Abstract
Many studies suggest strong hydrophilicity of plasma treated polyester surfaces. However, no studies have been reported on the influence of plasma on the antibacterial activity of polyethylene terephthalate. First samples were padded with triclosan as antibacterial agent with different concentrations. Second samples were treated by oxygen plasma with different operating frequency and treating time, respectively. Afterwards, plasma treated samples were padded with triclosan in same conditions. The results revealed that the antibacterial activity slighlty increased after treating with triclosan. SEM images and FTIR spectra showed that horizontal channels were brought about on the fiber surface and then better surface roughness and wettability were obtained by plasma. Fibers were fully coated with triclosan after plasma and the antibacterial activity increased with increasing operating frequency and reaction time. Finally, the samples treated with triclosan after plasma gave acceptable results and showed the best antibacterial activity for Staphylococcus aureus and Escherichia coli.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Textiles are carriers of microorganisms such as fungi, bacteria, and viruses and are ideal medium since their porous structures retain water, oxygen, and nutrients, providing a perfect environment for their growth. The most textiles are also conductive to cross infection or transmission of diseases caused by microorganisms. Antibacterial products have therefore shown huge potential in the textile industry. Many commercial chemicals, including phenoxy phenols, quaternary ammonium salts, phosphonium compounds, metal and metal salts, organo silicones, and halamines are used for antimicrobial application of textiles. Over the last 30 years, among them, triclosan has become the most potent and widely used bis-phenol for antimicrobial applications, including soaps, creams, toothpastes, mouthwashes, underarm deodorants, and polymers [1–11].
Having high productivity and low cost for producing antibacterial textiles, among synthetic fibers, polyethylene terephthalate (PET) fibers have superior chemical and physical properties due to the high degree of orientation and structural order of macromolecular chains. In spite of excellent characteristics, they have undesirable properties including low moisture regain, tendecy to pill, formation of static charge, and difficulty in dyeability. They exhibit also lower antibacterial properties after chemical finishing treatments using aqueous solutions because they do not possess any chemically active groups. Surface modification using plasma technique with different monomers is one of the ways to improve these undesired properties. The use of plasma for enhancing the adhesion of textiles has been known for over 30 years. Low-temperature plasma has been used in a lot of applications such as surface etching and material processing. It is particularly suited to apply to textile processing because most textile materials are heat sensitive polymers [12–14].
Plasma treatment generally changes the upper layers of surface without affecting bulk properties. Treatment is known to form polymer radicals on surfaces and the polarity of surface increases due to the polar groups formed. Plasma is dry process and does not require any water or wet chemicals. Chemical consumption is low and process is safer and much more environmentally friendly. Cold plasma at near the room temperature can be safely used for surface modification of textile polymers. Consequently, plasma applications in textile industry have been used to increase the surface energy, hydrophilicity, dyeability, adhesion to other materials, and to impart different functional finishes [13–27].
Hereby, PET fibers have high productivity and low cost for producing antibacterial textiles, but they have lower wet pick-up after aqueous treatment because they do not possess any chemically active groups. This study was therefore aimed to highlight the possibility of using the plasma treatment for surface modification, which could facilitate the more loading of triclosan onto the surface of PET fibers. It has been shown that the antibacterial activity of PET fibers can improve by plasma treatment through surface modification.
Experimental
Material
We used 100% PET fabric (39 × 28 courses/wales and 60 g/m2) due to their increasing presence in the production of medical, healthcare and hygiene materials.
Antibacterial Treatment
The esterified triclosan derivative, including fatty alcohol ethoxylate, 2-(2-butoxyethoxy) ethanol and triclosan [5-chloro-2-(2,4-dichlorophenoxy) phenol] was used as antibacterial chemical and provided from Thomson Research Associates, Canada. The antibacterial solution was applied to PET fabric by laboratory type padder through conventional pad-dry process. The fabrics were padded through squeeze rollers made by Mathis with two dips and two nips, at a setting 0.5 bar to give a wet pick-up of between 30 and 35% on weight of fabric. Treatment conditions were as follows: 40, 60, and 80 g/l of antibacterial chemicals, drying at 150°C for 5 min in Mathis oven.
