Abstract
The TiO2 etching characteristics and mechanisms in HBr/Ar and Cl2/Ar inductively-coupled plasmas were investigated under fixed gas-mixing ratio and bias power conditions. It was found that in both systems, an increase in gas pressure from 4 to 10 mTorr results in a non-monotonic TiO2 etching rate, while a variation of input power in the range 500–800 W causes a faster-than-linear acceleration of the etching process. Plasma diagnostics performed by Langmuir probes and zero-dimensional plasma modeling provided data on plasma parameters, steady-state densities, and fluxes of the active species on the etched surface. The model-based analysis of the etching mechanism showed that for the given set of processing parameters, the TiO2 etch kinetics correspond to the transitional regime of ion-assisted chemical reaction in which a chemical-etch pathway dominates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent research into micro- and nanoelectronic technologies has focused on alternative materials with the aim of developing new devices, as well as improving the characteristics of conventional devices. For example, titanium dioxide (TiO2) thin films have found numerous applications in solar cells, optical waveguides, light emitting diodes (LEDs), capacitors in planar integrated circuit construction, and as replacements for SiO2 in gate stacks for field-effect transistors [1–4]. For these applications mentioned above, the development of an anisotropic etching process for TiO2 is important to obtain an accurate pattern transfer as well as stable device parameters. Previous works have reported developments related to wet patterning of TiO2 thin films using both HF/HNO3 and HCl/HNO3 chemistries [5, 6]. However, these methodologies do not satisfy the modern requirements of process purity, uniformity, and resolution. To date, only few studies related to the application of plasma-assisted etching of TiO2 thin films using fluorine-based (CF4 or SF6), chlorine-based (Cl2), or hydrogen-based (H2, CH4) chemistries [7, 8] have been reported. By analyzing the limited data that is available, one can make the following conclusions: (1) The fastest etch rate is obtained by fluorine-based plasma chemistry. (2) Both fluorine- and chlorine-based chemistries show the chemical enhancement of the TiO2 etching rate to a greater extent than pure Ar sputtering, while the hydrogen-based chemistry works more slowly than the physical sputtering of Ar ions. (3) All three chemistries provide good film surface morphologies. It is important to note that the above mentioned works only provide information regarding the dependencies of the TiO2 etching rate and etching profile on the main operating parameters, as well as on the influence of the working gas on device performance. Furthermore, the basic relationships between plasma parameters, plasma chemistries, and etch kinetics were not fully explored. Hence, the etch mechanism is not clearly understood, and this retards development or optimization of a TiO2 dry-etch process.
In this work, the etching characteristics of TiO2 thin films in HBr/Ar and Cl2/Ar inductively-coupled plasmas have been investigated, under the condition of fixed gas-mixing ratio (40 sccm HBr or Cl2 + 10 sccm Ar) and a fixed bias power of 200 W. The main goal was to understand how the variations of gas pressures and input power influence the TiO2 etch rate through changes to the internal plasma parameters, the densities of neutral and charged species, and their fluxes on the etched surface. For these purposes, a model-based analysis of plasma chemistry and etch kinetics was applied.
Experimental and Modeling Details
Film Preparation and Etching Technique
TiO2 films were formed by spin-coating a precursor solution, followed by pre-baking, and annealing. Ti(i–C3H7O)4 was used as Ti–O precursor with a solvent mixture of butyl acetate and isopropyl alcohol. The precursor solution was then coated onto a Si substrate by spin-coating and pre-baked at 120°C for 10 min. The coated film was then fired at 420°C in air for 30 min. Auger Electron Spectroscopy (AES) analysis confirmed that a stoichiometric TiO2 film was formed for all samples in this work.
Etching and plasma diagnostic experiments were performed in a planar inductively-coupled plasma (ICP) reactor. The reactor had a cylindrical chamber (r = 16 cm) made from anodized aluminum, and with a 5-turn copper coil located above a 10-mm-thick horizontal quartz window. The coil was connected to a 13.56-MHz power supply. The distance (l) between the window and the bottom electrode that was used as a substrate holder was 12.8 cm. The bottom electrode was connected to a 12.56-MHz power supply in order to maintain a negative DC bias voltage. The temperature of the bottom electrode was stabilized at 17°C using a water-flow cooling system.
