Abstract
Novel apparatus for the generation of underwater plasma based on DC diaphragm discharge excited in a vapor bubble has been developed for decontamination and disinfection of conductive water. The apparatus allows deposition of relatively high applied power into the discharge (order of kW) and the treatment of a relatively large volume of liquid (order of L/min). The apparatus is operated at the quasi-pulse regime with self-terminating discharge pulses (with a repetition rate of 15–20 Hz) generated upon the formation of the vapor bubble inside the diaphragm (capillary) and its subsequent breakdown. The effects of input power, solution conductivity and the method of liquid flow through the reactor on the plasmachemical yield of H2O2 production and degradation of phenol have been determined. The biocidal effects of the apparatus were evaluated on inactivation of bacteria E. coli and E. faecalis suspended in aqueous NaCl solutions and on growth inhibition of the cyanobacterium Planktothrix sp. in natural lake water. The apparatus proved to be capable of efficiently reducing biological contamination in water, especially when operated in the plug-flow regime (up to a 5-log reduction in bacteria after 3 passes through the reactor). In the case of cyanobacteria, the growth inhibition further proceeded after exposure to the discharge and one pass of the biomass through the reactor was sufficient to reduce the algae in the water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrical discharges generated directly in water have been demonstrated to initiate a variety of chemical and physical effects, which have been shown to be effective at degrading a variety of organic compounds and also in the inactivation and destruction of microorganisms in water [1–4]. Since a very highly localized electric field of the order of 1 MV/cm is needed for the electrical breakdown of water, pulsed high voltage and electric field enhancing electrode systems are often used to generate an electrical discharge in water [1, 4]. However, from the technological and application points of view, the need for pulsed high voltage brings the cost and complexity disadvantages of these systems.
In this work, we present novel apparatus for the generation of underwater plasma based on a DC diaphragm discharge excited in a vapor bubble [5]. The apparatus is operated at the pulse regime, although it is charged by a DC power supply, since pulses are generated by physical processes in the device, and allows the deposition of relatively high power into the discharge (order of kW) and the treatment of a relatively large volume of liquid. This is a significant improvement compared to previously reported similar systems excited in vapor bubbles based on pinhole (diaphragm) or capillary discharges [6–20] which, although charged by DC or AC voltage, face problems with their electrical arrangements or wear problems associated with the pinhole in the diaphragm layer, which permit deposition only of low power into the discharge (order of tens to hundreds watts). Thus, only a small volume of generated plasma, a narrow range of operating conditions and the lifetime of the diaphragm (pinhole) are very limiting factors in these systems. The design of the presented DC diaphragm discharge system seeks to solve some of these limitations. Performance of the apparatus was evaluated based on the chemical and biological effects induced in water by plasma produced by the DC diaphragm discharge. The production of H2O2, degradation of phenol, inactivation of bacteria E. coli, E. faecalis, and the growth inhibition of cyanobacterium Planktothrix sp. was studied in dependence on the applied power, the solution conductivity and operating regime used for flow of the liquid through the reactor.
Experimental
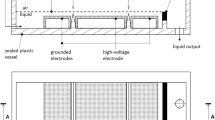
Figure 1 shows the scheme of the high power DC diaphragm discharge apparatus used in this work [5]. Two chambers (1,2) made from polymethyl methacrylate and provided with inlet (3) and outlet (4) ports were filled with aqueous solution (volume of each chamber was 600 mL). The chambers were separated from each other by a dielectric barrier (5) made from polymethyl methacrylate. Electrodes (6,7), one grounded and the second connected to the DC high voltage power source (HV), were placed in the chambers (1,2). A high voltage capacitor (8) was connected in parallel to the DC power supply to lower its output impedance. The chambers (1,2) were electrically connected through a connecting hole (capillary) in the dielectric barrier (9), which was lined with ceramic material made from sintered alumina (relative dielectric permittivity ~10, zero porosity). The liquid did not flow through the connecting hole except for the self-pumping effect of the capillary [20] and no visible changes or erosion were observed on the ceramic material of the capillary through the operation of the discharge. By proper choosing of the connecting hole dimensions (length and diameter) and the capacitance of the high voltage capacitor (8) it was possible to establish in the connecting hole an electrical current of sufficient density needed for the evaporation of a small amount of liquid, and thus, the formation of the vapor bubble inside the capillary and its subsequent electrical breakdown. The net flow of liquid through the capillary would intercept the formation of the vapor bubble inside the capillary since the water in the connecting hole would be continuously cooled down by flowing liquid. In this work, a capillary with an inner diameter of 2 mm and a length of 6 mm was used as the connecting hole in the dielectric barrier between the chambers (1,2). The capacitance of the high voltage capacitor (8) was 200 nF.