Plasma Treatment
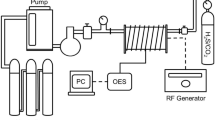
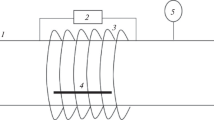
Plasma treatments were done in Diener Pico (Ebhausen, Germany) plasma generator (13.56 MHz/200 W) with a cylindrical vacuum chamber, made of stainless steel, with a diameter of 150 mm and a height of 300 mm. In the typical experiment, the samples were introduced by a load-lock system and placed on a grounded aluminium holder; the distance between plasma and the fabric was about 60 mm. The chamber was then closed, and pressure was created in the system. The oxygen was introduced into the generator under the pressure and flow-rate conditions after plasma generator was decontaminated. When the plasma source was on, the discharge was sustained for the desired frequency and reaction time values at near the room temperature. The gas pressure was fixed at 1 mbar, flow rate of oxygen was adjusted at 2.50 cm3 per min and the discharge power was set to 100 W. The following experimental conditions were employed during the plasma treatments: operating frequency was set to 50, 75, and 100 kHz, treating time was set to 1, 5, and 10 min. At the end of reaction, the power was disconnected and the base pressure was restored in the system. The generator was then repressurized by introducing air, and the fabrics were removed.
In order to acquire the antibacterial activity after plasma treatment, the samples were treated with triclosan in same treatment conditions. During study each sample was classified in accordance with the following form; untreated (U), treated with triclosan (T), treated with plasma (P) and treated with triclosan after plasma (P + T), respectively.
Measurements
Water spreading time was measured according to AATCC Test 39-1977. The fabric was taut in fixed frame to avoid contact with any surface. The average 37 μl distilled water droplet was placed at the centre of the fabric (4 cm2). The time for the water drop to disappear in surface was measured and recorded as wetting time. The results were the mean of five measurements. The shorter the average wetting time, the more readily wettable was the textile.
A JEOL JSM 6060 scanning electron microscope (SEM) was used for investigating morphological changes on the fabric surface after treatments. The standard procedure was followed, where samples were coated with gold for 150 s before SEM examination.
The attenuated total reflection Fourier transforms infrared spectroscopy (ATR-FTIR) spectra of the samples were recorded on Perkin-Elmer Spectrum 2000 Explorer spectrometer. The samples were scanned at frequencies from 400 to 4,000 cm−1, the number of scan times was 32, and the resolution was set at 2 cm−1.
The antibacterial efficiencies of the fabrics were quantitatively evaluated by the AATCC Test Method 100-1999. The Gram-positive Staphylococcus aureus (S. aureus-ATCC 6538) and the Gram-negative Escherichia coli (E. coli-ATCC 35218) were used as test microorganisms because they are the major cause of cross-infection in hospitals. After test and control samples were in contact with the bacteria for 24 h at 37°C, the diluted solutions were plated on Muller-Hinton II agar and incubated for 24 h at 37°C. The viable colonies of the bacteria on the agar plate were counted, and the reduction in the number of the bacteria was calculated using the following equation:
where A = the number of bacteria recovered from the inoculated treated test specimen samples in the jar incubated over the desired contact period (24 h) and B = the number of bacteria recovered from the inoculated treated test specimen samples in the jar immediately after inoculation (at “zero” contact time). During the antibacterial testing, all measurements were completed in microbiology laboratory environment of about 24°C and 55% relative humidity and repeated four times.
