Abstract
Atmospheric pressure air plasma discharges generate potential antimicrobial agents, such as nitrogen oxides and ozone. Generation of nitrogen oxides was studied in a DC-driven self-pulsing (1–10 kHz) transient spark (TS) discharge. The precursors of NOx production and the TS characteristics were studied by nanosecond time-resolved optical diagnostics: a photomultiplier module and a spectrometer coupled with fast intensified camera. Thanks to the short (~10–100 ns) high current (>1 A) spark current pulses, highly reactive non-equilibrium plasma is generated. Ozone was not detectable in the TS, probably due to higher gas temperature after the short spark current pulses, but the NOx production rate of ~7 × 1016 molecules/J was achieved. The NO2/NO ratio decreased with increasing TS repetition frequency, which is related to the complex frequency-dependent discharge properties and thus changing NO2/NO generating mechanisms. Further optimization of NO2 and NO production to improve the biomedical and antimicrobial effects is possible by modifying the electric circuit generating the TS discharge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-thermal (cold) plasmas generated by electrical discharges can be maintained at low gas temperatures, but have high electron energies responsible for their chemical activity. For this reason, several plasma based technologies were successfully implemented in the recent decades, in processes such as surface modification, flue gas cleaning, water purification, plasma assisted combustion, or hazardous liquid waste processing [1–4]. In the recent years, the plasma community witnesses a fast development of bio-medical applications [5, 6], since cold plasmas provide multiple agents that can efficiently kill bacteria and other hazardous microbes, and cause multiple biomedical and therapeutic effects in higher organisms [7–9].
In biomedical applications, the fundamental research to assess the roles of various plasma agents (e.g. UV radiation, electric field, reactive neutral and charged particles) involved is very important. At present, the major role in atmospheric pressure plasmas generated in air is typically attributed to reactive oxygen and nitrogen species (RONS) [10–13]. Plasmas in contact with water can simultaneously generate several aqueous RONS, such as nitrite NO2 −, nitrate NO3 −, peroxynitrite ONOO−, dissolved ozone O3, or hydrogen peroxide H2O2 from the hydroxyl radicals OH. It is reasonable to assume that the overall bactericidal effect of the air plasma discharge involves synergistic effect of several of these species [12, 14].
The aqueous RONS are produced from the gaseous RONS, e.g. nitrogen oxides NO, NO2, ozone O3 and OH radicals, generated by the plasma in gas and subsequently dissolved in water. Depending on the used electrical discharge, different gaseous RONS can dominate in the treated gas. For example, the O3 generation is very efficient by cold plasma sources, such as dielectric barrier or streamer corona discharges [15–17]. Water ozonation technology has been commercially used for more than a century. Various plasma sources can also generate significant amounts of NOx [18–21]. The antibacterial potential of NO2 is known for a long time [22, 23], and has been already commercially introduced [24], but new types of discharges optimized for bio-medical plasma applications are still being developed and studied. Several recent papers report investigating the NOx formation and their bactericidal effects in a hybrid glow-spark discharges or in dielectric barrier discharges in air [25–27].
In the present paper we focus on the synthesis of NO, NO2 and their precursors (O and N radicals) by the transient spark (TS) discharge in air. The TS is a DC-driven self-pulsing discharge with the repetition frequency 1–10 kHz. The TS is initiated by a streamer transforming to a short spark current pulse. Streamers are considered to be critical with respect to the efficiency of plasma induced chemistry at atmospheric pressure. The electric field in the streamer’s head can reach more than 200 kV/cm [28, 29], so that the chemical and ionization processes are very efficient there. On the other hand, transition of streamers to spark or arc discharge [30, 31], often generating thermal plasma, is usually not desired in environmental and bio-medical applications. High power requirements limit the utilization of thermal plasmas usually only to applications where one can either expect valuable products, or needs to completely destroy dangerous pollutants [32–34].
