Abstract
The influence of nitrogen addition (up to 10 %) to an argon surface-wave atmospheric-pressure discharge has been investigated by spectroscopic means. Nitrogen introduction results in a significant increase of gas temperature, from 2000 to 4000 K, and a drastic reduction of electron density, from 1014 to 1013 cm−3. These variations come along with the disappearance of the discharge contraction and the apparition of a diffuse, less bright zone (postdischarge) in the downstream zone. Modifications of the argon discharge kinetics leading to a significant decrease of the density of argon excited states due to the presence of nitrogen have been discussed to the light of the spectra emitted by the discharge and a simplified kinetic model, showing the relevance of argon species in Penning excitation and charge transfer reactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasmas have been proven to be very useful in scientific applications involving the treatment of material surfaces. However, in some other cases these treatments can also result in undesired damages on the material surface. In order to avoid these effects, Helix proposed to work outside of the plasma region, in the so-called remote plasma or postdischarge [1]. In this region the density of charged particles is very low, but still exist a significant fraction of molecules, radicals or atoms in long-lived excited levels, which are created in the plasma and afterwards transported to the postdischarge. These species are able to induce chemical reactions for the treatment of materials. Furthermore, the lower temperature of the postdischarge as compared to that of the plasma allows for the treatment of materials that can be degraded at high temperatures.
Nevertheless, the performance of all these applications depends on many parameters including, but not limited to, the gas composition, the geometry and electrodynamics of the discharge or the supplied power. All these parameters affect the amount of energy available in the discharge, either in the form of electron temperature or gas temperature, as well as the capability of the discharges to generate the active species of interest for each application.
Regarding gas composition, plasmas sustained in argon–nitrogen mixtures produced in a flowing gas have been successfully applied for thin film deposition [2–4], surface modification of material [5, 6] and the synthesis of nitride film [7]. However, most of these applications have been developed to work in low pressure regimes which makes difficult their implementation on industrial scale since a pumping system is required thus being more expensive.
Among the different plasmas sources that can be used to sustain an Ar–N2 mixture at atmospheric pressure, surface-wave discharges (SWDs) are characterized by their operational flexibility in terms of pressure, frequency, and gas composition. In SWDs a plasma column is generated inside a dielectric tube and extends beyond the field applicator; being the increase of the column length with the absorbed power a specific characteristic of this microwave plasma. This feature also allows for controlling the residence time of the molecules and atoms constituting the plasma gas, thus enhancing the control over the excitation and dissociation processes of nitrogen molecules.
In the past, several fundamental studies, both theoretical and experimental, have been carried out to exhaustively characterize Ar–N2 SWDs, specially at reduced pressures [8, 9]. More recently, the need of understanding the behavior of atmospheric pressure discharges has led to the investigation of these discharges [10–15]. Among them, a thorough study [12, 13] of argon–nitrogen discharges was carried out where experimental results fit with the modeling of a surface wave torch. However that study did only present results for a mixture containing 20 % nitrogen.
In the present research, a spectroscopic characterization of a surface wave discharge generated with Ar–N2 mixtures at atmospheric pressure has been carried out. Several macroscopic discharge parameters of interest, namely power density, electron density and gas temperature have been measured. To gain some insight in the behavior of the Ar–N2 discharges considered in the current work, a simplified global kinetic model has been applied to understand some of the excitation/ionization mechanisms of these plasmas. With this study, we aim to contribute to a more complete knowledge of SWDs generated with Ar–N2 mixtures for N2 concentrations below 10 %, which is the most frequently range of concentrations used in the aforementioned applications.
Experiment Setup
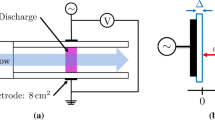
Plasmas were created by using a surfaguide [16] as SWD coupling device sustained at atmospheric pressure. The plasma created by means of this microwave source extends to both sides of the wave launcher, resulting in the appearance of direct and inverse columns [17], being the former where both the gas flux and the wave propagation take place in the same direction, contrary to what happens in the inverse column (Fig. 1).
Microwave (2.45 GHz) power was supplied to the discharge by a SAIREM GMP kG/D generator of 2000 W maximum power in continuous mode, equipped with a water cooled circulator to avoid power reflection damage. In the current research, the incident power (P i ) ranged from 80 to 700 W; two impedances matching by means of a short circuit movable plunger and a triple stub were used to minimize the reflected power (P r ) from the applicator back to the generator and to keep the reflected power lower than 5 % of incident power.
The plasma was sustained in different quartz tubes using high purity (99.999 %) argon and nitrogen gases. Different argon–nitrogen mixtures (Ar/N2) were considered and controlled by a set of mass flow controllers from Bronkhorst keeping constant the total gas flow at 1 slm. A first set of experiments with nitrogen concentrations up to 3 % V/V (percentage in volume, henceforth %) were carried out using a tube of 1.25 mm of internal radii. However, for the largest nitrogen amounts, up to 10 %, the physical integrity of the tube was compromised due to the higher powers required to sustain the discharge, the increase of the gas temperature and the disappearance of the radial contraction (see next sections). Consequently, a second set of experiments were carried out using a tube of 3 mm internal radii surrounded by a cooling system. A complete description of the cooling system can be found e.g. in [18].
The plasma capability for inducing reactions for exciting, ionizing and dissociating nitrogen molecules was analyzed using the radiation emitted by the discharge. This radiation was collected perpendicularly to the discharge tube by a PCS 1000 optical fiber with a silicon core of 1000 ± 40 μm diameter and guided to the entrance slit of a Czerny–Turner monochromator (Jobin–Yvon–Horiba), previously calibrated, with a 1 m focal distance and holographic grating of 2400 lines/mm. A Hamamatsu R928P photomultiplier (PMT) and a Symphony charge-coupled device (CCD-1024 × 256-OPEN-STE) were used as the radiation detectors. The recorded spectra allowed us to gain information about the different species existing in the plasma as well as to estimate the gas temperature (CCD camera) and the electron density (PMT). In order to compare the results, the spectra emitted by the plasma in the 200–750 nm range were registered at 1 cm measured from the end of the plasma column in all cases.
