Abstract
Cold atmospheric pressure plasma treatment is currently being explored as an alternative way to improve the germination and growing parameters of plant seeds. However, it is important to pay attention to the effect of plasma treatment on DNA damage of the seeds as well as detailed characteristics of plasma composition and parameters. The aim of this work was to study the DNA damage of plasma-treated pea seeds (Pisum sativum L.) and plasma parameters such as the chemical composition of plasma gaseous compounds and plasma radiation. Seeds were treated with plasma using the diffuse coplanar surface barrier discharge generated in different working gases (ambient air, nitrogen, oxygen and different mixtures of oxygen and nitrogen) at atmospheric pressure and at 60 s, 180 s and 300 s exposure times. DNA damage was studied using the single cell-gel electrophoresis called the comet assay and the plasma parameters were investigated by Fourier transform infrared spectroscopy and optical emission spectroscopy. Experiments in different ratios of oxygen and nitrogen were realized in order to understand the reaction mechanism between the ambient air plasma and the treated seeds. Based on our results, ambient air plasma appears to be the most advantageous for the plasma treatment due to no significant DNA damage because of the proper combination of plasma composition in combination with water vapor present in ambient air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Low-temperature plasma (LTP) generated at atmospheric pressure, often referred in literature as cold atmospheric pressure (CAP) plasma, is currently frequently used for the surface modification of different materials. This is because plasma generally represents a low-cost and environmentally friendly alternative to the usual way using harmful chemicals. In addition, CAP plasma is generated without the need of low-pressure equipment, and ambient air can be used as a working gas. Plasma technology based on CAP devices is also appropriate considering the increased demands for cost reduction and environmental protection [1,2,3].
CAP plasma consists of several different kinds of reactive species, electrons, ions, UV radiation, radicals and metastable particles and various gaseous products of elemental reactions in plasma. Non-equilibrium state of CAPP means that heavy particles like ions and neutrals have low temperatures and do not damage the surface of the material. Electrons have enough energy to enter into different plasma-chemical reactions. The interaction of plasma particles with the surface can cause changes in the surface energy. It leads to increased surface adhesion, and removes organic or inorganic contamination [4,5,6,7,8]. Nowadays, due to the confirmed sterilization and disinfection effects of plasma [9,10,11], increased attention is focused on research into plasma applications in medicine and agriculture [12,13,14,15]. One of the rapidly increasing areas of plasma application is the treatment of biomaterials such as plant seeds, crops, cereals, spices and many others. Traditionally, biomaterials are chemically treated for protection from various fungi and pathogens on the surface and for increasing germination and growing parameters. These methods are advantageous for improving material properties but also have some adverse effects, being harmful to the environment. CAP plasma treatment could be used as an alternative way of improving material properties. For example, plasma inhibits growth of microorganisms on the seed surface and improves germination and growing parameters or vigor of seedlings [16,17,18,19,20,21]. Although much scientific work has been published in recent years, the interaction of plasma and biological material is still under investigation by researchers from various scientific fields. Cooperation between physicists and biologists, plant physiologists and geneticists is needed to better understand the effect of plasma on biological material, because it is a very complicated system. It is necessary to investigate many aspects of interaction from the point of view of botany, physics, genetics, molecular biology [18,19,20,21,22,23,24]. Possible effects of plasma components on DNA damage in germinated plants, changes in enzyme activities or changes in surface properties are studied.
Another important goal is to better understand the chemical and physical plasma processes and interaction processes between plasma and the treated material. The physical impact of plasma components on the surface of the treated material can be monitored using various physical diagnostic methods that characterize the surface in detail, such as changes in surface energy by measuring the water contact angle (WCA), changes in chemical bonding on the surface by attenuated total reflectance—Fourier transform infrared spectroscopy (ATR-FTIR), changes in surface morphology by scanning electron microscopy (SEM) [19]. The obtained results should be correlated with plasma characteristics. Plasma is usually characterised by different methods, among which the most usual are electrical measurements of plasma parameters with interpretation of time development of current and voltage waveforms, mass spectrometry and optical emission spectroscopy [25].