The TS current pulses are sufficiently short (~10–100 ns) to avoid the plasma thermalization. Non-equilibrium plasma generated during the spark phase of the TS is highly reactive, since the electron density as high as 1017 cm−3 can be achieved [35], and its chemical activity is comparable with nanosecond repetitively pulsed (NRP) spark discharges [36]. The advantage of TS is no need of special and expensive high voltage pulsers with high repetitive frequencies and nanosecond rise-times used to generate NRP discharges [37–39].
The TS has already been successfully tested for several environmental and bio-medical applications [11, 12, 40, 41], and extensive fundamental research of TS using several electrical and optical diagnostic methods has been performed [35, 42–46]. However, further research is needed for better understanding of chemical processes initiated by the TS. In this work we present research of TS using optical emission spectroscopy (OES) combined with the post-discharge gas composition analysis, focused at the synthesis of NO, NO2 and their precursors (O and N radicals).
Experimental Set-Up
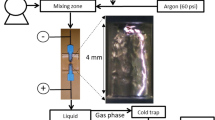
DC high voltage (HV) power supply Technix SR20-R-1200 (0–20 kV, 0–60 mA) connected to the anode via a series resistor (R = 4.92–9.84 MΩ) was used to generate a positive TS discharge between two stainless steel electrodes in point-to-point configuration with anode at the top. The distance d between the electrodes was varied between 4 and 6 mm. The electrodes were made of a 2-mm diameter rod. The anode tip was sharpened whereas the cathode was blunt. The curvature radius of the anode tip was of the order of 100 μm. The curvature radius of the cathode was of the order of a millimeter. The electrodes were placed in a 10 cm long gas cell with the inner radius 4 cm and two valves for the gas inlet and outlet. One side of the glass tube was enclosed by Teflon window with openings for the electrodes. The other side was enclosed by the CaF2 window enabling optical diagnostics. The schematic of the experimental set-up is depicted in Fig. 1.
The discharge voltage was measured by a HV probe Tektronix P6015A and the discharge current was measured on a 50 or 1 Ω resistor shunt. The 1 Ω resistor shunt was used to measure the spark current pulses; whereas the 50 Ω resistor shunt was used to measure the streamer current pulses. Electric signals were recorded by a 200 MHz digitizing oscilloscope Tektronix TDS2024 (200 MHz, sampling rate up to 2 Gs/s). The mean discharge current I mean was estimated from the current I o delivered and directly measured by the DC power supply, taking into account losses due to the parasitic current through the HV probe.
The experiments were carried out in synthetic air driven from the pressure tanks (N2:O2 = 4:1, N2: 99.99 % purity, O2: 99.5 % purity), with a flow rate 1.3–2.6 l min−1. A gas analyzer Kane KM9106 Quintox was used to measure the gas composition after passing through the discharge reactor. It detects NO and NO2 in the range of 0–5000 ppm, or 0–1000 ppm, respectively, with an accuracy of ±5 ppm (<100 ppm), or ±5 % (>100 ppm). In addition, Fourier transform infrared (FT-IR) spectrophotometer Shimadzu IRAffinity-1S (wavenumber range 7800–350 cm−1, best spectral resolution 0.5 cm−1) was used for the detection of O3 and other nitrogen oxides, such as N2O or N2O5. We also used the FT-IR spectrophotometer to verify the Kane gas analyzer measurement of the nitrogen oxides density in the treated post-discharge gas.
For fast recording of time-integrated broadband emission spectra (200–1100 nm), we used a dual-channel fiber optic compact emission spectrometer Ocean Optics SD2000 (spectral resolution 0.6–1.7 nm). The time-resolved emission spectra were obtained using a 2 m monochromator Carl Zeiss Jena PGS2 (spectral resolution 0.04–0.09 nm), covering the UV and VIS regions (200–800 nm), coupled with an intensified charge-coupled device (iCCD) camera Andor Istar (minimum gate 2 ns). The light collection was provided by a pair of parabolic mirrors focused on a small area near the anode tip. These mirrors, together with the optical fiber core diameter, determined the spatial resolution of 300 μm.