The experiments were performed with fixed gas-mixing ratios (40 sccm HBr or Cl2 + 10 sccm Ar) and a fixed bias power (W dc = 200 W), while the gas pressure p and input power W inp were varied within the ranges 4–10 mTorr and 500–800 W, respectively. The etched samples (with sizes of about 2 × 2 cm2) were placed at the center of the bottom electrode. The TiO2 and Si-etched depths were measured using a surface profiler (Alpha-step 500, Tencor).
Plasma diagnostics were performed using a double Langmuir probe (LP) (DLP2000, Plasmart Inc.). The probes were installed through the viewport on the sidewall of the reactor chamber, at a point that was 5.7 cm above the bottom electrode and centered in a radial direction. The treatment of I–V curves to obtain electron temperature (T e) and total positive ion density (n +) was carried out using the software supplied by the equipment manufacturer. Calculations were performed based on Johnson and Malter’s double probe theory [9] and by using the Allen-Boyd-Reynolds (ABR) approximation for ion saturation current density j i ≅ 0.61en + v [10].
Plasma Modeling
A simplified global (zero-dimensional) model was used, operating with volume-averaged plasma parameters possessing a Maxwellian approximation for the electron energy distribution function (EEDF) [11–14]. The experimental data on T e and n + were directly applied as input model parameters. The model used included simultaneous solutions to the following equations: (1) balanced equations for neutral and charged species in a steady-state approximation and (2) quasi-neutrality conditions for both volume densities and fluxes of the charged species. The output model parameters were the rate coefficients for electron-impact processes, densities of charged species (electrons, negative ions), densities of neutral ground-state species (Cl2 and Cl for Cl2/Ar plasma and HBr, H, Br, H2, Br2 for HBr/Ar plasma) as well as their fluxes on the etched surface. For more details on the modeling algorithm, the list of processes taken into account by the model, and the rest input data set (rate coefficients for atom-molecular reactions, heterogeneous recombination probabilities for atomic species), see our earlier works [13, 14].
Results and Discussion
TiO2 Etching Rate and TiO2/Si Selectivity
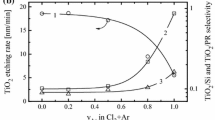
From Fig. 1, it can be seen that an increase in gas pressure and input power results in similar etch rate behaviors for TiO2 thin films in both HBr/Ar and Cl2/Ar plasmas. In particular, as the gas pressure is increased from 4 to 10 mTorr, the TiO2 etching rate changes non-monotonically and exhibits a maximum (ca. 17 A/min for HBr/Ar and ca. 123 A/min for Cl2/Ar) at p = 6 mTorr. The etching rate of Si changes in the same non-monotonic manner, but its reduction after reaching the maximum is more gradual than the TiO2 etching rate. Hence, the TiO2/Si etch selectivity decreases with increasing gas pressure. The significantly lower TiO2/Si etch selectivity for Cl2/Ar plasma compared to that for HBr/Ar plasma (e.g., 0.91–0.47 for HBr/Ar vs. 0.09–0.07 for Cl2/Ar at p = 4–10 mTorr and W inp = 700 W) is attributed to the considerably higher Si etching rate produced in the case of the chlorine-based plasma chemistry. An increase in the input power by 500–800 W (at constant pressure) results in a fast almost-exponential increase in TiO2 etching rates for both HBr/Ar (12.2–29.8 A/min, i.e., a 2.4-times increase) and Cl2/Ar (107.2–403.5 A/min, i.e., a 3.8-times increase) plasmas, while the behaviors of TiO2/Si etch selectivities for these two systems are different. For instance, in the case of the HBr/Ar plasma, the Si etching rate increases more slowly than the TiO2 etching rates, so that the TiO2/Si etch rate ratio increases from 0.56 to 1.01 with increasing W inp. In contrast, the etching rate of Si in Cl2/Ar plasma increases more quickly than the TiO2 etching rate does. As a result, the TiO2/Si etch selectivity decreases slightly from 0.095 to 0.075.
TiO2 etching rate and TiO2/Si etching selectivity as functions of gas pressure (a, b) and input power (c, d) in HBr/Ar (a, c) and Cl2/Ar (b, d) inductively coupled plasmas. The process conditions are as follows: a, b W inp = 700 W, W dc = 200 W, 40 sccm HBr or Cl2 + 10 sccm Ar; and c, d p = 6 m Torr, W dc = 200 W, 40 sccm HBr or Cl2 + 10 sccm Ar. The solid lines are provided only to guide the eye
From the data represented above, one can see at least two principal issues that need to be solved for further understanding of the TiO2 etching mechanism. The first is the reason for the non-monotonic etch rate as a function of gas pressure. The second is the difference between the absolute values of the TiO2 etching rates in HBr/Ar and Cl2/Ar plasmas (under the same operating conditions). For these reasons, data on plasma parameters, densities, and fluxes of active species are needed.