It is also the aim of this work to study plasma in content with the seed parameters especially chemical and radiation composition of the plasma related to the impact of plasma on the level of DNA damage of pea seeds. Our work is focused on two types of diagnostics methods. One of them is optical emission spectroscopy (OES) and the other is Fourier transform infrared (FTIR) spectroscopy. Plasma was generated by a DCSBD plasma source which can produce macroscopically homogeneous and diffuse plasma at atmospheric pressure in ambient air and in conventional gases like nitrogen, oxygen and their mixtures.
OES is a very common method for characterising plasma from the aspect of radiation, its composition and for determination of plasma parameters such as vibrational and rotational temperatures or densities of excited species. Many works often used the OES to study also low-temperature plasma [26,27,28,29]. The most observable systems in ambient air plasmas are the second positive system of nitrogen, first positive system of nitrogen, first negative system of nitrogen, system of hydroxyl radical and nitric oxide system [30].
The chemical composition of the gaseous products of LTP plasma was studied in many works by FTIR spectroscopy [31,32,33]. The gas of low-temperature plasma generated in air is typically composed of reactive oxygen and nitrogen species such as NO2, N2O, NO, O3, HNO3, HNO2, CO2.
As was mentioned, plasma is a very complex system which contains a lot of species in different states, electrons or photons. Many different reactions occur between the individual compounds in plasma or with the sample. In this work, we try to explain the mechanism of interaction between the plasma and the treated material partially by investigating the relationship between the results of the DNA damage of plasma treated pea seeds and plasma diagnostics. Plasma was generated in ambient air, which is used for the treatment of seeds, and a mixture of nitrogen and oxygen in various ratios.
Materials and Methods
Plasma Source and Plasma Treatment
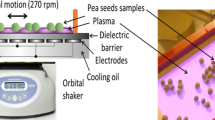
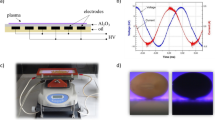
In our work, diffuse coplanar surface barrier discharge [34] as a source of low-temperature plasma was used for the plasma treatment of seeds and consequently for the diagnostics. Many parallel electrodes, 20 cm long and 1.5 mm wide, were situated from the bottom side of the ceramics in a mutual distance of 1 mm and cooled effectively by mineral oil cooling system. The temperature of the ceramics surface was not higher than 55–60 °C. Plasma was created as a thin layer of 0.3 mm effective thickness on the ceramics surface (Al2O3) (Fig. 1). The generation of plasma was performed at the input power of 400 W by the high voltage sinusoidal signal of 20 kV peak-to-peak and frequency of 14 kHz. Plasma was generated in ambient air and in different synthetic gases such as nitrogen, oxygen and different ratios of oxygen to nitrogen (O2:N2 = 20:80; 40:60; 60:40; 80:20) at atmospheric pressure. The humidity of ambient air was at the level of 40–50%.
Dried pea seeds (Pisum sativum L.) var. Prophet obtained from the Central Control and Testing Institute in Agriculture in Bratislava, Slovakia were used in this work as a biological material for the plasma treatment and to study the DNA damage. The seeds were stored in the dark at 23 °C. During the plasma treatment process the seeds were put into the plasma generated in different working gases for 60 s, 180 s, and 300 s exposures. These exposure times were selected according to a previous study by Kyzek et al. [24]. The number of seeds per one plasma treatment was in range 15–20 pieces, depending on planned number of biological experiments in one set. The orbital shaker moved the discharge panel and thus ensured the rotating of the seeds for the purpose of a homogeneous treatment. The seed´s temperature was checked immediately after switching off the plasma, it was not higher than 40 °C.
In case of ambient air, the treatment was realized without the cover and the seeds were put into the plasma at the input power of 400 W. For other treatments (in different synthetic gases), the process has more steps. Firstly, the seeds were put on the ceramics, then covered with a plastic reactor chamber and then the chamber was purged and filled with gas with a 3 L/min flow rate. After 1 min gas flow, the orbital shaker and the plasma source were switched on and the pea seeds were treated for the required time. After the treatment, the seeds were taken from the plasma field and left in the atmosphere for 24 h before the biological experiments started according to the conditions of the experiment.
Seeding of Seeds
Before seeding, plasma treated and control seeds were left to imbibe in the sterile water for 5 h in the dark at 23 °C. After that, seeds were placed on sterile Petri dishes covered by wet filter paper and wood pulp. After 3 days of germination, seedlings were used for experiments.