The iCCD camera was triggered by a 5 V rectangular pulse generator with <5 ns rise time. This generator was triggered directly by the discharge current signal, causing an additional delay of <10 ns. This delay, plus the delay caused by the transmission of the signal by BNC cables, was compensated by using a 10 m long optical fiber Ocean Optics P400-10-UV-VIS. For measurements with the iCCD camera, an additional small resistor r = 1 kΩ was attached directly to the anode. The purpose of additional r was to eliminate the current signal oscillations induced by the internal inductances of the grounding wire and the HV cable connecting R with the anode that caused problems with the iCCD camera triggering. More details about the role of r on the characteristics of the TS discharge can be found in Ref. [43].
For fast measurements of the emission intensity evolution, we used a photomultiplier tube (PMT) module Hamamatsu H955 (rise time 2.2 ns). In order to isolate a specific spectral transition for PMT measurements, a bandpass interference filter, e.g. Melles Griot 03 FIU127 for the N2 (C–B, 0–0) transition, was inserted into the optical path. The PMT module signal was recorded using the oscilloscope.
Results and Discussion
Discharge Properties
Transient spark is initiated by a streamer, when the potential on the stressed electrode V reaches voltage V TS , characteristic for the TS (corresponding time ≈ −500 ns in Fig. 2). The streamer forms a relatively conductive plasma channel between the electrodes. This leads to partial discharging of the electric circuit internal capacitance C. The current from the partial discharging of C heats up the gas inside the plasma channel generated by the streamer. The transition to spark occurs when gas temperature inside the plasma channel increases to ~1000 K [44].
During the spark phase, the electric circuit internal capacitance discharges completely, the voltage on the HV electrode drops to almost zero (time 0 ns in Fig. 2), and the discharge current given approximately by
reaches a high value (~1–10 A). Transition to an arc after the spark phase is restricted by the large ballast resistor R. It limits the DC current delivered to the plasma after C is discharged. For R ≈ 10 MΩ, even for maximum possible applied voltage 20 kV, the DC current is certainly below 2 mA: not high enough to maintain DC discharge after the TS spark phase. As a result, the plasma decays after the spark pulse. Eventually, the plasma resistance exceeds the R and the potential V on the anode gradually increases, as the capacitance C recharges. A new TS pulse, initiated by a new streamer, occurs when the potential V reaches the breakdown voltage V TS again. The TS is thus based on repetitive charging and discharging of C. The repetition frequency f of this process can be controlled by the DC power supply voltage V o and is typically in the range 1–10 kHz [43].
The TS repetition frequency increase is accompanied by the increase of the mean discharge current I mean and discharge power P (Fig. 3), estimated as
Here, E p is the energy delivered to the gap per pulse. The E p can be estimated using two different formulas:
where the time interval τ is chosen so that voltage and current waveforms cover the whole active phase of TS discharge, from beginning of the streamer to the end of the spark (Fig. 2). Both approaches were tested to determine the E p and the difference between the results was within their uncertainties. We further used the first approach because of its simplicity. The capacitance C was estimated from the measured current and voltage waveforms using Eq. (1).
Besides I mean and P, several other TS discharge characteristics change with frequency as well [43, 44]. The breakdown voltage V TS decreases, and the spark pulses are getting broader and smaller. Due to decrease of V TS , the energy delivered to the gap per pulse E p decreases also with the increasing TS repetition frequency (Fig. 3), as can be deduced from Eq. (3).
The analysis of the electric circuit representing the TS, and the measurement of the discharge channel diameter by the time-resolved imaging enabled us to estimate the electron density n e in TS from the current and voltage waveforms [35, 43]. After the streamer, n e ≈ 1014 cm−3 was obtained, which is in an agreement with values found in the literature [28, 47–49]. During the spark phase, the electron density as high as 1017 cm−3 can be achieved at f < 4 kHz. Generated plasma is thus strongly ionized for a short time. However, the TS spark current pulses are sufficiently short to avoid thermalization of generated plasma. With increasing TS repetition frequency, smaller and broader spark current pulses occur and the peak electron density decreases to ~1016 cm−3 [35].