Plasma Parameters and Composition
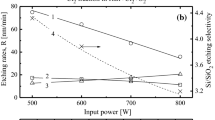
From Fig. 2, it can be seen that variations in both gas pressure and input power result in similar changes in electron temperature, total positive ion density, and electron density in HBr/Ar and Cl2/Ar plasmas. For example, an increase in gas pressure (Fig. 2a and b in the range of 4–10 mTorr reduces T e (3.5–3.2 eV for HBr/Ar and 3.8–3.5 eV for Cl2/Ar) due to an increase in electron-neutral collision frequency and electron energy loss. The higher electron temperature in Cl2/Ar plasma is connected to the high degree of dissociation for Cl2 that results in the domination of Cl atoms over Cl2 molecules in the gas phase. Since Cl atoms have higher excitation and ionization thresholds and lower cross-sections than those for HBr [13, 14], the Cl2/Ar plasma provides lower electron energy loss in the medium section of the EEDF, and thus, a higher T e. It was also found that in both plasma systems, a decrease in ionization rate coefficients with increasing gas pressure (for example, \( {\text{K}}_{\text{iz}}^{\text{HBr}} \) = 1.3 × 10−9 − 9.4 × 10−10 cm3/s for HBr/Ar and \( k_{\text{iz}}^{{{\text{cl}}_{ 2} }} \) = 3.1 × 10−9–2.3 × 10−9 cm3/s for Cl2/Ar at p = 4–10 mTorr and W inp = 700 W) is compensated by an increasing density of neutral species, so that the effective ionization frequency increases slightly (\( v_{\text{iz}} \approx k_{\text{iz}}^{\text{HBr}} n_{\text{HBr}} + k_{\text{iz}}^{\text{Ar}} n_{\text{Ar}} = 6.5 \times 10^{4} - 1.1 \times 10^{5} \,s^{ - 1} \) for HBr/Ar plasma, and \( v_{\text{iz}} \approx k_{\text{iz}}^{{{\text{cl}}_{ 2} }} n_{{{\text{cl}}_{ 2} }} + k_{\text{iz}}^{\text{cl}} n_{\text{cl}} + k_{\text{iz}}^{\text{Ar}} n_{\text{Ar}} = 7.0 \times 10^{4} - 1.2 \times 10^{5} {\text{s}}^{ - 1} \) for Cl2/Ar plasma). At the same time, the growth in the frequency of dissociative attachment (\( v_{\text{att}} \approx k_{\text{att}}^{\text{HBr}} n_{\text{HBr}} = 1.0 \times 10^{4} - 3.1 \times 10^{4} \,{\text{s}}^{ - 1} \) for HBr/Ar plasma and \( v_{\text{att}} \approx k_{\text{att}}^{{{\text{cl}}_{ 2} }} n{\text{cl}}_{ 2} = 3.3 \times 10^{3} - 2.2 \times 10^{4} \,{\text{s}}^{ - 1} \) for Cl2/Ar plasma) appears to be faster than that for viz because of increasing dissociative attachment rate coefficients (due to the threshold-less attachment processes for both HBr and Cl2) and decreasing degrees of dissociation for both HBr (n Br /n HBr = 0.9–0.07) and Cl2 (\( n_{\text{cl}} /n_{\text{cl2}} \) = 2.2–0.9) molecules. This produces a decreasing model-predicted electron density (n e) and results in a near-to-constant total ionization rate and n +. However, the flux of positive ions, Γ+, exhibits a decreasing tendency (8.9 × 1015–6.7 × 1015 cm−2 s−1 for HBr/Ar and 6.6 × 1015–4.2 × 1015 cm−2s−1 for Cl2/Ar, at p = 4–10 mTorr and W inp = 700 W) due to decreasing ion Bohm velocity. The last fact is clearly illustrated by decreasing T e (Fig. 2a). Both the absolute and relative (n − /n e) densities of negative ions follow the behavior of attachment rate, and increase monotonically with increasing gas pressure. For example, n Br− = 3.2 × 1010 − 5.4 × 1010) cm−3 for HBr/Ar plasma and n Cl− = 3.1 × 1010–4.8 × 1010 cm−3 for Cl2/Ar plasma, which correspond to n − /n e = 0.6–1.6 and 1.1–4.0, respectively. The influence of gas pressure on HBr and Cl2 dissociation kinetics is also similar in both cases. A loss in the dissociation efficiency (\( k_{\text{dis}}^{\text{HBr}} n_{e} = 118 - 60\,{\text{s}}^{ - 1} \) and \( k_{\text{dis}}^{{{\text{cl}}_{ 2} }} n_{e} = 778 - 308\,{\text{s}}^{ - 1} \) at p = 4–10 mTorr and W inp = 700 W), and therefore, in the degree of dissociation, is compensated by the increasing densities of HBr or Cl2 molecules. As a result, both formation rates and volume densities of Br and Cl atoms show a slow increase toward higher gas pressures. These same behaviors are also exhibited for the fluxes of the corresponding species.