Morphology of the Discharge
Introducing a certain amount of a second gas into an argon plasma results in a variation of its morphology. First and foremost, if the second gas has a higher ionization threshold, this results in larger powers required to sustain the discharge as compared to the previous situation which is translated into the shortening of the discharge column for a given set of experimental conditions in the particular case of SWDs, as reported in Ar–He and Ar–Ne mixtures [19, 20].
If a molecular gas, such as nitrogen, forms part of the gas utilized to create the discharge, a shortening of the plasma length is also observed as happens in Ar/He and Ar/Ne mixtures. However, a specific and important change is observed upon nitrogen introduction: the appearance of a diffuse zone after the direct column, which cannot be considered a plasma region and is known as remote plasma, afterglow or postdischarge [1]. In this weakly ionized or neutral medium, reaction pathways are more restricted than in the discharge, where electrons play a fundamental role in the internal kinetic. In plasmas generated with Ar–N2 mixtures, it is possible to observe different postdischarges: the orange afterglow or the better known Lewis–Rayleigh (yellow), pink, blue and green afterglows. Although in both orange and Lewis–Rayleigh postdischarges the emission of the first positive system of nitrogen is found, the vibrational levels are different [21]. In the current research, the orange afterglow or postdischarge is observed. In this afterglow, spectral observations [14, 21] reveal the presence of the emission in the orange-infrared (500–1000 nm) and UV regions (230–340 nm). These emissions are due to the first positive nitrogen system and the nitrogen Vegard–Kaplan band, respectively. The existence of this zone shows that the Ar–N2 discharge can be used to generate active species that can be used for remote surface treatment [10, 14, 22].
In Figs. 2 and 3, photographs corresponding to Ar/N2 discharges generated by using a surfaguide as energy coupling device are shown. These pictures were taken using a digital camera Casio Exilin (EX-FH20). As it can be seen, both the direct and inverse plasma columns are shortened after the introduction of different percentages of nitrogen. Besides, the characteristic filaments observed in the inverse column (plasma radial contraction) that occur in pure Ar plasma disappear [24]. The length of both discharge and postdischarge was measured by only taking into account the visible part for ease of comparison.
External aspect of the discharge for different experimental conditions: P = 250 W; Total gas flow = 2.5 slm. a [N2] = 0 %. b [N2] = 0.2 %. c [N2] = 0.4 %. d [N2] = 0.6 %. e [N2] = 2.6 %. Exposure time = 1s [23]
Figure 2 shows pictures of Ar–N2 discharges sustained with 200 W in all cases. In this figure, the addition of small amounts of N2 (in percentage) to a pure Ar discharge leads to a noticeable reduction of the plasma column length and the appearance of a postdischarge with a length significantly higher than that of the discharge when nitrogen added to the Ar plasma is increased (Table 1). However, N2 concentrations higher than 1 % give place to a drastic shortening of the discharge (Fig. 2e) and then, the plasma shows a morphology similar to that of pure nitrogen plasmas. It can be attributed to an important decrease of the mestastable Ar atoms in the discharge and, consequently, to plasma kinetics dominated by the nitrogen species (See Sect. 6.2). A similar result was found for a TIAGO plasma torch generated in air ambiance with low Ar flows [25]. In that case, the entrance of nitrogen gave place to a significant reduction of the plasma dart (discharge) as the plasma kinetics was more influenced by the nitrogen ions and molecules. Also, a slight change in the discharge color can be also observed, which is a probe of the existence of new species formed into the plasma.
Another important effect noticed when N2 is added to an Ar discharge is the disappearance of radial contraction due to the higher thermal conductivity of the mixed plasma gas relative to that in pure Ar gas [24]. As shown in Fig. 2, an increase of the N2 percentage added to the Ar plasma originates a leading discharge that fills the discharge tube almost completely.
Figure 3 shows several pictures of Ar–N2 discharges for the same power and nitrogen concentration, with the total gas flow varying from 1 to 4 slm. A discharge of pure Ar is added to the figure for comparison. It can be observed that, while the discharge length is only slightly affected, the postdischarge length significantly increases (Table 2). This increase is due to the transport of mestastable nitrogen species by the gas flow towards positions away from the end of the plasma column. As mentioned before this is a very important fact from the point of view of technological applications, since it allows for the contact of the active species in the postdischarge with soft materials such as biological samples without exposing them to high temperatures or charged particles bombardment [26, 27].
Plasma Parameters: Linear Power Density, Electron Density and Gas Temperature
In surface wave discharges, the power transferred from the electromagnetic field to the plasma is expressed through the power per unit length or linear power density (L, in W/cm) [28]. It provides information about the energy available at a given axial position, z, of the discharge since the processes (kinetics) that take place within the discharge depend on this energy. In this way, the plasma parameters such as electron density and gas temperature at a given position have a close relation to the L(z) value.
From an experimental point of view, in a SWD, an approximation to the L value can be obtained from the ratio between the absorbed power (P abs ) and the plasma column length (l) [29] with P abs being the difference between the incident power (P i ), supplied to the generator, and the reflected power (P r ). The L value calculated by this ratio can be considered as an average value for the plasma column and can be represented by \(\bar{L}\). The linear power density is a function of the radius of the discharge tube (e.g. see Eq. 2.1 in [30]). So, comparison of \(\bar{L}\) for discharges contained in tubes with different internal radii (R) requires rather the calculation of \(\bar{L}/\pi R^{2}\) (W/cm3) that can be considered as an estimation of the power dissipated in the discharge volume or power density of the discharge [19].
Figures 4a and 4b show the \(\bar{L}/\pi R^{2}\) values calculated for different Ar–N2 concentrations in discharge tubes of 1.25 and 3 mm of internal radius, respectively. The \(\bar{L}\) value for a pure Ar discharge was introduced in Fig. 4a for comparison purposes. In both cases, one observes that for a given nitrogen concentration in the discharge, increasing the supplied power results in a growth of the power density. This is related to the well known fact that, upon increasing the power supplied to the discharge, the newly created column-portion has larger power and electron densities [31]. On the other hand, for the two used tubes and for a given absorbed power, the power density increases with nitrogen concentration as a result of the decrease of the plasma length (Fig. 2). This behavior is similar to that found in the case of Ar/He discharges [19].