The time-integrated emission spectroscopy study confirmed that TS pulses generate highly reactive non-equilibrium plasma with excited atomic radicals (\( {\text{O}}^{*} \), \( {\text{N}}^{*} \)), excited molecules \( {\text{N}}_{2}^{*} \) and ions \( {\text{N}}_{2}^{ + *} \) (Fig. 4). The rotational temperature T r derived from the N2 2nd positive system of the time-integrated spectra, ranging from 500 to 1500 K depending on f, is much lower than the vibrational temperature (3800–5000 K). The TS emission characteristics change with increasing frequency as well. Below ~3 kHz, atomic lines emission (\( {\text{O}}^{*} \), \( {\text{N}}^{*} \)) and molecular N2 2nd positive system dominates in the spectra. The absolute intensities of N2 2nd and 1st positive systems per single TS pulse do not change significantly with the increasing frequency, but the intensities of atomic lines emission weaken with increasing f (Fig. 4). This might be interpreted as a decrease of the TS ability to produce radicals at higher frequencies, and the measurement of the generated NOx density should reflect it as well.
Typical time-integrated spectra of the TS discharge in air at repetition frequencies 2 and 6 kHz. In the UV range, the differences between 2 and 6 kHz were small, resulting only from different rotational and vibrational temperature of N2(C) species. Thus, the spectrum at 6 kHz is not displayed for clarity. In the VIS range, the spectrum at 2 kHz is normalized to unity, while the spectrum at 6 kHz is normalized by the same factor multiplied by three, taking into account three times higher number of pulses per unit time at 6 kHz than at 2 kHz
Nitrogen Oxides Generation Efficiency
The density of both NO and NO2 increase with f (Fig. 5) due to increasing discharge power P. However, the rate of this increase weakens as the frequency grows. This is related to the logarithmic-like dependence of P on f, resulting from the decrease of E p with increasing frequency (Fig. 3). In order to asses the NO and NO2 generation efficiency at different TS frequency, it is thus necessary to take into account the changes of the input energy density. Other nitrogen oxides can be neglected in the first approximation, due to their absence in the measured infrared spectra.
Figure 6 shows the generation rate of NO and NO2 as number of molecules generated per input energy 1 J. The highest NOx (NO + NO2) generation rate achieved is around 7 × 1016 molecules/J. This is higher than in the work of Pavlovich et al. [25], where similar type of a discharge was used (initial production rate 1.5 × 1016 molecules/J). On the other hand, higher generation rates were obtained using different types of discharges ([20] and references therein). For example, NOx production rate as high as (10–40) × 1016 molecules/J was reached in an arc discharge [50]. We assume that we can still improve NOx generation efficiency our system by varying parameters of the electric circuit generating the TS discharge (external resistor, internal capacitance, distance of electrodes). Moreover, the NOx generation rate in the gas phase is not the only parameter to be considered for water bio-decontamination application. Here, the NOx transfer from the gas to the liquid phase can be a limiting step. From this point of view, the TS represents a promising alternative, as it can operate in direct contact with the treated water [41]. With water being supplied via a hollow anode [12, 41], the TS can even induce electrospray of the treated water and the gas–liquid interaction area enhancement to facilitate the mass transfer towards bacteria or cells suspended in water solutions.
The NOx generation rate can be also used to evaluate the TS ability to induce chemical changes with increasing repetition frequency. The NO generation rate tends to improve with increasing f, while the NO2 generation rate decreases. As a result, the total NOx generation rate does not change significantly and does not support the hypothesis of weaker chemical reactivity of TS at higher frequencies. It seems it is rather the NOx synthesis mechanism that changes with f, leading either preferentially to NO or to NO2 generation.
Nitrogen Oxides Generation Mechanism
Despite the fact that TS generates ‘cold’ non-equilibrium plasma, the temperature during the spark phase of the TS can be as high as ~3000 K (T spark , Fig. 7). We therefore have to consider the Zeldovich thermal mechanism of the NOx generation. This mechanism is initiated by the thermal decomposition of N2 and O2 into their atomic states at high temperature (above ~1500 K). Next, both N and O atomic radicals are able to produce NO:
The rate coefficient of the second reaction with N atoms is four orders of magnitude higher at 3000 K, but production of N atomic radicals via the thermal decomposition of N2 is very slow even at 3000 K (rate coefficient ~1.5 × 10−24 cm3 s−1) [51]. As a result, the thermal decomposition of N2 is rate-limiting step of the thermal NO formation. In non-thermal plasmas, alternative reaction pathways for the generation of N atomic radicals exist. Moreover, reactions (4) and (5) can be much faster if one of the reactants is in excited state [51, 52].