Alkaline Comet Assay
The alkaline comet assay (single cell alkaline gel electrophoresis) is a method used for the measurement of DNA damage in eukaryotic cells. Cells with damaged DNA exhibit increased migration of the chromosomal DNA from the nuclei that moves from the cathode to the anode during electrophoresis. This method is applicable for detection of various DNA defects, like single-strand breaks, double-strand breaks, cross-links, apyrimidine and apurine sites [35, 36].
Our experiments were performed according to Gichner et al. [37]. Briefly, two roots for each sample were cut by a razor blade ensuring DNA release in the 150 µL of 0.4 M Tris-HCl buffer solution (pH 7.5) (Sigma-Aldrich), because of the mechanical disruption of the cell and nuclear walls. The DNA slicing and releasing were realized in the dark and on ice. After that, 100 µL of the DNA and buffer suspension was mixed with 100 µL of 1% low melting point agarose (Roth). The final solution was then placed on a slide covered by 1% normal melting point agarose (Roth) and then covered by the coverslip. The coverslips were removed after 5 min and the slides were placed in the electrophoretic chamber filled with cold electrophoretic buffer solution containing 1 mM EDTA (Sigma-Aldrich) and 300 mM NaOH (Centralchem) for 8 min. After that, electrophoresis was launched on 1 V cm−1 for 15 min at 4–8 °C. Slides were then neutralized three times with 0.4 M Tris-HCl buffer solution (pH 7.5) and stained with fluorescent dye ethidium bromide (0.05 mM, 80 µL for each slide) (Serva) for 5 min. The DNA damage was observed using fluorescent microscope OLYMPUS BX 51 with green excitation filter UMWIG3 under 400× magnification (see Fig. 2) and evaluated by Comet visual software.
Statistical Analysis
Each experiment was performed at least three times. Cold atmospheric pressure plasma treated samples were compared to plasma untreated samples using Student’s t test: *p < 0.05; **p < 0.01; ***p < 0.001.
Plasma Properties
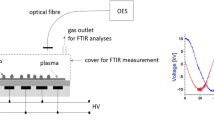
Experimental set-up for diagnostics measurements is shown in Fig. 3. OES and FTIR spectroscopy were used for characterising the DCSBD plasma which was situated in the plastic reactor chamber.
Using FTIR spectroscopy, the chemical composition of the gaseous product of the plasma was determined by the absorption of infrared radiation passed through the cuvette with gaseous plasma compounds, situated in the spectrometer. A Bruker Optics Vector 22 spectrometer in the range (4000–500) cm−1 with the resolution of 2 cm−1 and a glass cuvette of 31 cm3 volume were used. Gas from the bottle or pumped by the compressor (in case of ambient air) was flowed through a plastic tube into the reactor chamber with a 3 L/min flow rate and then into the cuvette. After the gas was flowed for 7 min, we measured background spectrum. Subsequently, we switched on the plasma at the 400 W applied input power. After a 3 min flow of gaseous products from plasma to the cuvette, we measured the absorbance spectra as a dependence of the wavenumber. Finally, we identified the characteristic peaks in spectra using the HITRAN database [38] and scientific articles.
In case of OES, radiation from the plasma was collected by an optical fibre through a round quartz glass and analysed by a Stellar Net EP-2000 spectrometer in the range (200–1100) nm. The perpendicular distance between the fibre and the ceramics was approximately 7 cm. The working gas was flowed into the reactor chamber at a flow rate of 3 L/min and the same process conditions were used as for the FTIR measurements.
