-4-(4-nitrophenyl)-1,4-dihydropyrimidine-5-carboxylate (4g). IR (νmax, cm–1); 3335 (N–H); 3226 (N–H); 1721 (C=O); 1510 (v; =C–H and ring C=C str.vib.); 1336 (C–O–C str. vib.).
Ethyl 4-methyl-6-(4-nitrophenyl)-2-thioxo-1,3,6-trihydropyrimidine-5-carboxylate (4h). IR (νmax, cm–1): 3643 (N–H); 3233 (N–H); 1697 (C=O); 1449 (v; =C–H and ring C=C str.vib.); 1089 (C=C str. vib.).
Results and Discussion
Synthesis of the title compounds have been carried out by using Biginelli reaction in a mixture of an aromatic/ heteroaromatic aldehyde (1 mmol), ethyl acetoacetate (1 mmol), urea/thiourea (1.5 mmol), and ZSM-5 (10 wt%), which was stirred at room temperature for 10 min. (the progress of the reaction was checked by TLC). The solid product obtained was extracted with hot ethanol and, upon concentrating the extract, the product was obtained in low yield (45%). It was found that, even after stirring the reaction mixture for a long time, there is no satisfactory increase in the yield. Hence, attempts have been made to heat the reaction mixture at 50°C for a longer period of tme (with TLC check). Following the above workup procedure, the product was obtained in 98% yield.
This methodology was effective for sensitive aromatic aldehydes such as anisaldehyde, cinnalmaldehyde, etc. in the synthesis of dihydropyrimidones with uniformly high yields (Table 1).
The structural variations in the aldehydes employed in the reaction did not affect the reaction yield, which remained high everytime. Therefore the significance of the use of zeolite consists in that neither the course nor the yield of the reaction was affected by the use of sensitive functional groups like Cl, OCH3 , C6H7, NO2 and conjugated double bonds.
The optimization study using different catalysts with variable reaction time, temperature and yield has been carried out and the results are given in Table 2. From the optimization study, it was found that, among various acidic/basic/inorganic supported catalysts used for the synthesis ofDHPMs via Biginelli reaction, ZSM-5 zeolite used in this present study is an efficient, green and eco-friendly catalyst that ensures high product yield at low temperature without solvent.
The most important and salient feature of the present reaction is the recyclability of the catalyst and the scleability of the reaction. Another exclusive feature of the reaction is that the catalyst could be reused without any loss in its original activity. This was supported by the fact that the use of the recycled catalyst in the reaction had affected neither the yield nor the quality of the product. Moreover, there is no formation of any side product in the reaction. Hence, we have developed an efficient and green procedure for the synthesis of DHPMs, which follows almost all the principals of green chemistry.
References
G.W. Rewcastle, A. J. Bridges, D.W. Fry, et al., J. Med. Chem., 40, 1820 (1997).
D.W. Fry, M. A. Becker, and R. L. Switzer, Mol Pharmacol, 47, 810 (1995).
J. E. Gready, C. McKinlay, and M. G. Gebauer, Eur. J. Med. Chem., 38, 719 (2003).
Y. S. Sanghhvi, S. B. Larson, S. S. Matsumoto, et al., J. Med. Chem., 32, 629 (1989).
R. B. Tenser, A. Gaydos, and K. A. Hay, Antimicrob. Agents Chemother., 45, 3657 (2001).
J. P. De la Cruz, T. Carrasco, G. Ortega, and Sanchez De la Cuesta, Lipids, 27, 192 (1992).
Nizamuddin, M. Mishra, M. K. Srivastava, and M. H. Khan, Indian J. Chem. B, 40, 49 (2001).
V. J. Ram, A. Goel, S. Sarkhel, and P. R. Maulik, Bioorg. Med. Chem., 10, 1275 (2002)
(a) K. Hirota, J. Huang, H. Sajiki, and Y. Maki, Heterocycles, 24, 2293 (1986); (b) R. Niess and R. K. Robins, J. Heterocycl. Chem., 7, 243 (1970); (c) K. Hirota, H. Kitade, H. Sajiki, and Y. Maki, Synthesis, 589 (1984); (d) M. Gohain, D. Prajapati, B. J. Gogoi, and J. S. Sandhu, Synlett, 1179; (e) J. L. Bernier, A. Lefebvre, C. Lespognol, et al., Eur. J. Med. Chem., 12, 341 (1977); (f) D. Prajapati and A. J. Thakur, Tetrahedron Lett., 46, 1433 (2005)
(a) A. Domling and I. Ugi, Angew. Chem. Int Ed., 39, 3168 (2000); (b) I. Ugi and A. Domling, Endeavour, 18, 115 (1994); (c) S. Heck and A. Domling, Synlett, 424 (2000).