Another important fact is that the temperature necessary for the thermal NO generation remains high only for a certain time after the spark current pulse. Mixing with the ambient air between two successive TS pulses cools the gas between the electrodes down. The ‘steady-state’ gas temperature, at which the initiating streamer of the next TS event appears, starts only at about 350 K at f ≈ 1 kHz (T str , Fig. 7). Unfortunately, we do not know exactly how long the temperature is high enough for the efficient thermal NOx formation after the spark phase of the TS discharge. We approximated T str and T spark by the rotational temperature T r of the N2(C) species, assuming that in atmospheric pressure air plasma T ≈ T r . The T r was obtained by fitting the time-resolved (time window 5–20 ns) experimental emission spectra of the N2 2nd positive system (0–0 transition) with the simulated ones, using Specair program [53] (Fig. 8). This emission can be observed only for a short time (a few tens of ns) after the streamer and spark current pulses (see Figs. 9, 10, respectively). For this reason we cannot measure the entire temperature evolution between two successive TS pulses and thus we are not able to asses the role of the thermal Zeldovich mechanism on the overall NOx production precisely.
However, the T str increases with TS repetition frequency up to ~600 K at f ≈ 10 kHz, and this could theoretically accelerate the thermal NOx generation. On the other hand, the T spark decreases with f and could be responsible for the opposite trend. In any case, we observed no significant effect of TS repetition frequency on the NOx generation rate (Fig. 6), and thus also no dependence on T str or T spark . We therefore assume that plasma processes, primarily initiated by high energy electrons, should play more important roles in the NOx generation by the TS discharge than the Zeldovich thermal mechanism.
Information on the plasma processes responsible for NOx generation can be deduced from the emission profiles obtained by the PMT measurements, using interference filters for selecting the N2(C) emission at 337 nm, and \( {\text{O}}^{*} \) emission at 777 nm. The N2(C) emission starts with the beginning of both streamer and spark current pulses. During the rising slope of the current pulses, the electrons must have enough energy to ionize, as well as to excite N2 molecules. We therefore assume that the majority of the N2(C) states are created by high energy electrons:
The fast quenching of excited \( {\text{N}}_{2}^{*} \) molecules, such as N2(C), with molecular oxygen is probably one of the major sources of atomic oxygen [54]:
The O atoms may generate NO (Eq. 4), but they can also generate ozone O3:
Next, ozone is able to oxidize NO to NO2:
These reactions should establish certain equilibrium concentrations of NO, NO2 and O3 molecules, depending on the initial concentrations of their precursors, gas temperature and composition. In the streamer corona and other discharges generating atmospheric pressure air plasma with the temperature very close to ambient, the dominant product is ozone, since the reaction (8) is much faster at 300 K then the reaction (4) [51].
In the TS, the streamer is followed by the spark with much higher gas temperature. Ozone generated during the streamer-to-spark transition phase, should be thermally decomposed and the Zeldovich reaction (4) enhanced. In fact, only traces of ozone with concentration below ~10 ppm were detected in the outlet gas, according to the obtained infrared spectra (Fig. 11). The negligible amount of O3 in the gas phase supports our previous findings that nitrites and nitrates generated in the aqueous phase from dissolved NO/NO2 play crucial role as antimicrobial agent in the water treated by TS, while the role of dissolved O3 was found negligible [12].
The role of the spark phase of TS is not only thermal decomposition of O3. Similarly to the streamer, during the initial phase of the spark with the current rising, the energy of electrons is high enough to ionize, dissociate and excite molecules. The spark phase can therefore strongly influence the overall plasma chemistry, since the electron density during the spark phase (n e > 1017 cm−3 at f < 3 kHz [35]) is much higher than during the streamer phase.