As for the electron density determination, the most commonly used method is the Stark broadening of atomic lines. Particularly, Hα (656.4 nm) and Hβ (486.1 nm) hydrogen lines from the Balmer series are usually chosen since its Stark broadening has been extensively studied [32] in a wide range of experimental conditions. In Fig. 5, a spectrum of the Hβ line is shown. Under our experimental conditions of pressure, the spectral line profiles can be approximated by a Voigt function stemming from the convolution between a Gaussian and a Lorentzian profiles. The Lorentzian width of the profile results from the Stark and van der Waals broadenings, while the Gaussian width is the combination of instrumental and Doppler broadenings. Both Lorentzian and Gaussian widths were separated using a commercial deconvolution process based on the Levenberg–Marquardt nonlinear algorithm. Given that the van der Waals broadening of the Hβ line is negligible under our experimental conditions [9], its Lorentzian width can be ascribed to Stark broadening.
In the spectra emitted by pure argon plasmas at atmospheric pressure, the Hα and Hβ lines are easily observed, however the introduction of nitrogen in the discharge leads to a strong decrease in the intensities of the Balmer lines. Simultaneously, even for amounts of nitrogen as low as 0.5 %, several molecular bands belonging to the first positive system \({{N}}_{2} \left( {{{B}}^{3}\Pi _{{g}} \to {{A}}^{3}\Sigma _{{u}}^{ + } } \right)\) for 503–730 nm, and the second positive system \({{N}}_{2} \left( {{{C}}^{3} \Pi_{{u}} \to {{B}}^{3} \Pi_{{g}} } \right)\) for 300–500 nm of nitrogen appear in the spectra overlapping with the emission of Hα and Hβ lines, making thus more difficult the measurement of electron density from the Stark broadening of the hydrogen lines.
In spite of the aforementioned difficulties, it was possible to estimate electron density in two cases: in a pure Ar plasma sustained in the 1.25 mm tube, and in a mixed gas discharge containing 0.5 % of nitrogen sustained in the 3 mm tube. For the first case, a value of (1.7 ± 0.1) × 1014 cm−3 was obtained, which is in agreement with the typical values of this parameter in pure argon discharges [33]. As for the Ar–N2 discharge, the result was estimated to be (8 ± 2) × 1013 cm−3. In this case, the higher relative uncertainty in the measurement is due to the difficulties for properly deconvoluting the spectral line shape to obtain the Stark width. These results point to a significant decrease in electron density upon the introduction of nitrogen in the discharge which are consistent with those reported for low pressure surface wave discharges sustained in Ar–N2 mixtures [8, 9] and, more significantly, with those obtained for atmospheric pressure Ar–N2 microwave discharges [12, 13], where a theoretical model was used to forecast an electron density of 1 × 1013 cm−3 for a nitrogen concentration of 20 %.
Gas temperature (T gas ) is a measurement of the kinetic energy of the heavy particles (atoms and ions) in the discharge. For its calculation, it is usual to use the rovibrational spectra of certain molecular species, such as OH [34, 35], N 2 [36], N 2 + [34, 37] or CN [38], depending on their availability. Such species can be present in the discharge either as impurities or, as is our case, as a part of the plasma gas. Assuming that the rovibrational levels of the molecules are in equilibrium with the gas atoms, the rotational temperature (T rot ) derived from their rovibrational spectra can be considered to be equal to T gas . This assumption is acceptable in atmospheric pressure plasmas, since the exchanges between translational energy of the molecules and their internal rotational levels are very efficient.
In our research, we have used the P branch of the first negative system (B 2Σ + u – X 2Σ + g ) of the N 2 + (0 – 0 molecular band; 391.4 nm) molecular ion, using the following relation
with I being the intensity of the line, A and B parameters corresponding to each rotational transition [39] (Table 3). The gas temperature is then obtained from the slope of the straight line fitting the points obtained by using (1).
Figure 6 shows the gas temperature results obtained applying the aforementioned technique to the spectra emitted by the Ar–N2 discharges. Uncertainties in the data shown in this figure were calculated as the standard deviation on sets of three measurements, this deviation is in every case below 10 %. For increasing nitrogen amounts up to 2 %, gas temperature increases from 2200 to 4000 K for the case of discharges sustained in the smallest tube, while it increases from 3000 to 4500 K for the case of discharges sustained in the largest tube, respectively. For nitrogen amounts larger than 2 %, T gas tends to remain stable around the maximum value, which is similar to that found in pure nitrogen SWDs sustained under conditions similar to those presented therein [24]. Besides, these results are similar to those found both experimentally [13] and theoretically [12] for Ar–N2 discharges with 20 % of nitrogen content.
Gas temperature as a function of nitrogen content in Ar–N2 discharges calculated from N 2 + rotational band. Experimental conditions as in Fig. 4
Just as the \(\bar{L}\) parameter was defined before, that is, as a measure of the energy available in the discharge, its value has a close relation with the rest of macroscopic parameters in the discharge, namely electron density and gas temperature. So, for a given geometry, an increase of \(\bar{L}\) value should be reflected in an increase of these parameters. However, with the addition of N2 to the plasma gas, only the gas temperature increases with the \(\bar{L}\) value, while the electron density is reduced. This leads us to think that a part of the increase in the energy in the discharge is not being used in processes leading to the ionization, but in elastic energy interchanges. On the other hand, regarding the gas temperature, the fact that it increases for increasing nitrogen amounts has been also reported in atmospheric pressure inductively coupled plasmas sustained in argon and nitrogen [40]. Since the nitrogen molecule possesses rotational and vibrational levels which are easily accessible from an energetic point of view, it is easier to redistribute electron energy among heavy particles.
Emission Spectra from the Atomic and Molecular Species of Ar/N2 Plasmas
As it has been mentioned before, the nitrogen introduction into the discharge induces significant changes in the external morphology and the color of the discharge, being the latter a result of the formation of excited nitrogen species into the plasma. In Fig. 7 a series of spectra in the 230–730 nm interval for Ar/N2 discharges appear depicted. Figure 7a–c corresponds to plasmas sustained in tubes with 1.25 mm of internal radius and Fig. 7d is representative of discharges sustained in the large tube (3 mm of internal radii) utilized in the current study. A pure argon discharge has been included for comparison (Fig. 7a).
Spectrum emitted by a pure argon such as that shown in Fig. 7a is dominated by emissions belonging to the atomic system of argon; but, emissions relative to OH (A 2Σ+ → X 2Π) and NH (A 3Π → X 3Σ) transitions reveals the presence of water and nitrogen impurities contained in the plasma gas.