Results and Discussion
Alkaline Comet Assay
For a better understanding of why the ambient air plasma is the most convenient, we realised the experiments with plasma treatment at the treatment time of 60 s, 180 s, and 300 s in different working gasses (N2; O2 and their mixtures in various ratios). We used O2 and N2 because they are the main components of ambient air. As shown in Figs. 4, 5 and 6, DNA damage of pea seedlings increased after CAP plasma treatment of seeds compared to the untreated control. Treatment with CAP plasma generated in ambient air for 60 s resulted in DNA damage increase on 24.7% (Fig. 4). The highest DNA damage in pea seedlings treated with CAP plasma for 60 s was measured using pure nitrogen as working gas (60.4%). However, with increasing concentration of oxygen and decreasing concentration of nitrogen in working gas, the DNA damage of pea seedlings decreased. Using pure oxygen for CAPP treatment resulted in DNA damage on 38.3%. The lowest DNA damage in CAPP treated samples for 180 s was measured again using ambient air as a working gas (Fig. 5). The DNA damage in this sample was on 31.5%. However, seeds treated with CAP generated in pure nitrogen for the same exposure time did not sprout, and there were no roots for comet assay analyses. With increasing concentration of oxygen and decreasing concentration of nitrogen in working gas, seeds sprouted. In the samples treated with CAPP generated in the mixture of nitrogen (80%) and oxygen (20%) DNA damage was on 59.8%. The DNA damage of pea seedlings slightly decreased with an increasing concentration of oxygen and a decreasing concentration of nitrogen in working gas on 45.1% when pure oxygen was used. Very similar results were obtained for exposure time of 300 s (Fig. 6). The lowest DNA damage was in samples treated with CAP generated in ambient air (32.5%), and seeds treated with CAPP generated in pure nitrogen did not sprout. With an increasing concentration of oxygen in the working gas, DNA damage of seedlings decreased. Treatment with CAPP generated in pure oxygen resulted in DNA damage of 46.8%. The results of our experiments suggest that CAPP generated in ambient air has the least negative effect on the DNA of pea seedlings. On the contrary, the most negative effect on pea DNA and sprouting is produced by CAP generated in pure nitrogen. Another result is the decreasing of differences in different mixtures with increasing treatment time.
DNA damage in nucleoids of pea seedlings after seeds treatment by CAPP generated in different mixtures of oxygen and nitrogen for 60 s evaluated by comet assay. NC, negative control (untreated seeds); PC, positive control (seedlings treated with H2O2); AA, seedlings treated by CAPP generated in ambient air for 60 s
DNA damage in nucleoids of pea seedlings after seeds treatment by CAPP generated in different mixtures of oxygen and nitrogen for 180 s evaluated by comet assay. NC, negative control (untreated seeds); PC, positive control (seedlings treated with H2O2); AA, seedlings treated by CAPP generated in ambient air for 180 s
DNA damage in nucleoids of pea seedlings after seeds treatment by CAPP generated in different mixtures of oxygen and nitrogen for 300 s evaluated by comet assay. NC, negative control (untreated seeds); PC, positive control (seedlings treated with H2O2); AA, seedlings treated by CAPP generated in ambient air for 300 s
Fourier Transform Infrared Spectroscopy
FTIR spectra of gaseous products of plasma are shown in Fig. 7 for ambient air and in Fig. 8 for different mixtures of oxygen and nitrogen. In case of ambient air, the spectrum is dominated by peaks related to NO2 and N2O. The most intensive peak of NO2 is in the region 1660–1560 cm−1 and N2O in the region 2265–2145 cm−1. Another smaller peak of NO2 is observable in 2935–2845 cm−1 and for N2O in 3515–3430 cm−1, 2600–2520 cm−1 and in region 1330–1210 cm−1 [38] overlapped with HNO2. The regions of 3630–3545 cm−1, 1735–1670 cm−1 and 890–760 cm−1 are also characterized by the HNO2 peaks [33] originated from the water vapor in the ambient air. NO is observable in the 1965–1770 cm−1 region [38] and can be produced by different reactions described in the work of Abdelaziz [39].