The voltage drop that occurs during the spark phase of the TS (Fig. 2) results in the decrease of the electric field intensity between the electrodes and the decrease of the electron mean energy. The electron temperature T e after the spark current pulse decreases to ‘only’ ~10,000 K [35], so they are unable further to ionize or excite higher electronically excited states of N2. However, they can still significantly contribute to the overall O and N atoms generation via dissociative electron–ion recombination reactions:
since the rate coefficients of these reactions are relatively high at T e = 10,000 K (2.3 × 10−8 and 4.6 × 10−8 cm3 s−1, respectively [51]), and the total density of positive ions must be approximately as high as the density of electrons. In the next step, the products of the reactions (10) and (11) can enhance the NOx synthesis, especially by N production bypassing the rate limiting step in the Zeldovich mechanism (Eq. 4).
The importance of dissociative recombination reactions could also explain higher NO2/NO ratio at low TS repetition frequency, where the density of charged particles is the highest. If the dissociative recombination reactions make a significant contribution to the production of atomic oxygen species, there might be enough O to produce not only NO, but also further oxidize it to NO2 in a three-body reaction (12):
With increasing f and decreasing degree of ionization in the TS spark phase, the total amount of produced oxygen atoms would decrease. As a result, less NO would be oxidized to NO2.
The NO2/NO ratio could be also decreased at higher TS frequencies due to the growing importance of another NO generation channel. Similarly to the NRP spark discharge [54], we can expect accumulation of atomic oxygen O from the previous TS pulses in the discharge zone at higher repetition frequency. These oxygen atoms can react with excited nitrogen molecules generated during the streamer phase of TS discharge to produce NO:
It was shown recently that this reaction can be an important NO synthesis channel in a nanosecond pulsed discharge [55].
At last, there is another possible mechanism leading to the changes of the NO2/NO ratio with f that can additionally contribute. At lower TS frequencies, the temperature right after the streamer is relatively low (<400 K). These conditions should be favorable to the generation of O3 (Eq. 8). During the streamer-to-spark transition time, these O3 molecules could oxidize a part of the available NO to NO2 (Eq. 9). With the increasing f, the T str grows and the generation of O3 after the streamer is suppressed. As a result, the contribution of ozone to NO2 generation becomes negligible. These chemical kinetics considerations can be, however, oversimplified. Further research including kinetic modeling is required to resolve this problem, taking into account more reactions and species.
Conclusions
Our objective was to investigate the generation of important biocidal agents such as NO, NO2 and O3, precursors of RONS in the plasma treated water, by the Transient Spark (TS) discharge in atmospheric pressure air. The TS is a streamer-to-spark transition type discharge initiated by a streamer, with the spark pulse duration and amplitude limited by a small amount of energy stored in the circuit internal capacitance C. The TS repetition frequency (1–10 kHz) can be controlled by the applied voltage. Similar type of plasma, even at higher pulse repetition frequencies, can be generated using nanosecond repetitive pulsed high voltage power supplies. The advantage of our approach is simpler and cheaper electric setup using DC high voltage power supply.
The TS discharge can be maintained at low energy conditions (~1 mJ/pulse), so that the generated plasma stays out of thermal equilibrium. The non-equilibrium state of plasma generated by TS was confirmed by the optical emission spectroscopic study. Our research confirmed high reactivity of the plasma generated by the TS, and its antimicrobial activity. In dry synthetic air, the TS leads to the formation of NOx: the sum of NO and NO 2 densities more than 600 ppm was achieved with power input below 6 W (NOx generation rate ~7 × 1016 molecules/J).
The TS characteristics change with the increasing TS repetition frequency. At higher frequencies, the preheating of the gas between the electrodes leads to the decrease of the breakdown voltage, smaller and broader spark pulses, and lower electron density during the spark phase of TS. These changes also influence the induced chemistry and generation of excited O and N atoms and nitrogen oxides. The atomic lines emission intensity and the NO2/NO ratio decreased with increasing TS repetition frequency.