When nitrogen is added to the discharge in amounts as small as 0.5 % (Fig. 7b), molecular bands from the nitrogen excited molecules and molecular ions (N 2 +) appear in the spectra emitted by the discharge. Table 4 summarizes the molecular bands observed in spectra recorded for the plasma considered in the current study. On the other hand, the intensities of the spectral lines corresponding to 5p and upper levels sharply decrease. When the nitrogen amount in the gas mixture is increased, the intensities of the band-heads corresponding to nitrogen molecular species growth and only the Ar atomic lines belonging to the 4p levels can be observed in the spectrum.
On the other hand, analyzing the spectra in Fig. 7 for the smallest tube and nitrogen concentration above 1.5 %, atomic silicon lines are also observed. Particularly a sextuplet of SiI appears around 250 nm (Fig. 7c). The observation of these lines is related to the disappearance of radial contraction of the discharge. So, in tubes of small radius, the discharge expansion leads to the erosion of the plasma tube by the interaction of the plasma species with the tube walls, thus eroding them and extracting silicon atoms which are excited by the discharge species. It was observed that for smaller diameter tubes an increase of nitrogen concentration on plasma gas mixture led to an increase in the intensity of the silicon spectral lines. This fact reveals a fast deterioration of the quartz tube. For this reason, in the case of experiments with the tube of 3 mm of inner diameter, the discharge tube was axially surrounded by another quartz tube 8.5 mm of internal radius as a jacket through which a dielectric liquid (α-tetradecene) was circulated. This liquid absorbs the ultraviolet radiation and then, the emissions below 350 nm are not detectable in the considered spectral range of the current study [41]. At 10 % of nitrogen in the Ar–N2 mixture, the spectrum is dominated by molecular emissions with an important drop of 4p Ar excited levels and a disappearance of the higher ones (> 5p Ar levels). This behavior differs from that found in plasmas sustained in Ar/He [31] and Ar/Ne [20] mixtures. In those cases, the spectra were mainly dominated by the emission of excited argon atoms up to reaching higher concentrations (>95 %) of the added gas (He or Ne).
These differences among the Ar–N2 plasmas and those generated by using Ar/He and Ar/Ne mixtures are due to the way (steps) that allows to the excitation/ionization of atoms and molecules in these plasmas; these processes depend on the energy of the species participating in them. As it is well known, the excitation processes in plasmas generated at atmospheric pressure take place mainly stepwise [42]. In a first step, the atoms or molecules are excited to metastable levels by means of inelastic collisions between the plasma electrons and the ground atoms. Due to their relatively longlife as compared to the rest of the species composing the plasma, these metastable levels are considered the departure for excitation and ionization processes. These reactions can be written as
In these processes A represents both atoms and molecules. Superscripts 0, m and * refer to ground, metastable and excited levels, respectively.
For a molecular gas in the plasma mixture, such as nitrogen, each one of the excited levels of the molecule has different vibrational states. These vibrational states can be populated by means of collisions with low energy electrons or with the heavy particles of the discharge which have enough energy to take part in these excitations reactions. Besides, for molecules, dissociation reactions have to be also considered; particularly, from upper vibrational levels belonging to an electronic state which allows for the formation of atoms.
In the case of Ar plasma, the first step demands the participation of electrons with energies higher than 11.5 eV. When another atomic gas like He or Ne is added to Ar, the excitation of the metastable states of He and Ne require collisions with electrons of higher energy than for Ar, 19.8 and 16.6 eV, respectively. This fact explains that the spectra emitted by Ar/He and Ar/Ne discharges are dominated by emissions of Ar atoms. However, when the N2 forms part of the gas mixture used as plasma gas, the metastable levels of nitrogen, represented by \(N_{2} \left( {A^{3}\Sigma _{u}^{ + } } \right)\) have an energy equal to 6.17 eV which is lower than the metastable level for Ar atom (Fig. 8).
From the previous discussion, it can be stated that nitrogen introduction in the Ar plasma gives place to a competition between the excitation of Ar atoms and the nitrogen molecules by collisions with the plasma electrons. This fact can be seen in the spectra (Fig. 7). With the increase of nitrogen concentration in the gas mixture, a quick increase on N2 molecular bands intensities is observed while the emission of excited Ar atoms decreases (4p Ar* levels) or almost disappears (≥ 5p levels).
Plasma Kinetics
Simplified Kinetic Model
A simplified colisional-radiative kinetic model was developed in order to gain some insight in the discharge kinetics. This model is a very rough approximation and is far from being complete, but its purpose is not to accurately describe the complexity of a molecular-gas plasma, but to account for the main reactions. The model takes into account the ground state for the neutral argon atom, the 4s excited states and the ground state of both the atomic and molecular ions, Ar + and Ar +2 . As for the nitrogen molecule, the model considers the ground state, \(N_{2} \left( {X^{1}\Sigma _{g}^{ + } } \right)\), together with the \(N_{2} \left( {A^{3}\Sigma _{u}^{ + } } \right)\), \(N_{2} \left( {B^{3}\Pi _{g} } \right)\) and \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) electronic excited molecular species. Besides, two levels of the nitrogen molecular ion, \(N_{2}^{ + } \left( {X^{2}\Sigma _{g}^{ + } } \right)\) and \(N_{2}^{ + } \left( {B^{2}\Sigma _{u}^{ + } } \right)\), are considered. No vibrational level is taken into account for any of the nitrogen species.
The model considers electron impact excitation and ionization processes for both the ground and the 4s levels of argon and the corresponding reversal processes. The collisional cross sections for the excitation and ionization reactions have been taken from Drawin [43], while the corresponding coefficients for the reversal processes, electron impact deexcitation and recombination where calculated using the detailed balance principle. Besides, Ar +2 ions are considered to be created by impact with neutral argon atoms (2) and lost by the reversal process, electron impact conversion (3) to atomic ions and dissociative recombination (4) resulting in ground state (30 %) and 4s atoms (70 %) [44].