In the nitrogen spectrum (Fig. 8), small traces of N2O peaks were observable because of oxygen impurities. For mixtures of oxygen with nitrogen in ratios of O2:N2 = 20:80, 40:60, 60:40, the spectra are similar to ambient air, dominant by the NO2, N2O and NO, but with different intensities and without the presence of HNO2. Production of NO molecules is important for NxOy generation, which is described by the chemical reactions [39]:
NO2 increases with increasing ratio of oxygen and decreasing ratio of nitrogen in working gas while NO is most intensive in ambient air, less intensive for O2:N2 = 40:60 and the least intensive for the other ratios of oxygen and nitrogen. N2O has the same tendency to change intensity as the peaks of NO molecules. In case of ratio O2:N2 = 80:20, ozone O3 appears in spectrum at 3055, 2122, 1125, 1053, 785–640 cm−1 [38] and three peaks of N2O5 are visible at 1719, 1245, 737 cm−1 [40] and some small peaks of HNO3 at 1712, 1325, 878 cm−1. The same peaks of N2O5 and HNO3 are visible also in case of O2:N2 = 60:40, but less intensive. An increasing concentration of ozone with an increasing amount of oxygen could be the reason for an increasing concentration of N2O5 and HNO3 as we observed from the FTIR spectra. Production of N2O5 and HNO3 could be described by the following reaction mechanism [39, 41]:
From the DNA study and the FTIR results, we found that not only the chemical composition of plasma is important at interaction with biological material, but other influences are present as well. The indicator is the finding that in nitrogen plasma is the highest the DNA damage, but we did not record any plasma reactive species in sufficient concentration in the FTIR spectrum. The lowest DNA damage in case of different mixtures of O2 and N2 is for pure oxygen. With increasing oxygen, ozone appears and DNA damage decreases. Thus, the hypothesis that nitrogen species damage DNA is open. We noticed that proper combination of reactive oxygen and nitrogen species in combination with water vapor is the most advantageous because the best results in term of DNA damage prevention are obtained in case of ambient air plasma. The effect of humidity on the production of reactive species in plasma is described in the work [39]. Water vapor positively supports the production of NO2 and negatively influences ozone production. Thus, seed DNA is better protected from ozone and less damaged.
Optical Emission Spectroscopy
Optical emission spectra of plasma radiations are shown in Fig. 9 for ambient air and Fig. 10 for nitrogen, oxygen and different mixtures of oxygen and nitrogen. For better visibility of the observed peaks, each spectrum is divided into three parts representing three spectral regions: 185–290 nm, 290–500 nm and 500–1125 nm. The most intensive system radiating in the plasma is the second positive system of nitrogen \({\text{N}}_{2} \left( {C^{3} \Pi_{u} \to B^{3} \Pi_{g} } \right)\), whose intensity increases with increasing ratio of nitrogen and decreasing ratio of oxygen. First negative system \(\left( {B^{2} \varSigma_{u}^{ + } \to X^{2} \varSigma_{g}^{ + } } \right)\) of N2+ is visible in ambient air spectrum and in spectra of mixtures of oxygen and nitrogen, not in pure oxygen and pure nitrogen. Also, the first positive system of nitrogen \({\text{N}}_{2} \left( {B^{3} \Pi_{g} \to A^{3} \varSigma_{u}^{ + } } \right),\) with low intensity is observed in spectra of nitrogen, mixture of O2 to N2 in ratio of 20 to 80% and ambient air plasma. The NO system \(\left( {A^{2} \varSigma^{ + } - X^{2} \Pi } \right)\) is also visible in the same spectra as the first positive system of nitrogen, the most intensive NO lines are in nitrogen spectrum and with lower intensity in spectrum of admixture of O2 to N2 in ratio of 20 to 80% and ambient air plasma. Furthermore, atomic nitrogen lines are visible in the spectrum of nitrogen plasma. Vibrational temperatures of DCSBD plasma decrease with increasing ratios of nitrogen and move approximately in the level (2200–3500) K as was estimated previously for ambient air and nitrogen [42]. Rotational temperatures are approximately in the level of 400 K.
By comparing the behaviour of DNA damage and behaviour of radiation depending on working gas, we have the assumption that plasma UV radiation damages the DNA. Radiation in different ratios of oxygen to nitrogen increases with increasing nitrogen, DNA damage also increases. After longer exposure time, DNA damage increases more for higher oxygen ratios. There are two factors, UV radiation and reactive oxygen species (ROS), in the plasma participating on DNA damage. Destruction effect of UV radiation and oxygen reactive species on DNA is confirmed by the work of Gill et al. [43]. Exposure times 180 s and 300 s in case of nitrogen plasma destroy the ability to germinate, because of the long-time exposure times. UV radiation has the ability to decrease metabolism rate in photosynthesis which negatively affects the growth capacity [44]. As was deeper studied in the work of Švubová et al. [45], the longer treatment time (180 s and 300 s) in pure nitrogen plasma with the most intensive UV radiation leads to the higher production of reactive oxygen species (˙O2− and H2O2) inside the pea seeds. Thus, it causes the oxidative stress and DNA damages. In case of ambient air plasma, the radiation is similar to the case of O2:N2 = 20:80 but the DNA damage is completely different. Therefore, we can state that water vapor has some additional positive effect in reducing DNA damage.