Results and Discussion
Water Spreading Time
For studing the influence of operating frequency and treating time on surface hydrophilicity, water spreading time was measured according to AATCC Test 39 and results are shown in Table 1. The untreated samples had water spreading time of 200 s and this value improved to between 30 and 13 s by plasma. This is because PET is essentially hydrophobic in nature, but the samples showed significant improvement in absorbency and spreading of water after plasma. The increase in absorbency is due to the enhanced capillary action on the surface caused by the formation of horizontal and vertical channels, which are expected to assist absorption and spreading of water [24, 28]. And, the oxygen containing groups of C–O and C=O introduced onto the surface by plasma improve the surface hydrophilicity [29, 30]. The best water absorbency was obtained when operating frequency and treating time were increased and so wet pick-up increased from 35% to above 60% after plasma.
Triclosan has a hydrophobic character due to aromatic rings, but it also contains a hydroxyl group which confers a degree of hydrophilicity to the compound. If PET fibers treat with triclosan, their surface will become less hydrophobic in the presence of hydrogen bounds [31]. Based on the results, it can be observed that the addition of triclosan decreased the hydrophobicity of surface and it had water spreading time of 124 s. The triclosan treated surfaces after plasma remained almost the same, which had water spreading time of 13 s.
SEM Analysis
Surface morphology of PET was analyzed by SEM and surface images are shown in Fig. 1. As can be seen in Fig. 1(a), the untreated fiber had relatively smooth surface, which had poor ability to hold water but the some impurities can be seen on the fiber surface. Compared with the untreated sample, surface in Fig. 1(b) was slightly covered by triclosan solution after finishing. We know that triclosan has moderate substantivity and it can be combined with different surfactants and special formulation additives to increase its substantivity. While triclosan solution is maintained in surfactant micelles by the solubilising group (fatty alcohol ethoxylates), the attachment group reacts with surface and then chemical coating on surface is slowly released into the environment. Due to the OH–O hydrogen bonding interactions and benzene–benzene interactions between adjacent PET and triclosan, these connections are further strengthened by the presence of bond between PET and triclosan. This co-operative effects lead to the formation of London dispersion forces (van der Waals contacts) and H bonding [31]. Otherwise, the relatively smooth untreated surfaces were roughened by plasma treatment to produce an even distribution of protrusions [30] and horizontal channels-like structures in Fig. 1c were developed on the surface. Therefore, the rough surface can have more capacity to capture water. The homogeneous surface topography indicates the uniformity of treatment. When comparing Fig. 1b–d, it can be seen that antibacterial chemical was intensively covered on fiber surface and therefore it can be concluded that there is more loading of triclosan on the PET surface by creating new polar groups on the surface through plasma.
FTIR Analysis
The ATR-FTIR spectra were used to determine the chemical changes caused by antibacterial and plasma treatments, and results are shown in Fig. 2. It is well known that the existence of an aromatic rings in a structure is normally readily determined from the C–H and C=C–C ring-related vibrations. The C–H stretching occurs above 3,000 cm−1 and is typically exhibited as a multiplicity of weak-to-moderate bands in Fig. 2a. The other important bands are the aromatic ring vibrations centered around 1,600 and 1,500 cm−1, which usually appear as a pair of band structures. The untreated PET have the following main absorption bands at 3,424 cm−1 (C=O, carbonyl overtone), 2,967 and 2,906 cm−1 (two strong glycol C–H stretching), 1,712 cm−1 (C=O stretching), 1,577 (ring C–C stretching), 1,504 cm−1 (ring C–H in-plane bending, ring C–C stretching), 1,469 cm−1 (CH2 bending, O–C–H bending), 1,407 cm−1 (ring C–H in-plane bending, ring C–C stretching), 1,338 cm−1 (CH2 wagging, O–C–H bending), 1,239 cm−1 (C(=O)–O stretching, ring ester C–C stretching, C=O in-plane bending), 1,091 cm−1 (glycol C–O stretching), 1,015 cm−1 (ring C–C–C bending, ring C–C stretching, ring C–H in-plane bending), 967 cm−1 (O–CH2 stretching, C(=O)–O stretching, chain folding), 870 cm−1 (ring C–H out-of-plane bending, ring ester C–C out-of-plane bending, C=O out-of-plane bending, ring torsion), 845 cm−1 (ring C–C stretching, C=O in plane bending, C–H2 rocking), 791 cm−1 (ring C–H out-of-plane bending, C=O rocking and CCO bending), 722 cm−1 (C=O out-of-plane bending, ring torsion, ring C–H out-of-plane bending) [29, 32–34].