The created inhomogeneity (i.e., formation of the vapor bubble inside of the connecting hole) allowed the electrical breakdown of the water and the initiation of an electrical discharge, which strongly expanded into the relatively large volume of surrounding water on both sides of the connecting hole, that is discharge of the positive and the negative polarity was generated simultaneously (see Fig. 2 showing a picture of the underwater plasma generated by the present apparatus). Expansion of the discharge led to the termination of the conditions required for the existence of the discharge and to the interruption of the discharge current between the electrodes. After the end of this process, the connecting hole began to fill back with water and the whole process was repeated. Thus, the apparatus was operated at the pulse regime although it was charged by a DC power supply; pulses were generated by physical processes in the device. Figure 3 shows a typical sequence of voltage and discharge current pulses produced by the presented apparatus under conditions used in this work.
The power input was 2 kW (with the applied voltage of 6 kV). The solution conductivity was 500 μS/cm. Figure 3 shows that the applied DC voltage dropped after the bubble breakdown from its initial value of 6 kV to approximately 4 kV accompanied by the increase in the discharge current with an amplitude of 0.5–2 A. The DC component of the current was approximately 60 mA. After approximately 10–20 ms of discharge duration, the voltage returned to its initial value. Such self-terminating discharge pulses were produced typically with a repetition rate of 15–20 Hz.
The chemical activity of the discharge apparatus was evaluated by measurement of the yield of plasmachemical production of H2O2 and degradation of phenol. The concentration of H2O2 was determined colorimetrically using the reaction of H2O2 with titanyl ions measuring absorbance of the peroxotitanium (IV) complex at 410 nm. The concentrations of phenol and its primary by-products (hydroquinone, catechol, 1,4-benzoquinone, hydroxy-1,4-benzoquinone) were analyzed using the HPLC system Shimadzu LC-10Avp with UV and fluorescence detection. Analyses were made using a 5-μm reversed phase Supelcosil LC-18 column (25 cm × 2.4 mm; Supelco). An isocratic method with a solvent mixture of 10 % acetonitrile and 0.5 % acetic acid in deionized water was used as the eluent with a flow rate of 0.4 mL/min. UV detection was performed at 250, 274 and 290 nm. Fluorescence detection was made with excitation and emission wavelengths 271 and 297 nm, respectively.
The biocidal effects of the experimental apparatus were evaluated on bacteria Escherichia coli CCM 3954 (ATCC 25922) and Enterococcus faecalis CCM 4224 (ATCC 29212). Bacterial suspensions were prepared by preculturing the bacteria cells in the growth medium and then dispersing in NaCl solutions with a final solution conductivity of 300 or 500 μS/cm. The discharge chamber was filled with a suspension of bacteria that was circulated through the reactor using a diaphragm liquid pump with the flow rate of 1 L/min. The total volume of bacterial suspension was 6 L with approximately 4.8 L of the liquid in the external flow circuit comprising from polyethylene tubings and a cooled water reservoir. In the case of plug-flow experiments (i.e., without re-circulation of the bacteria suspension back into the discharge reactor) the initial volume of bacterial suspension was 20 L, which was used in aliquot volume parts in three subsequent experiments. The treated liquid was cooled to maintain isothermal conditions of about 23 °C. The number of bacteria cells in the liquid suspension was assayed by counting colony forming units (CFUs) cultivated on agar plates. The initial amount of bacteria was about 105 CFU/mL in all experiments performed in this study. The viability of the bacteria was determined as the ratio of the concentration of surviving bacteria to the total concentration. The effects of DC diaphragm discharge on cyanobacterium Planktothrix sp. was assayed using the algal growth inhibition test. Aqueous solutions of Planktothrix sp. were prepared from freshwater and cyanobacteria biomass taken from the Plumlov water reservoir, Czech Republic. The total volume of cyanobacteria solution was 20 L. Experiments were performed using the same procedure as with E. coli and E. faecalis. The initial conductivity of cyanobacterial solutions was 285 μS/cm. The inhibition of cyanobacterium growth by the discharge was evaluated by measuring the cell density of discharge-exposed cyanobacteria cells against the unexposed control cultures at 24, 72 and 120 h after treatment by the discharge.