Conclusion
This work was focused on a study the degree of DNA damage as a biological analysis of the impact of plasma on pea seeds and optical diagnostics of plasma. The study contributed to better understanding the correlation between the produced active species in plasma generated in ambient air and mixture of oxygen and nitrogen with different ratios and its influence on DNA damage. While for the improvement of germination and growth of pea seed, the exposure time up to 120 s are efficient, in presented experiments, we exceeded the optimal times (180 s, 300 s) due to detection and analysis of the potential damage of DNA by plasma. Results of this work showed that ambient air plasma is the most advantageous for plasma treatment of seeds not only for improvement of germination but also from the aspect of the lowest DNA damage. DNA of pea seeds treated by plasma was damaged more with increasing amount of nitrogen, due to much more intensive UV radiation, and also with increasing treatment time, depending on working gas, because of longer exposure of reactive oxygen species. Ambient air seems to be the most suitable because of the combination of the plasma chemical composition with water vapor. Radiation, water vapor or different reactive particles in plasma and probably their combined synergetic effect have some positive influence on the seeds. It is necessary to ensure the correct ratios of the compound for no or minimal negative effect on the seeds, which could be the ambient air with appropriate humidity. In the future, it will be useful to investigate the influence of gaseous products of plasma and the radiation from plasma separately. It would help us better understand the mechanisms participated in the beneficial bio-application utilization of atmospheric pressure plasma generated in ambient air.
References
Surowsky B, Schlüter O, Knorr D (2015) Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: a review. Food Eng Rev 7:82–108. https://doi.org/10.1007/s12393-014-9088-5
Tendero C, Tixier C, Tristant P et al (2006) Atmospheric pressure plasmas: a review. Spectrochim Acta B 61:2–30. https://doi.org/10.1016/j.sab.2005.10.003
Adamovich I, Baalrud SD, Bogaerts A et al (2017) The 2017 Plasma Roadmap: low temperature plasma science and technology. J Phys D Appl Phys 50:323001. https://doi.org/10.1088/1361-6463/aa76f5
Homola T, Matoušek J, Kormunda M et al (2013) Plasma treatment of glass surfaces using diffuse coplanar surface barrier discharge in ambient air. Plasma Chem Plasma Process 33:881–894. https://doi.org/10.1007/s11090-013-9467-3
Johnsen K, Redford K (1996) Modification of polyolefin surfaces by plasma-induced grafting. J Appl Polym Sci 59:1651–1657. https://doi.org/10.1002/(SICI)1097-4628(19960307)59:10%3c1651:AID-APP17%3e3.0.CO;2-Z
Homola T, Matoušek J, Kormunda M et al (2012) Activation of poly(ethylene terephthalate) surfaces by atmospheric pressure plasma. Polym Degrad Stab 97:2249–2254. https://doi.org/10.1016/j.polymdegradstab.2012.08.001
Prysiazhnyi V, Cernak M (2012) Air plasma treatment of copper sheets using diffuse coplanar surface barrier discharge. Thin Solid Films 520:6561–6565. https://doi.org/10.1016/j.tsf.2012.06.069
Akram M, Jansen KMB, Ernst LJ, Bhowmik S (2011) Atmospheric pressure plasma surface modification of titanium for high temperature adhesive bonding. Int J Adhes Adhes 31:598–604. https://doi.org/10.1016/j.ijadhadh.2011.05.009
Laroussi M (2005) Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym 2:391–400. https://doi.org/10.1002/ppap.200400078
Lerouge S, Wertheimer MR, Yahia LH (2001) Plasma sterilization : a review of parameters, mechanisms, and limitations. Plasmas Polym 6:175–188. https://doi.org/10.1023/A:1013196629791
Moisan M, Barbeau J, Crevier M et al (2002) Plasma sterilization. Methods and mechanisms. Pure Appl Chem 74:349–358. https://doi.org/10.1351/pac200274030349