For triclosan, the strong absorption of halogenated hydrocarbons arises from the stretching vibrations of the carbon-halogen bond and the most prominent and informative bands in the spectra of aromatic compounds occur in the low-frequency range between 900 and 670 cm−1. After triclosan application, such new bands were detected between 3,000 and 2,800 cm−1 for aromatic C–H stretching bands and 1,800–1,600 cm−1 for weak combination and overtone bands in Fig. 2b. These strong absorption bands result from the out-of-plane bending of the ring C–H bonds. In-plane bending bands appeared in the 1,300–1,000 cm−1 region. Skeletal vibrations, involving C–C stretching within the ring, are absorbed in the 1,610–1,585 and 1,500–1,400 cm−1 regions. When several chlorine atoms are attached to one carbon atom, the band is usually more intense and at the high-frequency end of the assigned limits. The C–CI absorption bands were observed at frequencies range of 722–570 cm−1 and strong CH2 wagging bands were observed for the CH2CI group in the 1,300–1,150 cm−1 region. We attribute such new bands to coating triclosan on surface of PET, which is consistent with the SEM pictures.
Plasma treatment is known to break the covalent bonds and generate radicals on the surface. After plasma application in Fig. 2c, the variations in the peak positions were small in all samples because plasma treatment can only alter the fiber surface. However, sharper and more intensive bands between 3,600 and 2,800 cm−1 were detected since the surface was exposed to oxygen. It is expected that hydrophilic groups would be generated by reaction of the active radicals with the surrounding oxygen molecules, leading to a polar surface. Similarly, sharper peaks at other wavelengths were also obtained by plasma [24, 35, 36]. It has been also found similar bands between the triclosan treated surfaces and triclosan treated surfaces after plasma in Fig. 2d and that triclosan treated surfaces have generally sharper bands.
Antibacterial Activity
The antibacterial activities against S. aureus and E. coli are shown in Tables 2 and 3. It can be seen that untreated PET caused 52.66 and 34.30% for both bacteria, as a result of adhesion of bacteria onto fiber surface. These values are higher than those for natural fibers like cotton since synthetic fibers did not contain much more water which improves the growth of bacteria [37–39].
Considering antibacterial treatments, triclosan is one of the most widely used biocides with broad-spectrum antimicrobial activity (MIC’s ranging from 0.1 to 33 mg/ml). It is generally more effective against gram positive than gram negative bacteria or moulds. In prokaryotes, triclosan acts mainly by inhibiting fatty acid biosynthesis through blocking lipid biosynthesis by specifically inhibiting the enzyme enoyl-acyl carrier protein reductase (ENR). By blocking the active site, triclosan inhibits the enzyme, and therefore prevents the bacteria from synthesizing fatty acid, which is necessary for building cell membranes and for reproducing. In eukaryotes, the primary effects from triclosan are on the membranes. Triclosan acts as a site-specific inhibitor by mimicking the natural substrate of enoyl-ACP in gram-negative and gram-positive bacteria as well as in the mycobacteria. It workes with the concept of controlled release and provides a killing zone of inhibition. This zone of inhibition is the area around the treated surface into which the antimicrobial agent leaches or moves to, killing or inhibiting microorganisms [40–48]. It is well known that antibacterial activity depends on the amount of triclosan on fiber surface, but PET has lower wet-pick up (35%). Therefore, the antibacterial properties were slightly improved (66.80% for S. aureus and 50.94% for E. coli) after treating with 80 g/l triclosan. It was also found that triclosan had more effective against S. aureus than against E. coli. Gram-negative bacteria are generally more resistant to biocides than Gram-positive bacteria, because of an extra layer (outer membrane) surrounding the cell wall, composed of polysaccharides, proteins, and phospholipids [49, 50]. The results frankly are not as good as we expect and are not enough to have a sufficient antibacterial effect.