Measured (solid line + symbols) and model-predicted (dashed line) plasma parameters as functions of gas pressure (a, b) and input power (c, d) in HBr/Ar (a, c) and Cl2/Ar (b, d) inductively-coupled plasmas. The process conditions correspond to those in Fig. 1
An increase in input power at constant pressure (Fig. 2c, d) leads to an increase in n e (as it simply determined by the power balance equation [11]) and to an increase in T e. This last effect can be attributed to the increasing degrees of dissociation of HBr and Cl2 (n Br /n HBr = 0.4–1.0 and \( n_{\text{cl}} /n_{{{\text{cl}}_{ 2} }} \) = 0.5–2.1 at W inp = 500–800 W and p = 6 mTorr). The enrichment of the plasma by the species with higher excitation and ionization potentials leads to a lowering of the electron energy loss in the medium section of the EEDF. An increase in total ionization rate also causes an increase in n + which, however, is a bit slower than that for n e. The reason is that the growth of W inp accelerates the decay of positive ions (through the increasing ion Bohm velocity) and, simultaneously, retards the loss of electrons (through the decreasing diffusion coefficient due to a decrease in plasma electronegativity). The acceleration of the dissociation efficiencies (\( k_{\text{dis}}^{\text{HBr}} n_{e} = 45 - 126\,{\text{s}}^{ - 1} \) and \( k_{\text{dis}}^{{{\text{cl}}_{ 2} }} n_{e} = 175 - 757{\text{s}}^{ - 1} \) at W inp = 500–800 W and p = 6 mTorr) leads to an increase in both HBr and Cl2 degrees of dissociation, so that the densities of Br and Cl atoms increase rapidly (by 1.7 times for n Br, and by 2.6 times for n Cl) while the densities of HBr and Cl2 molecules decrease. Though this somewhat retards the attachment rate, it does keep its tendency to slightly increase in the case of both plasma systems, and therefore, results in the same behaviors of n − (n Br− = 3.41 × 1010 − 4.4 × 1010 cm−3 and n Cl− = 3.1 × 1010 − 4.8 × 1010 cm−3 at W inp = 500–800 W and p = 6 mTorr). However, the relative density of negative ions decreases due to the faster growth of n e (n − /n e = 1.5–0.8 for HBr/Ar and 4.5–1.5 for Cl2/Ar).
Thus, one can conclude that HBr/Ar and Cl2/Ar plasmas exhibit similar behaviors for neutral and charged active species with varying gas pressures and input power. In fact, this explains the similarity in the changes of TiO2 etching rate in both plasma systems. However, for answering the remaining questions, a comprehensive analysis of the TiO2 etch mechanism is required.