Fridman G, Friedman G, Gutsol A et al (2008) Applied plasma medicine. Plasma Process Polym 5:503–533. https://doi.org/10.1002/ppap.200700154
Morfill GE, Kong MG, Zimmermann JL (2009) Focus on plasma medicine. New J Phys 11:115011. https://doi.org/10.1088/1367-2630/11/11/115011
Randeniya LK, De Groot GJJB (2015) Non-thermal plasma treatment of agricultural seeds for stimulation of germination, removal of surface contamination and other benefits: a review. Plasma Process Polym 12:608–623. https://doi.org/10.1002/ppap.201500042
Hertwig C, Reineke K, Ehlbeck J et al (2015) Decontamination of whole black pepper using different cold atmospheric pressure plasma applications. Food Control 55:221–229. https://doi.org/10.1016/j.foodcont.2015.03.003
Hertwig C, Leslie A, Meneses N et al (2016) Inactivation of Salmonella Enteritidis PT30 on the surface of unpeeled almonds by cold plasma. Innov Food Sci Emerg Technol 44:242–248. https://doi.org/10.1016/j.ifset.2017.02.007
Măgureanu M, Sîrbu R, Dobrin D, Gîdea M (2018) Stimulation of the germination and early growth of tomato seeds by non-thermal plasma. Plasma Chem Plasma Process 38:989–1001. https://doi.org/10.1007/s11090-018-9916-0
Stolárik T, Henselová M, Martinka M et al (2015) Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem Plasma Process 35:659–676. https://doi.org/10.1007/s11090-015-9627-8
Zahoranová A, Hoppanová L, Šimončicová J et al (2018) Effect of cold atmospheric pressure plasma on maize seeds: enhancement of seedlings growth and surface microorganisms inactivation. Plasma Chem Plasma Process 38:969–988. https://doi.org/10.1007/s11090-018-9913-3
Zahoranová A, Henselová M, Hudecová D et al (2016) Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem Plasma Process 36:397–414. https://doi.org/10.1007/s11090-015-9684-z
Lotfy K (2017) Effects of cold atmospheric plasma jet treatment on the seed germination and enhancement growth of watermelon. Open J Appl Sci 07:705–719. https://doi.org/10.4236/ojapps.2017.712050
Misra NN, Pankaj SK, Segat A, Ishikawa K (2016) Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci Technol 55:39–47. https://doi.org/10.1016/j.tifs.2016.07.001
Dobrynin D, Fridman G, Friedman G, Fridman A (2009) Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys 11:115020. https://doi.org/10.1088/1367-2630/11/11/115020
Kyzek S, Holubová Ľ, Medvecká V et al (2018) Cold atmospheric pressure plasma can induce adaptive response in pea seeds. Plasma Chem Plasma Process 39:475–486. https://doi.org/10.1007/s11090-018-9951-x
Bradley JW, Bryant PM (2007) The diagnosis of plasmas used in the processing of textiles and other materials. Plasma Technol Text. https://doi.org/10.1533/9781845692575.1.25
Förster S, Mohr C, Viöl W (2005) Investigations of an atmospheric pressure plasma jet by optical emission spectroscopy. Surf Coatings Technol 200:827–830. https://doi.org/10.1016/j.surfcoat.2005.02.217
Machala Z, Janda M, Hensel K et al (2007) Emission spectroscopy of atmospheric pressure plasmas for bio-medical and environmental applications. J Mol Spectrosc 243:194–201. https://doi.org/10.1016/j.jms.2007.03.001
Rodero A, García MC (2017) Gas temperature determination of non-thermal atmospheric plasmas from the collisional broadening of argon atomic emission lines. J Quant Spectrosc Radiat Transf 198:93–103. https://doi.org/10.1016/j.jqsrt.2017.05.004
Pacheco M, Pacheco J, Moreno H, Mercado A, Valdivia R, Santana A (2008) OES analysis in a nonthermal plasma used for toxic gas removal: rotational and excitation temperature estimation. Laser Phys 18:303–307. https://doi.org/10.1134/S1054660X08030183
Ono R (2016) Optical diagnostics of reactive species in atmospheric-pressure nonthermal plasma. J Phys D Appl Phys 49:83001. https://doi.org/10.1088/0022-3727/49/8/083001
Sivachandiran L, Khacef A (2017) Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment. RSC Adv 7:1822. https://doi.org/10.1039/c6ra24762h