The plasma treated samples had almost same antibacterial properties (56.38% against S. aureus and 42.80% against E. coli) comparing untreated samples. It can be depend on the amount of reactive particles which generated on the fiber surface by plasma. In addition, the formation of horizontal channels on fiber surface after plasma can be caused by these unexpected results in antibacterial activity. It also can be enhanced the more biofilm formation and bacterial attachment onto fiber surface, so that antibacterial activity can negatively be affected by plasma.
When applying the antibacterial treatment after plasma, all samples had the best antibacterial activity (90.64% against S. aureus and 74.60% against E. coli). These results pointed that increasing operating frequency and reaction times accounted for the increase in antibacterial efficacies. In here, the fibers polarity increased and fiber became more wettable through plasma. These changes caused the increasing wet pick-up of PET and then fiber surface was fully covered with antibacterial chemical. Finally, the results showed the best antibacterial activity for both bacteria.
Conclusions
The antibacterial products have huge potential and many commercial chemicals have been used in textile industry for 6 decades. For producing antibacterial textiles, while PET fibers have superior properties and significant advantages, they have lower wet pick-up because they do not possess any chemically active groups. Therefore, surface modification using plasma is one of the ways to overcome this undesired property.
In this work, it is observed that horizontal channels occur on the surface after plasma and then fibers become more wettable. However, the results are not high enough to get satisfactory antibacterial effect. The formation of channels on surface can be caused by these unexpected results in antibacterial activity.
In addition, these changes after plasma bring about that fiber surfaces are fully coated with triclosan after finishing. SEM and FTIR results have also approved of loading more triclosan on surface. The antibacterial activity gets better with the increasing frequency and plasma time and as a consequence of this, the samples treated with triclosan after plasma have the best antibacterial activity.
Finally, the results indicate that plasma can be effectively used to improve the hydrophilicity and surface roughness of PET and then the more chemicals are able to attach to fiber surface after finishing. Therefore, plasma offers a potential pretreatment and an attractive prospect to finishing treatment of PET fibers.
References
Edward M (2002) Int Dyer 13:16
Holme I (2002) Int Dyer 9:11
Kim YH, Sun G (2001) Textile Res. J. 71:318
Lindemann B (2000) Melliand 9:E205
Mao J, Murphy L (2001) Am Assoc Textile Chem Color Rev 11:28
Mao J (2001) Am Assoc Textile Chem Color Rev 12:15
Mucha H, Hofer D, Abfalg S, Swerev M (2002) Melliand Int 8:148
Nakashima T, Sakagami Y, Ito H, Matsuo M (2001) Textile Res J 78:688
Service D (1998) Chem Fibers Int 48:486
Vigo T, Leonas K (1999) Textile Chem Color 1:42
Vigo TL, Donna GF, Goynes WR (1999) Textile Chem Color 31:29
Hall JR, Westerdahl CAL, Devine AT, Bodnar M (1969) J Appl Polym Sci 13:2085
Kaelble DH, Dynes PJ, Cirlin EHJ (1974) J Adhes 6:23
Liston EM, Martinu L, Wertheimer MR (1993) J Adhes Sci Technol 7:1091
Beake B, Ling J, Leggett G (1998) J Mater Chem 8:1735