The excited nitrogen molecules \( {\text{N}}_{2}^{*} \) are produced by excitation by high energy electrons during the rising slope of the current pulses, both streamer and spark. These excited \( {\text{N}}_{2}^{*} \) molecules can lead to the sufficient atomic oxygen production necessary for the synthesis of NO. The spark phase is probably very important for the NOx generation. The dissociative recombination of N2 + and O2 + ions with electrons provides additional atomic nitrogen and oxygen for the generation of NO, and oxidation of NO to NO2.
Only traces of ozone were detected in the outlet gas, most probably due to its thermal decomposition in the high temperature TS spark phase, as well as due to the oxidation of NO to NO2 that consumes O3. Further research including kinetic modeling and measurement of O species in the ground state is required to prove these hypotheses. In future, we also plan to optimize the NOx production by varying parameters of the electric circuit (external resistor, internal capacitance, distance of electrodes).
References
Civitano L (1993) Non-thermal plasma techniques for pollution control (NATO series vol 1). Springer, New York
Cernak M, Rahel J, Kovacik D, Simor M, Brablec A, Slavicek P (2004) Contrib Plasma Phys 44:492–495
Sahni M, Locke BR (2006) J Hazard Mater 137:1025–1034
Messerle VE, Karpenko EI, Ustimenko AB (2014) Fuel 126:294–300
Machala Z, Hensel K, Akishev Y (2012) Plasma for bio-decontamination, medicine and food security (NATO science for peace and security series a—chemistry and biology). Springer, Dordrecht
Pawlat J (2013) Eur Phys J Appl Phys 61:24323
Laroussi M, Kong MG, Morfill F, Stolz W (2012) Plasma medicine: applications of low-temperature gas plasmas in medicine and biology. Cambridge University Press, Cambridge
Fridman A, Friedman G (2013) Plasma medicine. Wiley, Oxford
von Woedtke T, Reuter S, Masur K, Weltmann K-D (2013) Phys Rep 530:291–320
Graves DB (2012) J Phys D Appl Phys 45:263001
Machala Z, Chládeková L, Pelach M (2010) J Phys D Appl Phys 43:222001
Machala Z, Tarabová B, Hensel K, Špetlíková E, Šikurová L, Lukeš P (2013) Plasma Process Polym 10:649–659
Lukeš P, Doležalová E, Sisrová I, Člupek M (2014) Plasma Sources Sci Technol 23:015019
Brisset JL, Pawlat J (2015) Plasma Chem Plasma Proc. doi:10.1007/s11090-015-9653-6
Eliasson B, Hirth M, Kogelschatz U (1987) J Phys D Appl Phys 20:1421–1437
Gordeyenya EA, Matveyev AA (1994) Plasma Sources Sci Technol 3:575–583
Knizhnik AA, Potapkin BV, Medvedev DD, Korobtsev SV, Shiryaevskii VL, Rusanov VD (1999) Tech Phys 365:336–339
Rehbein N, Cooray V (2001) J Electrostat 51–52:333–339
Rahman M, Cooray V (2003) Opt Laser Technol 35:543–546
Kim T, Song S, Kim J, Iwasaki R (2010) Jpn J Appl Phys 49:126201
Rahman M, Vernon C, Montaño R, Liyanage P, Becerr M (2011) J Electrostat 69:494–500
Shank JL, Silliker JH, Herper RH (1962) Appl Microbiol 10:185–189
Maricinelli RL, McKay CP (1983) Appl Environ Microbiol 46:198–202
Carbone P, Benedek K, Ruffo M, Luongo W (2013) Device and method for gas sterilization. US Patent 2013/0230430A1
Pavlovich MJ, Ono T, Galleher C, Curtis B, Clark DS, Machala Z, Graves DB (2014) J Phys D Appl Phys 47:505202
Moiseev T, Misra NN, Patil S, Cullen PJ, Bourke P, Keener KM, Mosnier JP (2014) Plasma Sources Sci Technol 23:065033