Etch Mechanism Analysis
From several published works [15–17], it can be understood that for the given combination of plasma-forming gas and etched material, the variation of etch rate versus main operating parameters depend on the volatility of reaction products (in fact, on the desorption mechanism for reaction products) and fluxes of active species on the etched surface. While direct data on saturated vapor pressures for TiBrx and TiClx are not available, we can refer to the melting (T mp) or boiling (T bp) points of the corresponding compounds if we assume a qualitative correlation between these parameters and volatility. For example, Ref. [18] gives T mp = 39°C and −24°C for TiBr4 and TiCl4, respectively. This allows one to conclude that saturated titanium bromide and titanium chloride are moderately volatile compounds (though the volatility of TiCl4 is expected to be the more noticeably higher of the two) that can then be spontaneously desorbed from the etched surface even at near-to-room temperatures. Another important issue is that direct bromination or chlorination of the TiO2 surface at temperatures close to room temperature appears to be impossible because a Ti–O bond (672.4 ± 9.2 kJ/mol) is much stronger than either Ti–Br (ca. 439 kJ/mol) or Ti–Cl (ca. 405 kJ/mol) bonds [18]. Therefore, for both HBr/Ar and Cl2/Ar plasmas, the TiO2 etch mechanism can be assumed to be an ion-assisted chemical reaction where ion bombardment is necessary for the destruction of the Ti–O bonds.
From Refs. [15–17] it can be understood that the rate of the ion-assisted chemical reaction can be expressed as follows:
where Γa is the Br or Cl atom flux, s0 is the Br or Cl atom’s sticking probability, δ is the stoichiometric coefficient for the reaction products, and (1 − θ) is the fraction of free surface sites at which the chemical reaction occurs. Since in our case, the free surface sites are produced by ion bombardment through breaking of the Ti–O bonds, the parameter θ is determined by the balance between the rates of the chemical reaction (which fills the surface with the reaction products) and the bond breaking rate (i.e., the rate of physical sputtering for the TiO2 surface):
where Y s is the ion-type-averaged sputtering yield and Γ+ is the total flux of positive ions on the etched surface. Accordingly, by combining (1) and (2), one can see that three principal regimes of ion-assisted chemical reaction do exist. In the case of θ → 0 (i.e., when high-efficiency Ti–O bond breaking occurs), one can obtain R ≈ δs0Γa, and the reaction-rate-limited etch regime exists. On the other hand, θ → 1 (i.e., when low-efficiency Ti–O bond breaking occurs), R ≈ Y sΓ+, which corresponds to the ion-flux-limited etch regime. In this regime, the behavior of the TiO2 etch rate can be simply characterized by ε 1/2i Γ+ [13, 14], where \( \varepsilon_{i} \approx e\left| { - U_{\text{f}} - U_{\text{dc}} } \right| \) is the ion-bombardment energy determined by the combination of floating potential (−U f) and the DC bias (−U dc) on the bottom electrode. For a transitional regime (when θ = 0.1–0.9), the etch rate is controlled by both Γa and ε 1/2i Γ+.
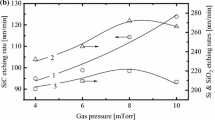
From comparison of Figs. 1 and 3, it can be seen that the monotonically increasing TiO2 etch rate as a function of W inp can be formally obtained in any mentioned reaction regime because of increasing ΓBr or ΓCl (i.e., through the increasing densities of the corresponding atoms), and ε 1/2i Γ+. The last effect is the result of a decrease in \( \left| { - U_{\text{dc}} } \right| \) (405–291 V for HBr/Ar and 411–284 V for Cl2/Ar at W inp = 500–800 W and p = 6 mTorr) that compensates for an increased flux of positive ions due to the growth of n +. At the same time, since in the Cl2/Ar plasma, the parameter ε 1/2i Γ+ is lower than that in the HBr/Ar plasma, the higher TiO2 etch rate in the Cl2/Ar plasma cannot be associated with the pure ion-flux-limited etching regime. Moreover, the non-monotonic TiO2 etching rate as a function of gas pressure has no direct correlation with either ΓBr or ΓCl, and with ε 1/2i Γ+. This allows one to neglect the pure reaction-rate-limited etching regime. In our opinion, a reasonable conclusion to draw from this is that in the transitional regime, a chemical-etching pathway will dominate. This conclusion is based on the fact that a higher etching rate in Cl2/Ar plasma is in agreement with ΓCl > ΓBr as well as with the fact that the volatility of Ti chlorides is higher than that of Ti bromides. In such a situation, the non-monotonic behavior of the TiO2 etching rate with increasing gas pressure in both plasma systems can result from the concurrence of increasing fluxes of chemically-active species and of decreasing Ti–O bond-breaking efficiency due to decreasing ε 1/2i Γ+. Several published works [17, 19, 20] report that similar situations occur in the case of the formation of moderate- or low-volatility reaction products. Thus, similar non-monotonic etching rates have been repeatedly obtained when the flux of chemically-active species increases and the efficiency of ion-stimulated desorption of reaction products decreases.
