A series of quaternary salts of adamantane-containing alkoxydialkylaminopropanols was synthesized. The structures of the reaction products were confirmed by IR and PMR spectroscopy. Some compounds were found to have high levels of antimicrobial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We have previously shown that a number of quaternary salts of adamantane-containing aminopropanols have antimicrobial activity [1].
With the aim of seeking new antimicrobial compounds, we have synthesized several quaternary salts of alkoxydialkylaminopropanols in which the alkoxy groups are adamantane substituents.
The initial 1-adamantylglycidyl ether was prepared as described in [2], and the 1-(4-(1-adamantyl)phenoxy)glycydyl ether was synthesized using the Williamson reaction (KCO, acetone, epichlorhydrin).

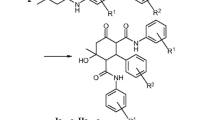
R = 4-(1-adamantyl)phenyl (I – X). R',R" = (CH2)2O(CH2)2, R'" = CH3, I– (I);R',R" = (CH2)5, R'" = CH3, I– (II); R',R" = (CH2)5, R'" = CH2C6H5, Cl– (III);R',R" = (CH2)2O(CH2)2, R'" = 4-NO2-C6H4CH2-, Cl – (IV);R' = CH3, R" = cyclo-C5H11, R'" = CH3, I–(V);R',R" = (C2H5), R'" = CH3, I– (VI);R', R" = (C2H5)2, R'" = CH2C6H5, Cl –(VII); R',R" = (CH2)4, R'" = CH3, I– (VIII); R',R" = (CH2)4, R'" = C2H5, I–(IX);R', R" = (CH3)2, R'" = CH3, I– (X).R = 1-adamantyl (XI – XIII). R', R" = (CH2)2O(CH2)2, R'" = CH3, I– (XI);R',R" = (CH2)5, R'" = CH3, I– (XII); R',R" = (C2H5)2, R'" = CH3, I– (XIII).Please insert scheme labeled (A) from R.p. 21.
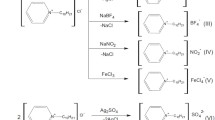
Alkoxydialkylaminopropanols were synthesized by interaction of epoxides with a 1.5-fold excess or an equimolar quantity of the corresponding secondary amine with heating in propan-2-ol. Excess regents were evaporated in vacuo and residues were dissolved in acetone or nitromethane; alkyl halide (CH3I, C2H5I, C6H5CH2Cl, p-NO2-C6H4CH2Cl) was added to 5% excess, and reaction mixtures were heated for 10 h to yield the corresponding quaternary salts of alkoxydialkylaminoalcohols.
Products were white or slightly yellow substances, soluble in water, ethanol, DMSO, and DMF. Elemental analysis data were consistent with expected values. Yields and melting temperatures are given in Table 1.
The PMR spectra contained typical proton signals for adamantane radicals (Ad, 1.57 – 2.09 ppm), a doublet of doublets from the CH2 group of the benzyl fragments (4.78 – 5.14 ppm), and a group of lines from aromatic protons (6.90 – 7.26 ppm).
The IR spectra (KBr, films) of the study compounds had characteristic absorption bands from hydroxyl groups at 3500 – 3200 and 2975 – 2845 cm–1 (CH2, CH3), oscillations of the ether bond at 1150 – 1100 cm–1, and absorption bands from the NO2 group at 1540 – 1510 and 1360 – 1340 cm–1 (compound IV).
Experimental chemical section
PMR spectra were recorded using a Varian VXP instrument with a working frequency of 299.945 MHz in DMSO-d6 and TMS as the internal standard. IR spectra were taken using a UR-20 instrument in KBr tablets.
1-(4-(1-Adamantyl)phenoxy)-3-(N-methylmorpholini um)-2-propanol iodide (I). A mixture of 1.85 g (0.005 mol) of 1-(4-adamantyl)phenoxy)-3-morpholino-2-propanol and 0.75 g (0.00525 mol) of methyl iodide in 10 ml of acetone was boiled for 10 h. After cooling, the reaction mixture was supplemented with 5 ml of dry diethyl ether and kept at +5°C for 6–8 h. The resulting precipitate was collected by filtration, washed with diethyl ether, and dried. The yield was 1.72 g (67%) (I). The melting temperature was 215 – 216°C.
Other quaternary salts (compounds II, V, VI, VIII – X) were prepared in the same way.
1-(4-(1-Adamantyl)phenoxy)-3-(N-benzylpiperidiniu m)-2-propanol chloride (III). A mixture of 1.84 g (0.00525 mol) of 1-(4-(1-adamantyl)phenoxy)-3-piperidino2-propanol and 0.66 g (0.00525 mol) of benzyl chloride in 5 ml of nitromethane was heated to 100°C for 10 h. The reaction mixture was evaporated in vacuo and the residue was crystallized from a mixture of acetone and diethyl ether. The yield was 1.33 g (52%) (III). Compounds IV and VII were prepared in the same way.
Experimental biological section
Antimicrobial and antifungal activities were assessed by microdilutions in liquid Mueller-Hinton and Sabouraud media. Antimicrobial activity was determined using Gram-positive (Staphylococcus aureus ATCC 25923) and Gram-negative (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853) bacteria. Antifungal activity was evaluated using the yeast-like fungus Candida albicans NCTC 885/653. Bacteria and fungi were grown on meat-peptone agar and solid Sabouraud medium respectively. Inoculum density was 106 cfu (colony-forming units) or 106 fungal elements per ml of growth medium. Cultures were incubated at 35 – 37°C for 18 – 24 h (bacteria) or 30 – 35°C for 24 – 48 h (fungi). Each experiment was performed in at least three repeats and included controls (for growth medium sterility and culture growth). The activities of the compounds were evaluated in terms of the minimum inhibitory concentration (MIC), i.e., the maximum dilution of substance at which there was no microbial growth.
Among the study compounds, II – VI and VIII – X had relatively high antimicrobial activity against Gram-positive bacteria (S. aureus), while compounds I, II, IV, V, IX, and X were active against Gram-negative bacteria (E. coli) (Table 2). Compounds I, II, IV – VI, and VIII – X also had marked antifungal activity (Table 3).
References
Ukrainian patent No. 42596 A; Byul. Izobret., No. 9 (2001).
A. K. Shiryaev, I. K. Moiseev, E. I. Boreko, et al., Khim.-Farm. Zh., 24(5), 23 – 25 (1990).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 1, pp. 21 – 23, January, 2011.
Deceased.
Rights and permissions
About this article
Cite this article
Korotkii, Y.V., Vrynchanu, N.A., Maksimov, Y.N. et al. Synthesis and antimicrobial and antifungal activities of quaternary salts of adamantane-containing alkoxydialkylaminopropanols. Pharm Chem J 45, 19–21 (2011). https://doi.org/10.1007/s11094-011-0552-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-011-0552-8