Hao XL, Mattson AM, Edelblute CM, Malik MA, Heller LC, Kolb JF (2014) Plasma Process Polym 11:1044–1056
Morrow R, Lowke JJ (1997) J Phys D Appl Phys 30:614–627
Kulikovsky AA (1998) IEEE Trans Plasma Sci 26:1339–1346
Loeb LB (1965) Electrical coronas, their basic physical mechanisms. University of California Press, Berkeley
Raether H (1964) Electron avalanches and breakdown in gases. Butterworths, London
Kobayashi A, Osaki K, Yamabe C (2002) Vacuum 65:475–479
Bromberg L, Cohn DR, Rabinovich A, Alexeev N (1999) Int J Hydrogen Energy 24:1131–1137
Kanzawa A (1993) Plasma Sources Sci Technol 2:58–62
Janda M, Martišovitš V, Hensel K, Dvonč L, Machala Z (2014) Plasma Sources Sci Technol 23:065016
Janda M, Machala Z, Lacoste DA, Stancu GD, Laux CO (2012) Stabilization of a lean methane-air flame using transient spark discharge. In: Janowski T et al (ed) International symposium on high pressure low temperature plasma chemistry HAKONE XIII (Kazimierz Dolny, Poland, 9–14 September), pp 185–189
Walsh JL, Shi JJ, Kong MG (2006) Appl Phys Lett 89:161505
Pancheshnyi SV, Lacoste DA, Bourdon A, Laux CO (2006) IEEE Trans Plasma Sci 34:2478–2487
Pai D, Lacoste DA, Laux CO (2008) IEEE Trans Plasma Sci 36:974–975
Hontanon E, Palomares JM, Stein M, Guo XA, Engeln R, Nirschl H, Kruis FE (2013) J Nanopart Res 15:1957
Hensel K, Kučerová K, Tarabová B, Janda M, Machala Z, Sano K, Mihai CT, Ciorpac M, Gorgan LD, Jijie R, Pohoata V, Topala I (2015) Biointerphases 10:029515
Machala Z, Janda M, Hensel K, Jedlovský I, Leštinská L, Foltin V, Martišovitš Morvová M (2007) J Mol Spectrosc 243:194–201
Janda M, Martišovitš V, Machala Z (2011) Plasma Sources Sci Technol 20:035015
Janda M, Machala Z, Niklová A, Martišovitš V (2012) Plasma Sources Sci Technol 21:045006
Gerling T, Hoder T, Brandenburg R, Bussiahn R, Weltmann K-D (2013) J Phys D Appl Phys 46:145205
Gerling T, Hoder T, Bussiahn R, Brandenburg R, Weltmann K-D (2013) Plasma Sources Sci Technol 22:065012
Bourdon A, Bonaventura Z, Celestin S (2010) Plasma Sources Sci Technol 19:034012
Kacem S, Eichwald O, Ducasse O, Renon N, Yousfi M, Charrada K (2012) J Comput Phys 231:251–261
Barni R, Esena P, Riccardi C (2005) J Appl Phys 97:073301
Wang Y, DeSilva AV, Goldenbaum GC, Dickerson RR (1998) J Geophys Res 103:19149–19159
Capitelli M, Ferreira CM, Gordiets BF, Osipov AI (2000) Plasma kinetics in atmospheric gases, Springer series on atomic, optical, and plasma physics. Springer, Berlin
Herron JT (1999) J Phys Chem Ref Data 28:1453–1484
Laux CO, Spence TG, Kruger CH, Zare RN (2003) Plasma Sources Sci Technol 12:125–138
Stancu GD, Kaddouri F, Lacoste DA, Laux CO (2010) J Phys D Appl Phys 43:124002
Shkurenkov I, Burnette D, Lempert WR, Adamovich IV (2014) Plasma Sources Sci Technol 23:065003
Acknowledgments
Effort sponsored by the Slovak Research and Development Agency APVV-0134-12, and Slovak Grant Agency VEGA 1/0918/15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janda, M., Martišovitš, V., Hensel, K. et al. Generation of Antimicrobial NOx by Atmospheric Air Transient Spark Discharge. Plasma Chem Plasma Process 36, 767–781 (2016). https://doi.org/10.1007/s11090-016-9694-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9694-5