Model-predicted fluxes of Br or Cl atoms (a) and the parameter ε 1/2i Γ+ (b) characterizing the ion-energy flux as functions of gas pressure and input power in HBr/Ar and Cl2/Ar inductively-coupled plasmas. The process conditions correspond to those in Fig. 1
Conclusion
In this work, we investigated the etching characteristics and mechanism of TiO2 thin films in HBr/Ar and Cl2/Ar inductively-coupled plasmas, under the conditions of fixed gas-mixing ratio (40 sccm HBr or Cl2 + 10 sccm Ar) and bias power (200 W). It was found that the influence of gas pressure (4–10 mTorr) and input power (500–800 W) on TiO2 etching rate in both systems is similar, with the absolute etching rates being noticeably higher in the Cl2/Ar plasma than in the HBr/Ar plasma. From model-based analysis of plasma chemistry and etch kinetics determined from the results of plasma diagnostics performed by Langmuir probes, it was shown that the TiO2 etching process occurs in the transitional regime of ion-assisted chemical reactions, whereby a chemical-etch pathway dominates. The non-monotonic behavior of the TiO2 etching rate with increasing gas pressure can result from the concurrence of increasing fluxes of chemically-active species and decreasing Ti–O bond-breaking efficiency due to decreasing ion-energy flux. The higher etching rates in Cl2/Ar plasma may be attributed to the flux of Cl atoms and the volatility of Ti chlorides being higher than those of bromides.
References
O’Regan B, Grätzel M (1991) Nature 353:737
Guglielmi M, Colombo P, Esposti LMD, Righini GC, Pellic S, Rigato V (1992) J Non Cryst Solids 147–148:641
Hsu YP, Chang SJ, Su YK, Chang CS, Shei SC, Lin YC, Kuo CH (2003) J Electron Mater 32: 5
Pan TM, Lei TF, Chao TS (2001) Appl Phys Lett 78: 1439
Srivastava KG (1960) Phys Rev 119: 520
Yeung KS, Lam YW (1983) Thin Solid Films 109:169
Noemaun AN, Mont FW, Cho J, Schubert EF, Kim GB, Sone C (2011) J Vac Sci Technol A 29:051302
Norasetthekul S, Park PY, Baik KH, Lee KP, Shin JH, Jeong BS, Shishodia V, Lambers ES, Norton DP, Pearton SJ (2001) Appl Surf Sci 185: 27
Johnson EO, Malter L (1950) Phys Rev 80: 58
Sugavara M (1998) Plasma etching: fundamentals and applications. Oxford University Press, New York
Lieberman MA, Lichtenberg AJ (1994) Principles of plasma discharges and materials processing. Wiley, New York
Lieberman MA, Ashida S (1996) Plasma Sour Sci Technol 5: 145
Efremov A, Min N-K, Choi B-G, Baek K-H, Kwon K-H (2008) J Electrochem Soc 155: D777
Kwon K-H, Efremov A, Kim M, Min NK, Jeong J, Kim K (2010) J Electrochem Soc 157: H574
Lee C, Graves DB, Lieberman MA (1996) Plasma Chem Plasma Process 16:99
Jin W, Vitale SA, Sawin HH (2002) J Vac Sci Technol A 20:2106
Efremov AM, Kim DP, Kim CI (2004) IEEE Trans Plasma Sci 32: 1344
Lide DR (1998) Handbook of chemistry and physics. CRC Press, New York
Efremov AM, Kim D-P, Kim C-I, J Vac Sci Technol A 21: 1837
Lee T, Efremov A, Ham Y-H, Yun SJ, Min NK, Hong MP, Kwon K-H (2009) J Micro/Nanolith MEMS MOEMS 8:021110
Acknowledgments
This work was supported by the New and Renewable Energy Program of the Korean Institute of Energy Technology Evaluation and Planning (KETEP), and funded by the Korean Ministry of Knowledge Economy (Contract No.: 2008-N-PV-08-P-03-0-000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, H., Efremov, A., Kim, D. et al. Etching Characteristics and Mechanisms of TiO2 Thin Films in HBr/Ar and Cl2/Ar Inductively-Coupled Plasmas. Plasma Chem Plasma Process 32, 333–342 (2012). https://doi.org/10.1007/s11090-012-9352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-012-9352-5