Abstract
This paper reports on experimental and theoretical studies of the direct current breakdown voltage characteristics for several gases (argon, nitrogen, helium, hydrogen, dry air, oxygen and carbon dioxide) in discharges with parallel-plane electrodes separated from 1 to 100 μm. The breakdown phenomena have been discussed in terms of field emission of electrons from the cathode. Based on the measured breakdown voltage curves, realistic values of the effective yields and the field emission thresholds for considered gases have been estimated. It was found that the secondary electron emission due to high electric field generated in microgaps depends primarily on the electric field E leading directly to the violation of the Paschen’s law. The effective yields due to the field emission for all gases are determined for the first time. Experimental data are supported by the theoretical predicitions that suggest departure from the scaling law and a flattening of the Paschen curves at higher pressures. The obtained results may provide better understending of the breakdown phenomena in microgaps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrical breakdown comprises an involved set of transient processes which are poorly understood even in the present day. Plasma breakdown as an important fundamental process in plasma science has been a subject of numerous investigations due to its relevance for both industrial applications [1–5] and a necessity of gaining a better understanding of the complex mechanisms of gas discharge phenomena [6–10]. Gas breakdown conditions in a discharge device are needed for optimization of plasma technological processes since devices with micrometer and sub-micrometer gaps can face a serious challenge due to electrical breakdown manufacturing, handling and operation. [11]. At he same time, these data also serve as input data for plasma discharge modelling [12, 13].
In large scale systems, the experimentally observed Paschen law has been successfully explained by the Townsend theory. The validity of the Paschen law which dictates the breakdown voltage for a particular gas as a function of the pd product (operating pressure times the electrode spacing) was confirmed for variety of discharge conditions [14]. This breakdown voltage curve represents a balance between the number of electrons lost by diffusion and drift in the interelectrode gap and the number of secondary electrons generated at the cathode [15]. The Paschen’s law is based on the observation that, over a large range of pressures and electrode separations, the probability of the ionization per collision in the gas and the probability of the production of electrons by ions by a secondary process are both dependent on the average kinetic energy of the electrons and ions and therefore on the reduced electric field E/N. However, Townsend mechanism when applied to breakdown at atmospheric pressure was found to have certain drawbacks [16, 17].
Electrical breakdown in microgaps can result from the mechanisms other than the Townsend avalanche within an ionized gas. These other mechanisms that are not considered in the theory behind the Paschen curve are closely related to the phenomena of electron field emission and tunneling [18, 19]. In spite of large number of publications devoted to the measurement of breakdown voltages at micro separation there exist only few studies in the literature for separation smaller than 300 μm and in homogeneous electric field [20, 21]. For this reason we built the new system to measure the direct-current (DC) breakdown from 100 μm down to 1 μm separations between plan-parallel electrodes in homogeneous electric field.
In this paper, results of measurements carried out in DC microdischarges for a number of gases (argon, nitrogen, helium, hydrogen, dry air, oxygen and carbon dioxide) are presented and analyzed. The primary goal of this paper is to demonstrate that the electrical breakdown in microgaps does occur at the voltages far below the Paschen curve minimum and that the modified Paschen curve should be used instead for micron and sub-micron gaps. Electrons from the field emission are one of the possible reasons why the breakdown and sparks occur at the voltages far below the Paschen curve minimum, which of course is not possible if one only considers the Townsend avalanche mechanisms for the gas phase and the surface ionization that are normally used to generate the Paschen curve. Based on the measured breakdown voltage curves, the dependence of the effective secondary yields on the E/p ratio (the electric field over the gas pressure) and on the electric field E have been evaluated. The effective yields versus the electric field clearly demonstrate that their values in microgaps are large (>1) due to field emission effect. The effective yields are then fitted in accordance with the expression that incorporates the field emission effect (taken from [22]) and parameters correpsonding to the expression for breakdown voltage in microgaps have been found. Due to the lack of experimental data in microgaps, there were no values for the effective yields for the most of the gases considered here. To the author’s knowledge, except for the yields for air [22] and recently hydrogen [23] and argon [24], the effective yields in microgaps for other gases are determined for the first time, now. Finally, obtained parameters have been used to determine the electric field strength in microgaps at various gas pressures.