Results and Discussion
Chemical Activity of DC Diaphragm Discharge
The performance of the present DC diaphragm discharge apparatus was evaluated in dependence on the applied power and solution conductivity. Hydrogen peroxide was used as a probe of the plasmachemical activity of the diaphragm discharge in these experiments, since H2O2 is one of the major chemical products of the underwater discharges [2, 21]. It should also be noted that the amounts of H2O2 presented in this work were in all experiments the sum of H2O2 productions obtained by the diaphragm discharge in both chambers (i.e., produced by the positive and negative polarity discharges simultaneously). The production of H2O2 by the diaphragm discharge in water was determined in dependence on the applied power (500–2,500 W) and the solution conductivity (200–500 μS/cm).
Figure 4 shows that the yield of H2O2 increased linearly with the increasing power input, with a maximum yield of 0.51 g/kWh for the highest applied power of 2.5 kW. On the other hand, the solution conductivity had a very negligible effect on the production of hydrogen peroxide under fixed applied power input. Figure 5 shows the production of H2O2 by diaphragm discharge generated in NaCl solutions with conductivity of 285, 350 and 500 μS/cm and power input of 2 kW. A similar result was reported also for AC capillary discharge [15, 16], however, this is quite a different result compared to the dependence of H2O2 energy yields on applied power and conductivity, which is typically observed for the production of H2O2 by the pulsed discharges in water generated with pulse energy input ~J/pulse [21, 22].
The reason for this difference is most likely to be the different mechanisms of the electrical breakdown of water between these two types of underwater discharges, that is higher power applied into the liquid in the diaphragm reactor advances the thermal effects inside the hole (capillary) and, thus, the formation of the vapor bubble inside the diaphragm (capillary) and its subsequent breakdown. Apparently, in such a mechanism, power input is one of the most important parameters. Solution conductivity seems to be a less important factor although it determines the resistance of water and thus the electrical current needed to heat the liquid and create the bubble inside the diaphragm. Concerning the energy efficiency of H2O2 production by DC diaphragm discharge, the obtained values are about one-third of the values typically determined for the production of H2O2 by the pulsed discharges in water generated with pulse energy input ~J/pulse [21]. The reason for such a difference can also be related to the thermal initiation mechanism of the diaphragm discharge, since a part of the total applied power is consumed in Joule heating of the liquid during the pre- and post-breakdown phases and does not contribute directly to the plasma formation and generation of chemical species. Depending on the discharge regime, this fraction can be quite significant [4]. The fraction of the power consumed for the thermal initiation of the discharge was estimated from the voltage and discharge current waveforms (Fig. 2) of approximately 400 W, which was about 25 % of the total average electrical power dissipated in the DC diaphragm discharge. In addition, the power applied into the diaphragm discharge was dissipated in the simultaneous generation of plasma of positive and negative polarity, compared to the pulsed streamer discharge, for which data for H2O2 production were obtained under the positive polarity of the applied power. Therefore, the comparison of the energy efficiency of H2O2 of both systems, considering only applied power into the discharge, is limited.
However, another reason for lower H2O2 energy efficiency might be the different properties of the plasma formed by diaphragm discharge in the vapor bubble and in the pulsed streamer discharge plasma in water. Plasma formation in vapor bubbles is a complex process due to the strong thermal effects in water and the dynamics of the vapor bubbles, which are accompanied by evaporation and condensation processes. The optical emission spectra of discharges in bubbles are quite similar to spectra from the pulsed streamer discharge in low conductive solutions and consist of OH-bands, H, O and atomic lines [4]. However, there are differences in the electron density of formed plasma. Electron densities reported for discharges excited in vapor bubbles are typically of the order of 1020 m−3 [17] while electron densities up to 1025 m−3 were reported in the case of pulsed streamer discharges in water [23–25]. In addition, as the electric field required for the discharge plasma formed in vapor bubbles is smaller (~30 kV/cm) compared to that for the pulsed streamer discharge plasma in water (~1 MV/cm), a lower electron mean energy might be expected in diaphragm discharge plasma. This assumption supports data of Bruggeman et al. [26] reporting a smaller electron temperature in the DC-excited discharge plasma formed in the vapor bubble than in water. The same authors also reported significantly smaller H2O2 production by the discharge formed in the vapor bubble than in the water. Therefore, while the magnitude of the temperature of the plasma and the energy distribution of the electrons in the plasma strongly influence chemical processes induced by electrical discharge plasma in water [27], it is likely that these factors might be the reason for the lower H2O2 production efficiency of diaphragm discharge.