As for the nitrogen molecule, electron impact excitation processes were taken into account for the generation of \(N_{2} \left( {A^{3}\Sigma _{u}^{ + } } \right)\), \(N_{2} \left( {B^{3}\Pi _{g} } \right)\) and \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) from the ground state of the neutral molecule and the excitation of \(N_{2}^{ + } \left( {B^{2}\Sigma _{u}^{ + } } \right)\) from the ground state of the molecular ion, \(N_{2}^{ + } \left( {X^{2}\Sigma _{g}^{ + } } \right)\). In the same way, electron collision ionization of the ground state molecular ion from the ground state and \(N_{2} \left( {A^{3}\Sigma _{u}^{ + } } \right)\), \(N_{2} \left( {B^{3}\Pi _{g} } \right)\) and \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) levels were also taken into account. The collisional cross sections for these reactions were taken from [45] and [46], while the coefficients corresponding to the reversal processes were calculated using the detailed balance principle. Penning excitation of the \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) due to the excitation transfer from metastable argon atoms and charge transfer reactions between argon and nitrogen species were also taken into account and the corresponding coefficients were taken from [47, 48]. Electron impact nitrogen dissociation of the ground molecular and ion levels was taken into account with coefficients taken from [45] and [47], respectively.
Radiation losses of the resonant levels of argon and the \(N_{2} \left( {B^{3}\Pi _{g} } \right)\), \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) and \(N_{2}^{ + } \left( {B^{2} \Sigma_{u}^{ + } } \right)\) species of nitrogen were taken into account with radiation coefficients \(\nu_{j}\) taken from [47]. Diffusion losses were also taken into account for the argon metastable and ion species, as well as for all the considered nitrogen species. Since the model does not take into account the spatial resolution of the discharge, the term \(D_{j} \cdot\nabla^{2} n_{j}\) that makes reference to these processes in the kinetic equations, was simplified in the form \(- \left( {D_{j} /\Lambda ^{2} } \right) \cdot n_{j}\), with D j being the diffusion coefficient for the jth species taken from [45] and Λ being the effective diffusion length given by
with R being the inner radius of the tube containing the discharge and l the length of the discharge.
Finally, the model assumes the electron energy distribution function to be maxwellian. While this assumption has been successfully applied to the modeling of atmospheric pressure argon plasmas [44], it is important to remark that this approximation may not necessarily hold while working with molecular gases.
For each nitrogen concentration, the model evaluates the reaction coefficients using the gas temperature and electron density data obtained from Sect. 4 as input, together with an initial estimation of the electron temperature based on the data available in the literature [29, 44]. After that, the set of kinetic equations is solved, obtaining the density of each of the considered species, together with the reaction coefficients. The estimation of electron temperature is changed in case the quasineutrality condition is not met.
Atomic Argon Lines
Figure 9 shows the variation of the intensity of a particular Ar spectral line of atomic argon associated to the 4p–4s transition. As it has been mentioned, in pure-argon surface-wave discharges, the excitation/ionization kinetics is known to be mainly stepwise [42]. However, some other processes, such as dissociative recombination of argon molecular ions, are also known to play a significant role in their population [44]. Similarly, Ar(4p) levels are populated by electron excitation from argon metastables and the density of both species are closely related. This allows us for plotting in Fig. 9 the density of the 4s levels calculated using our simplified model together with the intensity of the 706.7 nm Ar spectral line which is proportional to the density of 4p levels. As can be seen, even for amounts of nitrogen as low as 1 %, the intensity of the Ar line, sharply decreases tenfold. This sharp drop is related to a decrease in the density of the upper levels of the transition, 4p. Other argon lines belonging to transitions associated with different upper levels could not be analyzed because either their intensities are too low after nitrogen introduction, or their emission is overlapped with that of nitrogen bands. Our simplified model also predicts a sharp drop in the 4s level, which is in agreement with the experimental results for the 4p levels.
Being nitrogen a molecular gas, its different electron configurations have plenty vibrational states that can be populated by inelastic collisions with electrons. Particularly, it has been shown that, at low pressures, the population of the vibrational levels of the ground state nitrogen molecule significantly affects the population of electrons with energies larger than 2 eV in pure nitrogen discharges [49]. This reduces the energy available for excitation/ionization processes, leading to lower electron densities as the power supplied to the discharge is used in gas heating.
According to the results provided by our model, the reduction in electron density upon nitrogen introduction significantly affects electron impact excitation rates of argon ground level atoms towards 4s levels, being the main reason for the reduction. Several studies [8, 9, 12, 13] have suggested that the decrease in the density of metastable atoms with nitrogen concentration takes place due to effective Penning excitation processes leading to \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) production by reaction (6).
This reaction is favored by the nearly resonant energies for these species; that is, 11.50 eV for Ar mestatasble atoms and 11.03 eV for \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) nitrogen excited molecules. However, reaction (6) should be accompanied by an increase in the signal of the second positive system corresponding to transition for this \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) level to \(N_{2} \left( {B^{3} \Pi_{g} } \right)\) state. In our case, the intensity of this emission only increases with nitrogen up to 1 % in the discharge. From this percentage, the signal of the second positive system tends to a slow decrease maintaining almost constant from 2 % in the plasma gas as it will be presented in the next section. So, another explanation for the decrease of the Ar(4p) levels should be found (see Sect. 6.3).
Nitrogen Molecular Bands
Figure 10 shows the intensity of the bandhead of the first negative system occurring at 391.4 nm for the experimental conditions considered in the present study, originated by the radiation emission of the \(N_{2}^{ + } \left( {B^{2} \Sigma_{u}^{ + } } \right)\) molecules. As can be seen, the intensity sharply increases at first upon introduction of amounts of nitrogen as low as 1.0 %. Later, for nitrogen concentrations over 2 %, the intensity, and thus the concentration, increases linearly. A similar behavior is found from the results of our simplified model, also represented in Fig. 9. Thought there is a small disagreement for low nitrogen concentrations, its results fit reasonably well the experimental ones in spite of its simplicity.
According to our model, \(N_{2}^{ + } \left( {B^{2} \Sigma_{u}^{ + } } \right)\) is mainly generated by electron impact excitation of the ground state ion with electrons of low energy (≈ 2 eV) and depopulated by radiation to this level. On the other hand, the ground state \(N_{2}^{ + } \left( {X^{2} \Sigma_{g}^{ + } } \right)\) ion is mostly populated by charge transfer reactions (7), due the resonance in energy for these species, 11.6 eV for \(N_{2} \left( {X^{1} \Sigma_{g}^{ + } } \right)\) and 5.9 eV for Ar +.