Experimental
Measurements were performed for the gas pressure of about 746.3 Torr (±6 Torr) and for the gap sizes in the range from 1 up to 100 μm by using the experimental arrangements shown in Fig. 1a. The used experimental set-up has been well documented in previous publications [23–26], so only a brief description will be given here.
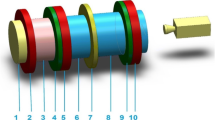
The vacuum chamber consists of three parts: positioner for centering the electrode position in three directions and tilting the upper electrode which is located in the upper part. In the middle part there is a glass crux with four fused silica windows. In the bottom part there is also positioning system for tilting electrode as well as an improved system for very ultra fine tilting. The electrode is fixed in the cradle with micrometric screw enabling to achieve parallelism of the electrodes with an accuracy of 1 μm. Molybdenum electrodes with Bruce profile shown in Fig. 1b demonstrates that the discharge burns on the edges stressing the importance of the isolation of the electrodes for the Paschen curve measurements. The electrodes were mechanically polished and chemically cleaned in ultrasonic bath. One of the electrodes was fixed while the other was moved continuously with micrometer scale linear feed-through. The 0 μm separation of the electrodes was established by checking the electrical contact between the electrodes and then the movable electrode was pulled away by the means of the micrometer screw at the upper electrode. The the electrode surface has been polished by the finest diamond paste (0.25 μm grain size) in order to achieve the average roughness of the electrode better then 0.25 μm and measured by using SEM.
Determination of the breakdown voltages were based on recording the current–voltage characteristics by using a digital oscilloscope and the AD card (National Instruments NI USB-6211). The potential across the discharge tube was increased with rate 0.3 to 20 V/s (depends on the pressure). Faster ramp voltage can be used in the left side of Paschen curve were free electrons are emitted easily from electrode surface by field emission.
Results and Discussion
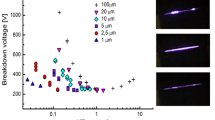
In all Figures, presented here, we used: orange symbols for carbon dioxide, green symbols for nitrogen, red symbols for hydrogen, blue symbols for oxygen, black symbols for dry air, pink symbols for helium and violet symbols for argon. The breakdown voltages versus the gap size in DC microdischarges for several gases are shown in Fig. 2. For all gases, for higher values of the gap, classical Paschen scaling is observed. For lower values of the gap sizes, however, significant deviations are seen. For all gases, departure from the similarity law take place for the left hand branch of the breakdown voltage curves when the electron mean free path is comparable to the interelectrode separation demonstrating difficulty in using the Paschen theory to fit over the full p·d range. As can be observed, the lowest breakdown voltages were achieved for helium, while breakdown voltage for carbon dioxide has the highest values. Actually, oxygen and the gases that contain oxygen, such as dry air and carbon dioxide, have the breakdown voltages higher as compared to other gases.
Following the procedure described in [27] and using the breakdown voltage curves shown in Fig. 2, we have estimated the dependence of the effective yield for various r gases on the: (a) E/p ratio and (b) the electric field E. The increasing of the yield with increasing the E/p ratio is depicted in Fig. 3a. In line with the expectations, the yield of helium has the highest values, while oxygen and oxygen-containing gases have low yields. Fig. 3b shows increase of the effective yields with increasing the electric field illustrating that when the electric field near the cathode is sufficiently large, electrons can be liberated from the surface by quantum mechanical tunnelling. Furthermore, as an ion approaches the cathode, it could narrow the potential barrier seen by the electrons in the metal resulting in an ion-enhanced electron field emission [28].