In addition to hydrogen peroxide, chemical activity of the present discharge apparatus was studied on the degradation of phenol, which is often used as an organic compound probe, especially in kinetic studies for the evaluation of oxidative effects caused by electrical discharges in water [2]. Figure 6 shows that about 50 % degradation of 500 μM phenol was obtained in 6 L of NaH2PO4 solution (with conductivity of 500 μS/cm) after 50 min of treatment by DC diaphragm discharge (applied voltage 6 kV, power input 2 kW). The rate of degradation was 5.4 × 10−7 mol/s and the energy yield of removal was 0.1 g/kWh. The degradation of phenol obeyed the first-order kinetics (typical for electrophillic oxidations) giving catechol (CC), hydroquinone (HQ), 1,4-benzoquinone (BQ) and hydroxy-1,4-benzoquinone (HBQ) as primary aromatic products of phenol (Fig. 6).
The pattern of analyzed products indicates that the degradation of phenol was achieved primarily through oxidation by the plasmachemically formed hydroxyl radical which leads to the production of hydroxylated phenol products [2]. The reason for the rather low energy efficiency of phenol degradation might be related to the partial consumption of applied energy for Joule heating of water during the pre- and post-breakdown phases of the DC diaphragm discharge and different properties of plasma generated in vapor bubbles (similar to the reasons for lower yields obtained for H2O2). In terms of the mechanism of phenol degradation, the content of organic carbon determined from concentrations of analyzed primary aromatic OH-products of phenol degradation corresponded to only about 20 % of the total removed phenol organic carbon. Many other phenol products, which were not detected by analytical method used in this work, were likely formed simultaneously (such as, ring cleavage products of saturated and unsaturated aliphatic C1–C6 hydrocarbons) [2]. However, taking into account the thermal initiation mechanism of the diaphragm discharge, the local temperature in the discharge can be very high. Therefore, it might be possible that, in addition to the chemical mechanism of phenol degradation, oxidative decomposition by radicals can be supplemented by thermal decomposition. For example, thermal pyrolysis in plasma generated in bubbles and OH radical reactions in the liquid near the interface were assessed for phenol degradation [28].
Biocidal Effects of DC Diaphragm Discharge
Inactivation of bacteria E. coli and E. faecalis and cyanobacterium Planktothrix sp. have been determined in dependence on the solution conductivity (300 and 500 μS/cm) and operating regime used for the flow of liquid through the reactor (re-circulation vs the plug-flow regime). The power input was 2 kW with an applied voltage of 6 kV. Figure 7 shows inactivation of E. faecalis as a function of the discharge treatment time in NaCl solutions with a conductivity of 300 and 500 μS/cm. The gradual decrease in the number of survival bacteria was observed with increasing the time of the discharge treatment, since the amount of bacteria decreased somewhat faster in conductivity of 500 μS/cm compared to 300 μS/cm. A 4- to 5-log reduction in the number of bacteria was obtained in 6 L volume solution within 15–20 min (required energy approximately 60 kJ per 1-log reduction per 1 L). Similar results were obtained for E. coli (Fig. 8). The faster bacterial inactivation with increasing solution conductivity might be partly related to the increasing contribution of UV light radiation emitted from the discharge, similar to the effect of UV which was reported in the inactivation of bacteria using the pulsed corona discharge in water [29]. The thermal effects of the plasma formed by DC diaphragm discharge might also contribute to bacterial inactivation and their role can rise with higher solution conductivity.