Other contributions to the population of excited levels of nitrogen molecular ion could be the stepwise ionization from \(N_{2} \left( {B^{3} \Pi_{g} } \right)\) and \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) with electrons of 11.4 and 7.7 eV, respectively. A similar conclusion was drawn in the theoretical model presented in [12, 13]. Besides, quenching of the \(N_{2}^{ + } \left( {B^{2} \Sigma_{u}^{ + } } \right)\) due to collisions with neutral (A 0) species (8) is also possible and, according to some studies [50, 51], it would play a significant role in the depopulation of this level, especially at high pressures, meaning that the population density calculated using our model would be overestimated.
Figure 11 shows the intensity of the bandhead of the second positive system occurring at 337.1 nm. This emission is originated by the radiation decay of the \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) molecules in the near ultraviolet region of the spectra. Due to the absorption of radiation by the external coolant in this spectral region, it was only possible to detect it for the first set of experiments, limited to a nitrogen content of 3.5 %. As happens in the case of the \(N_{2}^{ + } \left( {B^{2}\Sigma _{u}^{ + } } \right)\) density, it peaks around 1 %. From there on, the experimental values show a slow decrease followed by a plateau.
Observing Fig. 11, the results of our model show an increase in the intensity of the second positive system which is in agreement with a significant loss of Ar(4s) by reaction (6). Nevertheless, the experimental values show the opposite behavior, seeming that electrons with energy of 11.5 eV are also contributing to the population of \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) by direct excitation from the nitrogen molecules in ground state. As a result, a competition between the Ar atoms and the nitrogen molecules with electrons belonging to EEDF tail is established in the discharge. This competition would also explain the drastic decrease of 4s and 4p levels shown in Sect. 6.2. Another important loss mechanism explaining the behavior of the second positive system would be the ionization of \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) towards the \(N_{2}^{ + } \left( {X^{2} \Sigma_{g}^{ + } } \right)\) level, requiring low energy electrons (3–4 eV). However, the theoretical calculations show that radiation losses towards the \(N_{2} \left( {B^{3} \Pi_{g} } \right)\) level are much more relevant.
There are a number of processes that can be invoked to explain the disagreement between the experimental and theoretical results for the \(N_{2} \left( {C^{3} \Pi_{u} } \right)\) species. On the one hand, accounting for metastable pooling (9) would lead to larger \(N_{2} \left( {C^{3}\Pi _{u} } \right)\), thus increasing the disagreement with the experimental results. On the other hand including quenching of the \(N_{2} \left( {C^{3}\Pi _{u} } \right)\) with neutrals (10) would lead to lower densities, partly compensating the dissagerement, especially at lower nitrogen concentrations. However, considering a non-Maxwellian EEDF would lead to significantly lower direct electron impact population rates, thus improving the agreement between experimental al theoretical results.
Figure 12 shows the variation of the intensity of the bandhead belonging to the first positive system of nitrogen, that is, the decay from \(N_{2} \left( {B^{3} \Pi_{g} } \right)\) to \(N_{2} \left( {A^{3} \Sigma_{u}^{ + } } \right)\) with nitrogen concentration in the discharge. The \(N_{2} \left( {A^{3} \Sigma_{u}^{ + } } \right)\) is a nitrogen molecule excited at metastable level with long-live time. These metastable molecules and nitrogen atoms are the species that are present in the nitrogen postdischarge [52].
As it can be seen, in this case, the results of the kinetic model are in good agreement with the experimental results. Examination of the reaction rates show that \(N_{2} \left( {A^{3} \Sigma_{u}^{ + } } \right)\) molecular level is mainly populated by radiative decay from the \(N_{2} \left( {B^{3} \Pi_{g} } \right)\).
The emission of the first positive system is bonded to population of nitrogen molecules at the \(B^{3} \Pi_{g}\) level. Two main processes can be followed for the generation of these molecules
Observing Figs. 11 and 12, the results point to reaction (11), rather than reaction (12) as the main contributor to the population of the \(N_{2} \left( {B^{3} \Pi_{g} } \right)\) level. This would imply that a significant concentration of nitrogen atoms exists into the discharge, arising mainly from dissociative recombination and dissociation of ground state nitrogen molecules by direct impact dissociation. The latter processes, requiring low energy electrons also needed for the Ar stepwise excitation described in Sect. 5, are another indication of the competition between Ar and N2 species for the discharge electrons; where nitrogen takes control of the discharge excitation kinetics even at low concentrations, as it can be observed in emission spectra shown in Fig. 7.
Finally, the formation of \(N_{2} \left( {A^{3} \Sigma_{u}^{ + } } \right)\) and nitrogen atoms within the discharge and the transport of these species by the gas flux towards positions outside the plasma zone gives place to the postdischarge in Ar–N2 plasmas. The presence of Ar atoms in the plasma gas contributes to the formation of excited nitrogen molecules and nitrogen molecular ions via reactions (6) and (7), which are involved in the reactions generating the aforementioned species. In our case, the theoretical calculations predict a 10−4 relative concentration of the metastable nitrogen molecule, a similar value to that found in more complex models [12].
Conclusions
An experimental spectroscopic characterization of a microwave (2.45 GHz) surface-wave argon–nitrogen discharge sustained at atmospheric pressure with nitrogen amounts up to 10 % has been performed. Upon introduction of small nitrogen concentrations, the discharge undergoes several aspectual changes, being the most relevant the apparition of a diffuse bright zone after the downstream column (postdischarge), revealing the existence of long-lived excited species in their transport out of the discharge zone due to the action of the flow. Besides that, the radial contraction of the discharge disappears due to the increase in thermal conductivity of the gas mixture
The introduction of nitrogen induces an increase in gas temperature together with a strong decrease of electron density as a consequence of the competition between argon and nitrogen in the excitation/ionization kinetics. These modifications reflect in the spectra emitted by the discharge, where a strong reduction of the emissions originated by argon excited species is accompanied by the appearance of molecular bands belonging to nitrogen. The use of a simplified kinetic model reveals that, besides the competition between argon and nitrogen, there is a significant contribution of Penning reactions between argon metastables and ground state nitrogen molecules and charge transfer reactions that ultimately contribute to the generation of nitrogen metastable molecules and atoms that are essential for the existence of the postdischarge.