Schiorlin M, Paradisi C, Brandenburg R et al (2015) Pollutant degradation in gas streams by means of non-thermal plasmas. In: Nejadkoorki F (ed) Current air quality issues. IntechOpen. https://doi.org/10.5772/60049
Reuter S, Sousa JS, Stancu GD, Hubertus Van Helden JP (2015) Review on VUV to MIR absorption spectroscopy of atmospheric pressure plasma jets. Plasma Sources Sci Technol 24:054001. https://doi.org/10.1088/0963-0252/24/5/054001
Černák M, Černáková L, Hudec I et al (2009) Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur Phys J Appl Phys 47:22806. https://doi.org/10.1051/epjap/2009131
Hartmann A, Agurell E, Beevers C et al (2003) Recommendations for conducting the in vivo alkaline comet assay. Mutagenesis 18:45–51. https://doi.org/10.1093/mutage/18.1.45
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 26:249–261. https://doi.org/10.1385/MB:26:3:249
Gichner T, Patková Z, Száková J et al (2008) DNA damage in potato plants induced by cadmium, ethyl methanesulphonate and γ-rays. Environ Exp Bot 62:113–119. https://doi.org/10.1016/j.envexpbot.2007.07.013
Gordon IE, Rothman LS, Hill C et al (2017) The HITRAN2016 molecular spectroscopic database. J Quant Spectrosc Radiat Transf 203:3–69. https://doi.org/10.1016/j.jqsrt.2017.06.038
Abdelaziz AA, Ishijima T, Osawa N, Seto T (2019) Quantitative analysis of ozone and nitrogen oxides produced by a low power miniaturized surface dielectric barrier discharge: effect of oxygen content and humidity level. Plasma Chem Plasma Process 39:165–185. https://doi.org/10.1007/s11090-018-9942-y
Wang H, Zhuang Z, Sun C et al (2016) Numerical evaluation of the effectiveness of NO2 and N2O5 generation during the NO ozonation process. J Environ Sci (China) 41:51–58. https://doi.org/10.1016/j.jes.2015.05.015
Kuwahata H, Mikami I (2014) Generation of nitric acid and nitrous acid in distilled water irradiated with atmospheric-pressure plasma jet. e-J Surf Sci Nanotechnol 12:410–413. https://doi.org/10.1380/ejssnt.2014.410
Tomeková J, Medvecká V, Hergelová B et al (2018) Spectroscopic study of diffuse coplanar surface barrier discharge plasma used for treatment of pea seeds. In: Šafránková J, Pavlů J (eds) WDS’18 Proceedings of contributed papers—physics. Matfyzpress, Prague, pp 124–129
Gill SS, Anjum NA, Gill R et al (2015) DNA damage and repair in plants under ultraviolet and ionizing radiations. Sci World J 2015:5–7. https://doi.org/10.1155/2015/250158
Pournavab RF, Mejía EB, Mendoza AB et al (2019) Ultraviolet radiation effect on seed germination and seedling growth of common species from northeastern Mexico. Agronomy 9:269. https://doi.org/10.3390/agronomy9060269
Švubová R, Kyzek S, Medvecká V et al (2020) Novel insight at the effect of cold atmospheric pressure plasma on the activity of enzymes essential for the germination of pea (Pisum sativum L. cv. Prophet) seeds. Plasma Chem Plasma Process. https://doi.org/10.1007/s11090-020-10089-9
Acknowledgements
This work was supported by the Slovak Research and Development Agency under the Contract No. APVV-16-0216, Project VEGA 1/0410/18 and the University Grant UK/366/2019.
Author information
Authors and Affiliations
Contributions
JT: diagnostics of plasma—Fourier transform infrared spectroscopy, optical emission spectroscopy: data measurements and analysis, writing—first draft preparation; SK: DNA damage analysis, writing—first draft preparation; VM: plasma treatment of seeds, writing—review and editing; EG: DNA damage analysis, writing—review and editing; AZ: plasma diagnostics, writing—first draft preparation, review and editing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomeková, J., Kyzek, S., Medvecká, V. et al. Influence of Cold Atmospheric Pressure Plasma on Pea Seeds: DNA Damage of Seedlings and Optical Diagnostics of Plasma. Plasma Chem Plasma Process 40, 1571–1584 (2020). https://doi.org/10.1007/s11090-020-10109-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10109-8