From the viewpoint of the potential use of underwater plasma technology for biological decontamination of water in real applications, it was interesting to evaluate the inactivation efficiency of the present discharge apparatus when operated in a plug-flow regime (i.e., without re-circulation of the bacteria suspension back into the discharge reactor using the initial liquid volume of 20 L). Figure 9 shows the inactivation of E. coli and E. faecalis in dependence on the number of passes of the treated bacterial suspension through the discharge reactor (conductivity of water was 500 μS/cm). As expected, bacterial inactivation increased with the number of passes through the reactor. However, a 4- to 5-log reduction in the number of bacteria was attained after 3 passes through the reactor (required energy approximately 20 kJ per 1-log reduction per 1 L) compared to 15–20 min in the continuous regime (Figs. 7, 8). Thus, using several discharge reactors coupled in series seems to be the option to enhance the time and energy efficiency and to increase the volume capacity of the treated liquid in underwater plasma technology in environmental applications.
Another advantage of operating the diaphragm discharge reactor in a plug-flow regime is shown in Fig. 10, which presents the growth inhibition of cyanobacterium Planktothrix sp. in lake water with biomass after treatment by the discharge in dependence on the number of passes through the reactor. Immediate inhibition of cyanobacteria increased with the number of passes through the discharge (similar to the results obtained for E. coli and E. faecalis in Fig. 9). However, growth inhibition of the algae further proceeded in the days after exposure to the discharge and the same degree of permanent inhibition was eventually attained after one pass as after two passes of the biomass through the reactor. Various mechanisms might be involved in growth inhibition of cyanobacteria. These include rupture of intracellular gas vacuoles in algae cells by physical effects (shockwaves) induced by the discharge in water [30, 31] (which is, however, not a permanent effect since cyanobacteria are able to recover within 5–7 days), and the oxidative stress and damage to cells by reactive oxygen species produced by the plasma in water—either by radicals (such as OH, O, etc.) or by long lived species (such as H2O2, etc.) [3, 32, 33].
Figure 11 shows concentrations of H2O2 produced by DC diaphragm discharge in lake water which was treated under the same experimental conditions as in the case of the cyanobacteria experiments. The concentrations of H2O2 formed in water were already quite high after one pass (~6 mg/L) and sufficient to cause damage to cyanobacteria, which are rather sensitive to the oxidative effects of H2O2 [34–37]. Moreover, it is likely that hydrogen peroxide formed in the lake freshwater upon treatment by plasma was partly decomposed by iron ions, which were present naturally in the lake water (~20 μg/L Fe2+), since lower concentrations of H2O2 were determined in this case compared to the same experiment performed with NaH2PO4 solution (both solutions were without cyanobacteria, see comparison in Fig. 11). Therefore, OH radicals, formed through Fenton’s reaction from hydrogen peroxide, might also contribute to the cyanobacteria inactivation in this case. Another reason for the prolonged and permanent inhibition of cyanobacteria might be the cell lyse of weakened cyanobacteria by other (more resistant) microorganisms (e.g., bacteria) present in the plankton biomass of the lake water. The bacteria-algae interactions were not evaluated in this study but it is evident that in natural aquatic ecosystems more factors have to be considered in addition to the inactivation effects caused directly by plasma. Nevertheless, it is seems that for growth inhibition of cyanobacteria, one pass of the biomass through the discharge might be sufficient, which is a very promising result and further study of these effects is in progress.
Conclusion
Novel apparatus for the generation of underwater plasma based on DC diaphragm discharge excited in a vapor bubble has been developed for the decontamination and disinfection of conductive water. Compared to previously reported similar systems excited in vapor bubbles based on pinhole or capillary discharge, the presented apparatus allows deposition of relatively high applied power into the discharge (order of kW) and treatment of a relatively large volume of liquid (order of L/min). The apparatus is operated at the quasi-pulse regime with self-terminating discharge pulses (with a repetition rate of 15–20 Hz) generated upon the formation of a vapor bubble inside the diaphragm (capillary) and its subsequent breakdown. Performance of the apparatus was evaluated on the chemical and biological effects induced in water by the DC diaphragm discharge in dependence on the applied power, solution conductivity and treatment method of the liquid flowing through the reactor. The chemical activity of the discharge apparatus was evaluated by measuring of the yield of plasmachemical production of H2O2 and the degradation of phenol. The yield of H2O2 increased linearly with increasing power input, with the maximum yield of 0.51 g/kWh for the highest applied power of 2.5 kW. The energy yield of phenol degradation was 0.1 g/kWh. Solution conductivity had a very negligible effect on the plasmachemical activity of the discharge. The biocidal effects of the apparatus were evaluated on the inactivation of bacteria E. coli, E. faecalis, and the growth inhibition of cyanobacterium Planktothrix sp. It was shown that the apparatus is capable of efficiently reducing biological contamination in water, especially when the diaphragm discharge reactor was operated using the plug-flow regime. Up to a 5-log reduction in the number of bacteria was obtained after 3 passes through the discharge reactor. In the case of cyanobacteria it was found that growth inhibition of algae further proceeded in the days after exposure to the discharge and the same degree of inhibition was eventually attained after one pass as after two passes of the biomass through the reactor. It seems that for the growth inhibition of cyanobacteria, one pass of the biomass through the discharge reactor might be sufficient.