References
Helix MJ, Vaidyanathan KV, Streetman BG, Dietrich HB, Chatterjee PK (1978) R.F. plasma deposition of silicon nitride layers. Thin Solid Films 55:143–148. doi:10.1016/0040-6090(78)90082-2
Lucovsky G (1987) Plasma enhanced chemical vapor deposition: differences between direct and remote plasma excitation. J Vac Sci Technol A Vac Surf Film 5:2231. doi:10.1116/1.574963
Kato I, Noguchi K, Numada K (1987) Preparation of silicon nitride films at room temperature using double-tubed coaxial line-type microwave plasma chemical vapor deposition system. J Appl Phys 62:492. doi:10.1063/1.339772
Baclez E, Mutel B, Dessaux O, Goudmand P, Grimblot J, Gengembre L (1997) Elaboration and study of an elastic hard Si-based coating obtained at room temperature from a far cold remote nitrogen plasma. Thin Solid Films 303:156–166. doi:10.1016/S0040-6090(97)00059-X
Normand F, Granier A, Leprince P, Marec J, Shi MK, Clouet F (1995) Polymer treatment in the flowing afterglow of an oxygen microwave discharge: active species profile concentrations and kinetics of the functionalization. Plasma Chem Plasma Process 15:173–198. doi:10.1007/BF01459695
Ricard A, Oseguera-Pena JE, Falk L, Michel H, Gantois M (1990) Active species in microwave postdischarge for steel-surface nitriding. IEEE Trans Plasma Sci 18:940–944. doi:10.1109/27.61507
Mérel P, Tabbal M, Chaker M, Moisan M, Ricard A (1998) Influence of the field frequency on the nitrogen atom yield in the remote plasma of an high frequency discharge. Plasma Sources Sci Technol 7:550–556. doi:10.1088/0963-0252/7/4/012
Henriques J, Tatarova E, Guerra V, Ferreira CM (2002) Wave driven N2–Ar discharge. I. Self-consistent theoretical model. J Appl Phys 91:5622. doi:10.1063/1.1462842
Henriques J, Tatarova E, Dias FM, Ferreira CM (2002) Wave driven N2-Ar discharge. II. Experiment and comparison with theory. J Appl Phys 91:5632–5639. doi:10.1063/1.1462843
Clement F, Svarnas P, Marlin L, Gkleios A, Held B (2011) Atmospheric-pressure plasma microjet of argon–nitrogen mixtures directed by dielectric flexible tubes. IEEE Trans Plasma Sci 39:2364–2365. doi:10.1109/TPS.2011.2159246
Huai Y, Li S-Z, Li H, Wu K, Zhang J, Wang S, Wang Y (2014) Characteristic study of an atmospheric-pressure radio-frequency capacitive argon/nitrogen plasma discharge. IEEE Trans Plasma Sci 42:1648–1653. doi:10.1109/TPS.2014.2320263
Henriques J, Tatarova E, Ferreira CM (2011) Microwave N2–Ar plasma torch. I. Modeling. J Appl Phys. doi:10.1063/1.3532055
Henriques J, Tatarova E, Dias FM, Ferreira CM (2011) Microwave N2–Ar plasma torch. II. Experiment and comparison with theory. J Appl Phys 109:023302. doi:10.1063/1.3532056
Callede G, Deschamps J, Godart JL, Ricard A (1991) Active nitrogen atoms in an atmospheric pressure flowing Ar–N2 microwave discharge. J Phys D Appl Phys 24:909–914. doi:10.1088/0022-3727/24/6/015
Ricard A, Tetreault J, Hubert J (1991) Nitrogen atom recobination in high pressure Ar–N2 flowing post-discharges. J Phys B At Mol Opt Phys 24:1115–1123. doi:10.1088/0953-4075/24/5/030
Moisan M, Etemadi E, Rostaing JC (1998) Device for gas excitation by means of a surface-wave plasma and gas treatment installation including such device. French Patent No. 2762748 (1998), European Patent No. EP. 0 874 537 A1
Martínez-Aguilar J, Castaños-Martínez E, García MC, Calzada MD (2007) Influence of the gas flow rate on the column length and the electron density in an argon surface wave sustained discharge at atmospheric pressure. In: Proceedings of the XXVIII international conference on phenomena in ionized gases, Prague, pp 923–925
Muñoz J, Bravo JA, Calzada MD (2009) Surface wave discharges generated with Ar/He and Ar/N2 gas mixtures at atmospheric pressure. Open Spectrosc J 3:52–57
Muñoz J, Calzada MD (2010) Experimental study on equilibrium deviations in atmospheric pressure argon/helium surface wave discharges. Spectrochim Acta Part B At Spectrosc 65:1014–1021. doi:10.1016/j.sab.2010.11.005
Castaños-Martínez E, Moisan M, Kabouzi Y (2009) Achieving non-contracted and non-filamentary rare-gas tubular discharges at atmospheric pressure. J Phys D Appl Phys 42:012003. doi:10.1088/0022-3727/42/1/012003
Piper LG (1994) Further observations on the nitrogen orange afterglow. J Chem Phys 101:10229. doi:10.1063/1.467903
Boisse-Laporte C, Chave-Normand C, Marec J (1997) A microwave plasma source of neutral nitrogen atoms. Plasma Sources Sci Technol 6:70–77. doi:10.1088/0963-0252/6/1/010
Bravo JA, Muñoz J, Sáez M, Calzada MD (2011) Atmospheric pressure Ar–N2 surface-wave discharge morphology. IEEE Trans Plasma Sci 39:2114–2115
Kabouzi Y, Calzada MD, Moisan M, Tran KC, Trassy C (2002) Radial contraction of microwave-sustained plasma columns at atmospheric pressure. J Appl Phys 91:1008. doi:10.1063/1.1425078
Rincón R, Muñoz J, Sáez M, Calzada MD (2013) Spectroscopic characterization of atmospheric pressure argon plasmas sustained with the Torche à Injection Axiale sur Guide d’Ondes. Spectrochim Acta Part B At Spectrosc 81:26–35. doi:10.1016/j.sab.2012.12.006
Rincón R, Yubero C, Calzada MD, Moyano L, Zea L (2015) Plasma Technology as a New Food Preservation Technique. In: Ravishanka Rai V, Jamuna Bai A (eds) Microb. Food Saf. Preserv. Tech. CRC Press, pp 415–429
Calzada MD, Jiménez M, Rincón R (2009) In: Proceedings of the 2nd international workshop on plasmas for environmental issues. Environ. Appl. Surf. wave discharges. Sofía (Bulgaria), pp 77–85
