Abstract
A method based on high performance liquid chromatography (HPLC), has been developed to measure hydroxyl radical (·OH) in plasma reactors. The determination was performed indirectly by detecting the products of the reaction of ·OH with salicylic acid (SAL). The applicability, and effect of time, specific input energy (SIE), relative humidity (RH), catalyst were investigated. It was found that 3 h was the optimal trapping time; concentration of ·OH was (5.9–23.6) × 1013 radicals/cm3 at SIE range. The highest ·OH yield and toluene removal efficiency (η) were achieved with a RH of 20%. With MnO x , η was two times that without catalyst, while ·OH yield in gas stream was one-sixth that without catalyst. However, if summed with ·OH adsorbed on catalyst surface, the total ·OH yield was the same as without catalyst. Experiments performed with/without toluene allowed to determine the role of ·OH on decomposition of toluene in air plasma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To understand the reactions taking place in non-thermal plasma system, the detection of radicals and active species is necessary [1]. It gives an insight into plasma chemical processes, enables an understanding of plasma system and optimizes the process of air pollution control. The hydroxyl radical (·OH) is one of the most frequently studied diatomic radicals for its importance in plasma-induced chemical processes. It has extremely high oxidation potential (2.8 V), which is only lower than that of F2 (3.0 V) [2]. A lot of studies have indicated the importance of ·OH in the plasma treatments of water pollutants, in biological system and for air pollution control [3–6]. ·OH is also believed to be the key reactive species for oxidation of VOCs and affects their removal efficiency in plasma system [7, 8]. Klamt [7] reported that the rate constant of ·OH in gas-phase is an important indication for the fate of organic compounds in the environment. Storch and Kushner [8] also pointed out that the destruction of HCHO was mainly due to the chemical attack by ·OH radicals and O atoms, which are primarily produced by electron impact dissociation of H2O and O2.
Though the importance of ·OH in VOCs decomposition by plasma have been affirmed, ·OH measurement techniques have long been a goal due to its short lifetime (less than 10−9 s). In recent studies, several methods have been developed to detect ·OH in non-thermal plasma systems, including electron spin resonance (ESR) [9], laser-induced fluorescence (LIF) [10, 11], colorimetric spectrophotometry [12], a radiocarbon method [13], and quantitative analysis of ·OH by oxidating CO to CO2 [14, 15]. However, the methods reported have great limitations. ESR and LIF need special equipment. Spectrophotometry is considered as a simple and practical approach, but the accuracy is low. The radiocarbon method is difficult to apply for the handicap of marking the carbon element. The method of measuring the density of ·OH by oxidating CO to CO2 is not suitable for the systems that produced CO2 itself.
High-performance liquid chromatography (HPLC) technique, which needs no expensive instruments, proves to be a simple and sensitive method for the measurement of ·OH in a biological system [4]. Furthermore, Jen [3] first applied the method to the measurement of ·OH in an advanced oxidation process. However, for the special characteristics of non-thermal systems, this method needs improvement. The most important issue is to choose an appropriate trapping agent. In this study, salicylic acid (SAL) was chosen, previous studies [3] show that it reacted with high selectivity with ·OH, provided a fast, reproducible and well-characterized response. The products of the reaction of ·OH with SAL could be measured with high sensitivity and selectivity. We have previously reported [16] some preliminary results by the application of this method in a DBD system. However, the range of humidity employed was narrow and correlation was not made between the ·OH yield and toluene removal, neither was the effect of catalyst.
In this paper, high performance liquid chromatography coupled with an ultraviolet detector (HPLC-UV) was used to measure ·OH indirectly by determining its main reaction products with SAL, 2,3-dihydroxybenzoic acid (2,3-DHBA) and 2,5-dihydroxybenzoic acid (2,5-DHBA). The present work aimed to investigate the feasibility of measuring the ·OH yield in air DBD plasmas at atmospheric pressure by HPLC method, to study the effect of specific input energy (SIE), relative humidity (RH) and catalyst on ·OH production. The relationship between ·OH yield and toluene removal efficiency have also been investigated.
Experimental
Experimental Setup and Reactor
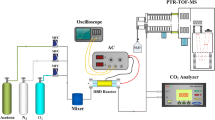
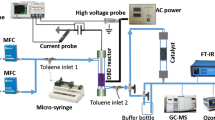
The experimental setup and reactor were similar to previous reports [17]. Air was used as the carrier gas and was generated by an air compressor. Mass flow controllers were used to regulate the gas flow rate. Toluene was analyzed by a gas chromatograph (GC-7890II, China) equipped with a hydrogen flame ionization. The wave form of current and voltage input into the reactor were measured with a digital oscilloscope (TDS1012, Tektronix) and a high voltage probe (P6105, Tektronix). HPLC (Ultimate 3000, USA) was used to determine the amount of hydroxylated products and residual SAL. The initial concentration of toluene was controlled to 400 mg/m3 and the gas flow rate was 450 ml/min. The applied SIE ranged from 140 to 320 J/L. Water was bubbled by air stream to adjust the RH. Figure 1 shows the wire-plate DBD reactor. Two epoxy resin boards were used to form the reactor walls and acted as dielectric barriers. The positive high voltage electrode was made of brass wire, which was shaped into a spiral in order to increase the energy density in the reaction volume. Two grounded copper nets, fixed on the two sides of the middle epoxy resin board, formed negative electrodes. The principal feature of the reactor is that it can be dismantled, which facilitates loading the films and catalyst.