References
Locke BR, Sunka P, Sato M, Hoffmann M, Chang JS (2006) Electrohydraulic discharge and non thermal plasma for water treatment. Ind Eng Chem Res 45:882–905
Lukes P, Locke BR, Brisset JL (2012) Aqueous-phase chemistry of electrical discharge plasma in water and in gas-liquid environments. In: Parvulescu VI, Magureanu M, Lukes P (eds) Plasma chemistry and catalysis in gases and liquids. Wiley-VCH, Weinheim, pp 241–307
Lukes P, Brisset JL, Locke BR (2012) Biological effects of electrical discharge plasma in water and in gas-liquid environments. In: Parvulescu VI, Magureanu M, Lukes P (eds) Plasma chemistry and catalysis in gases and liquids. Wiley-VCH, Weinheim, pp 309–352
Locke BR, Lukes P, Brisset JL (2012) Elementary chemical and physical phenomena in electrical discharge plasma in gas-liquid environments and in liquids. In: Parvulescu VI, Magureanu M, Lukes P (eds) Plasma chemistry and catalysis in gases and liquids. Wiley-VCH, Weinheim, pp 183–239
Babicky V, Clupek M, Lukes P, Sunka P (2009) Apparatus for decontamination and disinfection of aqueous solutions. PCT Application WO 2009/033436 A1, Mar 19, 2009
Monte M, De Baerdemaeker F, Leys C, Maximov AI (2002) Experimental study of a diaphragm discharge in water. Czech J Phys 52 (Suppl. D):724–730
Stara Z, Krcma F (2004) The study of H2O2 generation by DC diaphragm discharge in liquids. Czech J Phys 54:C1050–C1055
Prochazkova J, Stara Z, Krcma F (2006) Optical emission spectroscopy of diaphragm discharge in water solutions. Czech J Phys 56:B1314–B1319
Makarova EM, Khlyustova AV, Maksimov AI (2009) Diaphragm discharge influence on physical and chemical properties of electrolyte solutions. Surf Eng Appl Electrochem 45(2):133–135
Sunka P, Babicky V, Clupek M, Lukes P, Balcarova J (2003) Modified pinhole discharge for water treatment. In: Giesselmann M, Neuber A (eds) PPC-2003: 14th IEEE International Pulsed Power Conference, Vol. 1 and 2, Digest of Technical Papers, New York, 2003. IEEE, pp 229–231
Krcma F, Stara Z, Prochazkova J (2010) Diaphragm discharge in liquids: fundamentals and applications. J Phys: Conf Ser 207:012010
Joshi R, Schulze RD, Meyer-Plath A, Friedrich JF (2008) Selective surface modification of poly(propylene) with OH and COOH groups using liquid-plasma systems. Plasma Process Polym 5(7):695–707
Nikiforov AY, Leys C (2007) Influence of capillary geometry and applied voltage on hydrogen peroxide and OH radical formation in ac underwater electrical discharges. Plasma Sources Sci Technol 16(2):273–280
Bruggeman P, Degroote J, Vierendeels J, Leys C (2008) DC-excited discharges in vapour bubbles in capillaries. Plasma Sources Sci Technol 17(2):025008
De Baerdemaeker F, Simek M, Leys C (2007) Efficiency of hydrogen peroxide production by ac capillary discharge in water solution. J Phys D Appl Phys 40(9):2801–2809
De Baerdemaeker F, Simek M, Schmidt J, Leys C (2007) Characteristics of ac capillary discharge produced in electrically conductive water solution. Plasma Sources Sci Technol 16(2):341–354
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42(5):053001
Zhang L, Sun B, Zhu XM (2009) Organic dye removal from aqueous solution by pulsed discharge on the pinhole. J Electrost 67(1):62–66
Sun B, Aye NN, Wang XM, Zhu XM, Sato M (2011) Eradication of invasive organisms from ballast water with electrodeless pulsed-discharge hybrid reactor. IEEE Trans Ind Appl 47(3):1079–1085
De Baerdemaeker F, Simek M, Leys C, Verstraete W (2007) Pump effect of a capillary discharge in electrically conductive liquids. Plasma Chem Plasma Process 27(4):473–485