The dependence of the effective yield on: a the ratio E/p and b the inverted electric field. Symbols correspond to the data obtained from the measured breakdown voltage curves, while lines represent fit based on Eq. (1)
Due to the fact that the highest electric field is generated at the smallest interelectrode separation, for all considered gases, the values of the yield are much larger for the 1 μm gap. We have compared the minimum and maximum values of the effective yields for various gases (shown in Table 1). For all the gases, the minimal values of the yields vary from around 1 × 10−8 from carbon dioxide up to around 0.1 for helium. On the other hand, for the gaps of 2.5 and 1 μm, the effective yields are much higher (in the case of helium even higher than 4) due to field emission effect which is in the line with our explanation about the departure from the standard scaling law observed in microgaps (see Fig. 2). An explicit expression for the electron yield per ion (γ) that incorporates this ion-enhanced field emission is given by [29]:
where K and D are material and gas dependent constants. As the electric field E near the cathode becomes larger than the threshold value D, the electron yield per ion increases rapidly. The effective yields shown in Fig. 3b can be fitted by the expreesion (1) with parameters D and K for various gases listed in Table 2. From the slopes of the straight lines plotted in Fig. 3b, one may conclude that the constant D does not depends on the cathode material only, but also on the gas. Actually, for the same cathod material, parameter D has was found to be equal to 1.156, 1.709, 1.501, 3.276, 4.462, 3.999 and 7.018 × 108V/cm for helium, argon, hydrogen, oxygen, dry air, nitrogen and carbon dioxide, respectively.
Previously determined values of the paramters D and K, can be used in the equation that governs the mechanism of the DC breakdown in micrometer derived in Ref. [30]:
in order to determine the breakdown voltages for various molecular gases in microgaps at different pressures. Contanst A and B for various gases can be found elsewhere, for example in [7, 8]. The variation of the electric field strength (the breakdown voltage versus the gap size) with the gap spacing at two different pressures are plotted in Fig. 4.
The electric field strength values based on Eq. (1), with parameters K and D listed in Table 2. The exponential dependency of the field emissions on the electric field strength pins the electric field during breakdown to the threshold for field emissions (see parameter D in Table 2) and allows for a rapid reduction of the breakdown voltage as gap size is reduced.
The effect of the pressure on the breakdown voltage at the interelectrode separations of 0.1 and 0.5 μm are depicted in Fig. 5. In both cases, the breakdown voltage decreases with increasing the pressure, although more evident decline is for 0.1 μm. One may noticed that at low pressures the breakdown voltage curve follows scaling law. At intermediate pressures, however, breakdown voltages are almost constant and correspond to the minimum in the Paschen curve for that gas and electrode material [5]. In accordance with the Ref. [5], delimiting these two regimes the pressure value for which the transition between two regimes occurs can be determined. Our theoretical predictions for 0.5 μm gap, indicate that the transition between two regimes occures at the lower pressure for nitrogen than for other gases.
Conclusions
Novel experimental results for the DC breakdown voltage curves for several gases (argon, nitrogen, helium, hydrogen, dry air, oxygen and carbon dioxide) for gap sizes ranging from 1 to 100 μm are presented and analyzed. For all gases, failure of the standard scaling law is observed due to electrons enhanced from field emission, which of course is not possible if one only considers the Townsend avalanche mechanisms for gas ionization used to generate the Paschen curve. At pressures below one atmosphere, care must be taken when applying the Paschen formula to gaps in the micron range. For operation at one atmosphere, standard Paschen behavior is observed for gaps larger than 10 μm. As gap size is reduced, however, the electric field increases and the ion-enhanced field emissions become important and a lowering of the breakdown voltage and a departure from the Paschen curve are observed. Based on the breakdown voltage curves, the effective yields for various gases have been determined. Parameters K and D were also determined as well as the field emission threshold for various gases. Theoretical predictions including field emission effect confirm a flattening of the Paschen curves at higher pressures and a fix gap as previosuly observed in [5].
The results shown and discussed here can be useful for microelectronic devices, in localized diagnostics of ICs during their manufacture, in choosing appropriate conditions for electro mechanical micro systems which may eventually lead to nanomachining, in localized treatment of materials and numerous applications in processing and diagnostics, especially in situation with the difficulty in achieving well-defined experimental conditions and the limited diagnostic techniques available for small scale.