Zakrzewski Z, Moisan M (1995) Plasma sources using long linear microwave field applicators: main features, classification and modelling. Plasma Sources Sci Technol 4:379–397. doi:10.1088/0963-0252/4/3/008
Calzada MD, Sáez M, García MC (2000) Characterization and study of the thermodynamic equilibrium departure of an argon plasma flame produced by a surface-wave sustained discharge. J Appl Phys 88:34. doi:10.1063/1.373619
Nowakowska H, Zakrzewski Z, Moisan M, Lubanski M (1998) Propagation characteristics of surface waves sustaining atmospheric pressure discharges: the influence of the discharge processes. J Phys D Appl Phys 31:1422–1432. doi:10.1088/0022-3727/31/12/005
Muñoz J, Calzada MD (2008) Experimental research on surface wave Ar–He discharges at atmospheric pressure. J Phys D Appl Phys 41:135203. doi:10.1088/0022-3727/41/13/135203
Gigosos MA, Cardeñoso V (1996) New plasma diagnosis tables of hydrogen Stark broadening including ion dynamics. J Phys B At Mol Opt Phys 29:4795–4838. doi:10.1088/0953-4075/29/20/029
Calzada MD, Moisan M, Gamero A, Sola A (1996) Experimental investigation and characterization of the departure from local thermodynamic equilibrium along a surface-wave-sustained discharge at atmospheric pressure. J Appl Phys 80:46. doi:10.1063/1.362748
García MC, Yubero C, Calzada MD, Martínez-Jiménez MP (2005) Spectroscopic characterization of two different microwave (2.45 GHz) induced argon plasmas at atmospheric pressure. Appl Spectrosc 59:519–528
Christova M, Castaños-Martinez E, Calzada MD, Kabouzi Y, Luque JM, Moisan M (2004) Electron density and gas temperature from line broadening in an argon surface-wave-sustained discharge at atmospheric pressure. Appl Spectrosc 58:1032–1037
Hasegawa T, Winefordner JD (1987) Spatially resolved rotational and vibrational temperatures of a neutral nitrogen molecule in the ICP. Spectrochim Acta Part B At Spectrosc 42:637–649. doi:10.1016/0584-8547(87)80120-9
Rodero A, Quintero MC, Sola A, Gamero A (1996) Preliminary spectroscopic experiments with helium microwave induced plasma produced in air by use of a new structure: the axial injection torch. Spectrochim Acta Part B At Spectrosc 51:467–479. doi:10.1016/0584-8547(95)01452-7
Cruden BA, Rao MVVS, Sharma SP, Meyyappan M (2002) Neutral gas temperature estimates in an inductively coupled CF4 plasma by fitting diatomic emission spectra. J Appl Phys 91:8955. doi:10.1063/1.1474614
Mermet J (1987) Inductively Coupled Plasma Emission Spectrometry. Applications and Fundamentales. Wiley-Interscience, New York, Part II
Montaser A, Zhang H (1998) Mass spectrometry with mixed-gas and helium ICPs. In: Inductively coupled plasma mass spectrom, pp 809–890
Muñoz J, Dimitrijević MS, Yubero C, Calzada MD (2009) Using the van der Waals broadening of spectral atomic lines to measure the gas temperature of an argon–helium microwave plasma at atmospheric pressure. Spectrochim Acta Part B At Spectrosc 64:167–172. doi:10.1016/j.sab.2008.11.006
Calzada MD, Rodero A, Sola A, Gamero A (1996) Excitation kinetic in an argon plasma column produced by a surface wave at atmospheric pressure. J Phys Soc Japan 65:948–954. doi:10.1143/JPSJ.65.948
Drawin HW (1967) Collision and transport cross-sections report EUR-CEA-FC-383. Fontenay-aux-Roses (France)
Sáinz A, Margot J, García MC, Calzada MD (2005) Role of dissociative recombination in the excitation kinetics of an argon microwave plasma at atmospheric pressure. J Appl Phys 97:113305. doi:10.1063/1.1922086
Plasma Data Exchange Project, www.lxcat.net. Accessed 15 Jun 2015
Phelps AV. Compilation of Electron Cross Sections. http://jila.colorado.edu/~avp/collision_data/electronneutral/ELECTRON.TXT. Accessed 22 Jun 2015
Kang N, Oh S, Gaboriau F, Ricard A (2011) Determination of the absolute nitrogen atom density in an Ar–N2 ICP discharge. J Korean Phys Soc 59:3031–3036
Kang N, Gaboriau F, Oh S, Ricard A (2011) Modeling and experimental study of molecular nitrogen dissociation in an Ar–N2 discharge. Plasma Sources Sci Technol 20:049802. doi:10.1088/0963-0252/20/4/049802
Ricard A (1996) Reactive plasmas. Société française du vide, Paris
Dilecce G, Ambrico PF, De Benedictis S (2010) On the collision quenching of N2 +(B2Σ +u , v = 0) by N2 and O2 and its influence on the measurement of E/N by intensity ratio of nitrogen spectral bands. J Phys D Appl Phys 43:195201. doi:10.1088/0022-3727/43/19/195201
Zhu X-M, Pu Y-K (2010) Optical emission spectroscopy in low-temperature plasmas containing argon and nitrogen: determination of the electron temperature and density by the line-ratio method. J Phys D Appl Phys 43:403001. doi:10.1088/0022-3727/43/40/403001
Ricard A, Besner A, Hubert J, Moisan M (1988) High nitrogen atom yield downstream of an atmospheric pressure flowing Ar–N2 microwave discharge. J Phys B At Mol Opt Phys 21:L579–L583. doi:10.1088/0953-4075/21/18/006
Acknowledgments
This work has been funded by the Adalusian Regional Council under project FQM-7489 and the FEDER Funds from the European Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bravo, J.A., Rincón, R., Muñoz, J. et al. Spectroscopic Characterization of Argon–Nitrogen Surface-Wave Discharges in Dielectric Tubes at Atmospheric Pressure. Plasma Chem Plasma Process 35, 993–1014 (2015). https://doi.org/10.1007/s11090-015-9647-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-015-9647-4