Borcia G, Anderson CA, Brown NMD (2006) Surf Coat Technol 201:3074
Errifai I, Jama C, Le Bras M, Delobel R, Gengembre L, Mazzah A, De Jaeger R (2004) Surf Coat Technol 180–181:297
Hochart F, Jaeger R, Grutzmacher J (2003) Surf Coat Technol 165:201
Jahagirdar CJ, Tiwari LB (2004) J Appl Polym Sci 94:2014
Lee HR, Kim K, Lee KH (2001) Surf Coat Technol 142–144:468
Liu D, Hu J, Zhao Y, Zhou X, Ning P, Wang Y (2006) J Appl Polym Sci 102:1428
Oktem T, Ayhan H, Seventekin N, Piskin E (1999) J Soc Dyers Colour 115:274
Poll HU, Schladitz U, Schreiter S (2001) Surf Coat Technol 142–144:489
Samanta KK, Jassal M, Agrawal AK (2009) Surf Coat Technol 203:1336
Ueda M, Tokino S (1996) Rev Pro Color 26:9
Vohrer U, Muller H, Oehr C (1998) Surf Coat Technol 98:1128
Yip J, Chan K, Sin KM, Lau KS (2002) J Mater Process Tech 123:5
Gupta B, Hilborn J, Hollenstein C, Plummer CJG, Houriet R, Xanthopoulos N (2000) J Appl Polym Sci 78:1083
Donelli I, Freddi G, Nierstrasz VA, Taddei P (2010) Polym Degrad Stabil 95:1542–1550
Greenwood OD, Hopkins J, Badyal JPS (1997) Macromolecules 30:1091–1098
Ramos AL, Braga SS, Paz FAA (2009) Acta Cryst Sect C Acta C65:404–405
Coates J (2000) Interpretation of infrared spectra, a practical approach. Wiley, New York
Roberts JD, Caserio MC (1977) Basic principles of organic chemistry, 2nd edn. Wiley, New York
Roberts JD, Webster FX (1997) Spectrometric identification of organic compounds. Wiley, New York
Boenig HV (1982) Plasma science and technology. Cornell University, London
Grill A (1994) Cold plasma in materials fabrication: from fundamentals to application. IEEE Press, New York
Kut D, Orhan M, Gunesoglu C, Ozakin C (2005) AATCC Rev 5:25
Orhan M, Kut D, Gunesoglu C (2007) Indian J Fibre Text 32:114
Orhan M, Kut D, Gunesoglu C (2009) J Appl Polym Sci 111:1344
Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO (1998) J Biol Chem 273:30316–30320
Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO (1999) J Biol Chem 274:11110–11114
Jones RD, Jampani HB, Newman JL, Lee AS (2000) Am J Infect Control 28:184–196
Levy CW, Roujeinikova A, Sedelnikova S, Baker PJ, Stuitje AR, Slabas AR, Rice DW, Rafferty JB (1999) Nature 398:383–384
McDonnell G, Russell AD (1999) Clin Microbiol Rev 12:147–179
McMurry LM, Oethinger M, Levy SB (1998) Nature 394:531–532
Regos J, Hitz HR (1974) Zentralbla Bakteriol Hyg Abt 1 Orig A 226:390–401
Roujeinikova A, Levy CW, Rowsell S, Sedelnikova S, Baker PJ, Minshull CA, Mistry A, Colls JG, Camble R, Stuitje AR, Viner R, Rice DW (1999) J Mol Biol 294:527–535
Stewart MJ, Parikh S, Xiao G, Tonge PJ, Kisker C (1999) J Mol Biol 290:859–865
Russell AD, Chopra I (1996) Understanding antibacterial action and resistance. Ellis Horwood, Chichester
Denyer SP (1995) Int Biodeterior Biodegrad 36:227
Acknowledgments
This study was supported by The Commission of Scientific Research Projects of Uludag University, Project Number 2009/33 and by Grant TUBITAK BIDEB 2219 (The Scientific and Technological Research Council of Turkey). We also thank reviewers for helpful and stimulating suggestions and questions, which have helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orhan, M., Kut, D. & Gunesoglu, C. Improving the Antibacterial Property of Polyethylene Terephthalate by Cold Plasma Treatment. Plasma Chem Plasma Process 32, 293–304 (2012). https://doi.org/10.1007/s11090-011-9342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-011-9342-z