(a) S. Balalaie and A. Arabanian,, Green Chem., 2, 274 (2000); (b) W. F. Hoelderich, Appl. Catal. A, 194, 487 (2000); (c) R. Sreekumar, R. Padmakumar, and P. Rugmini, Tetrahedron Lett., 39, 2695 (1998); (d) H. Zhang, H. Li, G. Lin, et al., Stud. Surf. Sci. Catal., 101, 1369 (1996); (e) A. J. Hoefnagel and H. Van Bekkum, Appl. Catal. A, 97, 87 (1993).
(a) D. W. Breck,, Zeolite Molecular Sieves, Wiley, New York (1974); (b) A. Dyer, An Introduction to Zeolite Molecular Sieves, Wiley, Chichester (1988).
(a) W. Hölderich, M. Hesse, and F. Näumann, Angew. Chem. Int. Ed., 27, 226 (1988); (b) K. Smith, Stud. Surf. Sci. Catal., 59, 55 (1991).
G. C. Rovnyak, S. D. Kimall, B. Beyer, et al., J. Med. Chem., 38, 119 (1995).
K. S. Atwal, G. C. Rovnyak, S. D. Kimball, et al., J. Med. Chem., 33, 2629 (1990).
H. Cho, M. Ueda, K. Shima, et al., J. Med. Chem., 32, 2399 (1989).
J. J. Baldwin, D. A. Claremon and D. E. McClure, US Patent No. 4609494 (1986).
J. J. Baldwin, S. M. Ptizenberger, and D. E. McClure, US Patent No. 4675321 (1987); K. S. Atwal, US Patent No. 4684656 (1987).
C. O. Kappe and S. F. Falsone, Synlett, 718 (1998).
J. Lu, Y. Bai, Z. Wang, et al., Tetrahedron Lett., 41, 9075 (2000).
J. S. Yadav, B. V. S. Reddy, R. Srinivas, et al., Synthesis, 1341 (2001).
F. Bigi, S. Carloni, B. Fraullanti, et al., Tetrahedron Lett., 40, 3465 (1999).
T. Boumoud, B. Boumoud, S. Rhouati, et al., E-Journal of Chemistry, 5(4), 688 – 695 (2008).
J. Lu, Y. Bai, Z. Wang, et al., Tetrahedron Lett., 41, 9075 – 9078 (2000).
M. B. Deshmukh, P. V. Anbhule, S. D. Jadhav, et al., Indian J. Chem. B, 46(9), 1545 – 1548 (2007).
D. Shobha, M. A. Chari, P. Sadanandam, and K. Mukkanti, J. Heterocycl. Chem., 45, 1225 – 1227 (2008).
N. Y. Fu, M. L. Pang, Y. F. Yuan, and J. T. Wang, Chinese Chem. Lett., 10, 921 – 922 (2002).
B. Gangadasu, P. Narender, B. C. Raju, and V. J. Rao, Indian J. Chem. B, 45(6), 1259 – 1263 (2006).
G. Aridoss and Y. T. Jeong, Bull. Korean Chem. Soc., 31, 868 (2010).
M. A. Pasha, N. R. Swamy, and V. P. Jayshankara, Indian J. Chem. B, 44(3), 823 – 826 (2005).
S. L. Jain, V. B. Sharma, and B. Sain, J. Heterocycl. Chem., 43(3), 777 – 779 (2006).
I. Suzuki, Y. Suzumura, and K. Takeda, Tetrahedron Lett., 47, 7861 – 7864 (2006).
T. Jin, S. Zhang, and T. Li, Synth. Comm., 32, 1847 – 1851 (2002).
Acknowledgements
Authors are thankful to the Department of Chemistry, Jankidevi Bajaj College of Science, Wardha for the support to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajaj, S.D., Mahodaya, O.A. & Tekade, P.V. ZSM-5 Catalyzed Solvent Free Ecofriendly Synthesis of Substituted Pyrimidine Derivatives. Pharm Chem J 48, 679–682 (2015). https://doi.org/10.1007/s11094-015-1170-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1170-7