References
Ito T, Izaki T, Terashima K (2001) Thin Solid Films 386:300–304
Kogelschatz U (2003) Plasma Chem Plasma P 23:1–46
Becker KH, Kogelschatz U, Schoenbach KH, Barker RJ (2005) Non-equilibrium air plasmas at atmospheric pressure. IOP Publishing Ltd, London
Makabe T, Petrović Z Lj (2006) Plasma Electronics: Applications in Microelectronic Device Fabrication. Taylor&Francis, New York
Patrick Carazzetti P, Shea HR (2009) J Micro/Nanolith MEMS MOEMS 8:031305 (1-9)
Druyvestein MJ, Penning FM (1940) Rev Mod Phys 12:87
Korolev YD, Meys GA (1998) Physics of pulsed breakdown in gases. URO-PRESS, Ural
Raizer YP (1991) Gas discharge physics. Springer, Berlin
Marić D, Malović G, Petrović ZLJ (2009) Plasma Sources Sci T 18:034009 (1–8)
Sameera I, Bhatia R, Ouyang J, Prasad V, Menon R (2013) Appl Phys Lett 102:033102 (1–5)
Terashima K, Howald L, Haefke H, Guntherodt H-J (1996) Thin Solid Films 281–282:634
Kushner MJ (2005) J Phys D Appl Phys 38:1633–1643
Walsh JL, Zhang YT, Iza F, Kong MG (2008) Appl Phys Lett 93:221505 (1-3)
Loeb LB (1939) Fundamental processes of electrical discharges in gases. Wiley, New York
Meek JM, Craggs JD (1953) Electrical breakdown of gases. Oxford University Press, Oxford
Radmilović-Radjenović M, Lee JK, Iza F, Park GY (2005) J Phys.D Appl Phys 38:950–955
Petrović ZLj, Škoro N, Marić D, Mahony C, Maguire PD, Radmilović-Radenović M, Malović G (2008) J Phys D Appl Phys. 41: 194002 (1-5)
Torres JM, Dhariwal RS (1999) Microsyst Technol 6:6–10
Torres JM, Dhariwal RS (1999) Nanotechnology 10(1):102
Chen CH, Yeh JA, Wang PJ (2006) J Micromech Microeng 16:1366–1373
Hourdakis E, Simonds BJ, Zimmerman NM (2006) Rev Sci Instrum N77:034702
Jones FL, Morgan CG (1951) Phys Rev 82:970–971
Klas M, Matejčik Š, Radjenović B, Radmilović-Radjenović M (2012) Phys Lett A 376:1048–1052
Radmilovic-Radjenovic M, Matejcik S, Klas M, Radjenovic B (2013) J Phys D Appl Phys 46:015302 (1-5)
Klas M, Matejčik Š, Radjenović B, Radmilović-Radjenović M (2011) Europhys Lett 95:35002 (1–5)
Radmilović-Radjenović M, Radjenović B, Klas M, Matejčik Š (2012) Micro Nano Lett 7:232–234
Guillot Ph, Belenguer Ph, Therese L, Lavione V, Chollet H (2003) Surf Interface Anal 35:590–592
Radmilović-Radjenović M, Radjenović B (2007) Plasma Sources Sci T 16:337–340
Boyle WS, Kisliuk P (1955) Phys Rev 97:255–259
Radmilović-Radjenović M, Radjenović B (2008) Europhys Lett 83:25001 (1-3)
Acknowledgments
The work has been carried out under Ministry of Education and Science Republic of Serbia 171037 and III41011 projects. This work has also been supported by the VEGA grant agency; project VEGA 1/0514/12, the Slovak Research and Development Agency Projects APVV-0733-11 and DO7RP-0025-11, and the Slovak Research and Development Agency under Contract SK-SRB-0026-11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radmilović-Radjenović, M., Radjenović, B., Matejčik, Š. et al. The Breakdown Phenomena in Micrometer Scale Direct-Current Gas Discharges. Plasma Chem Plasma Process 34, 55–64 (2014). https://doi.org/10.1007/s11090-013-9488-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9488-y