Locke BR, Shih KY (2011) Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci Technol 20(3):034006
Lukes P (2002) Water treatment by pulsed streamer corona discharge in water. PhD Dissertation, Institute of Plasma Physics AS CR, Prague, Czech Republic. http://www.ipp.cas.cz/Ips/public/lukes_dissert.pdf
Sunka P (2001) Pulse electrical discharges in water and their applications. Phys Plasmas 8:2587–2594
An W, Baumung K, Bluhm H (2007) Underwater streamer propagation analyzed from detailed measurements of pressure release. J Appl Phys 101(5):053302
Namihira T, Sakai S, Yamaguchi T, Yamamoto K, Yamada C, Kiyan T, Sakugawa T, Katsuki S, Akiyama H (2007) Electron temperature and electron density of underwater pulsed discharge plasma produced by solid-state pulsed-power generator. IEEE Trans Plasma Sci 35(3):614–618
Bruggeman P, Schram D, Gonzalez MA, Rego R, Kong MG, Leys C (2009) Characterization of a direct dc-excited discharge in water by optical emission spectroscopy. Plasma Sources Sci Technol 18(2):025017
Locke BR, Mededovic Thagard S (2012) Analysis and review of chemical reactions and transport processes in pulsed electrical discharge plasma formed directly in liquid water. Plasma Chem Plasma Process 32(5):875–917
Polyakov OV, Badalyan AM, Bakhturova LF (2004) Relative contributions of plasma pyrolysis and liquid-phase reactions in the anode microspark treatment of aqueous phenol solutions. High Energ Chem 38(2):131–133
Lukes P, Clupek M, Babicky V, Sunka P (2008) Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci Technol 17(2):024012
Li Z, Sakai S, Yamada C, Wang D, Chung S, Lin X, Namihira T, Katsuki S, Akiyama H (2006) The effects of pulsed streamerlike discharge on cyanobacteria cells. IEEE Trans Plasma Sci 34:1719–1725
Li Z, Ohno T, Sato H, Sakugawa T, Akiyama H, Kunitomo S, Sasaki K, Ayukawa M, Fujiwara H (2008) A method of water-bloom prevention using underwater pulsed streamer discharge. J Environ Sci Health A 43:1209–1214
Wang CH, Li GF, Wu Y, Wang Y, Li J, Li D, Wang NH (2007) Role of bipolar pulsed DBD on the growth of Microcystis aeruginosa in three-phase discharge plasma reactor. Plasma Chem Plasma Process 27(1):65–83
Wang CH, Wu Y, Shen XQ (2010) A multi-wire-to-cylindrical type packed-bed plasma reactor for the inactivation of M. aeruginosa. J Electrost 68(1):31–35
Drabkova M, Admiraal W, Marsalek B (2007) Combined exposure to hydrogen peroxide and light—selective effects on cyanobacteria, green algae, and diatoms. Environ Sci Technol 41(1):309–314
Drabkova M, Matthijs HCP, Admiraal W, Marsalek B (2007) Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 45(3):363–369
Barrington DJ, Ghadouani A (2008) Application of hydrogen peroxide for the removal of toxic cyanobacteria and other phytoplankton from wastewater. Environ Sci Technol 42(23):8916–8921
Matthijs HCP, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, Talens R, Huisman J (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res 46(5):1460–1472
Acknowledgments
This work was supported by the Grant Agency of AS CR (project No. IAAX00430802) and the Czech Science Foundation (No. 104/09/H080).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lukes, P., Clupek, M., Babicky, V. et al. High Power DC Diaphragm Discharge Excited in a Vapor Bubble for the Treatment of Water. Plasma Chem Plasma Process 33, 83–95 (2013). https://doi.org/10.1007/s11090-012-9432-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-012-9432-6