Chemicals and Reagents
All the chemicals and reagents were HPLC-grade. 2, 3-DHBA and 2, 5-DHBA were obtained from Sigma, SAL and methanol were purchased from Darmstadt and Merck (Germany), respectively. Both ethanol and Phosphoric acid were from Kermal (China). The deionized water (≥18 MΩ/cm) used for preparing the chemical solutions was processed by a Milli-Ultrapure R/O System (Millipore, USA) to ensure the quality of the prepared solution.
HPLC Conditions
A HPLC Ultimate 3000 (USA) and a C18 reversed-phase column (5 μm, 250 mm × 4.6 mm) were used to separate the analytes. The sample was run isocratically with a mobile phase consisting of 40% (v/v) methanol, 60% deionized water, at a flow rate of 1 ml/min. Phosphoric acid was used to adjust pH of the buffer solution to 4.5. All eluents were filtered through a 0.45 mm membrane filter and degassed ultrasonically. The temperature of the column was set at 35°C. The HPLC system coupled with an UV detector was used to monitor SAL and hydroxylated derivatives at 300 nm. The injection volume of sample was 20 μl.
Trapping and Detection of ·OH
The trapping of ·OH was achieved by introducing a film impregnated with SAL into the DBD reactor. In order to identify the influence of films on the characteristic of electric field, digital oscilloscope and high voltage probe were used to investigate the power at the same voltage 16 kV with/without the films placed in the discharge zone. It was found that the powers were 1.339 and 1.338 W respectively, which were nearly the same. Hence, unlike the packed bed, the films placed in the reactor had little effect on discharge.
Preparation of a Sampling Film
To trap ·OH produced during the plasma reaction, one piece of filter paper, whose size should be 300 mm × 22.5 mm, was needed. An excess of 0.1 g SAL was dissolved in 5 ml anhydrous ethanol (99.9%) to ensure the effective trapping. The solution was then dripped evenly onto the filter paper by an injector. It was dried until all the solution had been loaded.
Sampling and Sample Extraction
Two prepared sampling films, which were separated by resin board in the middle of reactor, were folded as shown in Fig. 2. The distance (d) between valleys should be as small as possible to capture ·OH effectively. As the reaction of SAL and ·OH was a gas–solid interface reaction, the mass transfer may influence the trapping reaction. However, the coarse surface of films used easily absorbed the ·OH in the gas stream and the reaction kinetics between ·OH and salicylic acid belonged to the type of diffusion, and its reaction rate was up to 2.2 × 1010 cm3 s−1 [18]. Moreover, the films were placed as in Fig. 2 to make effective the trapping for short-range collisions. Therefore, sampling film collection efficiency was approximated to be 100%.
Subsequently, the films were removed for analysis. There were two ways to extract the products. One was ultrasound extraction, in which the films were washed with deionized water (10 ml), then, extracted by an ultrasonic cleaner for half an hour to accelerate dissolution of products. The other was a soxhlet extraction method, it could extract the products more completely, but it took a long time (4 h). So in this experiment, ultrasound extraction had been chosen. If we assume that the methodology of soxhlet extraction used can extract products completely, the extraction efficiency of ultrasound extraction could be obtained: η = C ul/C sol (C ul: the concentration of total hydroxylated derivatives extracted with ultrasound method; C sol: that with soxhlet method). To obtain the value of η, two samples were collected at the same condition, and then one was extracted by ultrasound method, the other with soxhlet method, got C ul and C sol respectively, η = C ul/C sol is about 75.6% in this study.
Quantification and Calculation
In our study, only 2,3-dHBA and 2,5-dHBA were found as the hydroxylated derivatives of salicylic acid in hydroxyl radical trapping. Catechol was ignored due to its insignificant distribution under the conditions used.
The total concentration of the hydroxylated derivatives can be calculated:
In this study, all SAL loss is due to its reaction with ·OH, which can be verified by a carbon balance. It is calculated by the amount of SAL consumed (residual SAL subtracts from SAL used) and the quantity of hydroxylated derivatives (2,5-DHBA + 2,3-DHBA). The ratio is 96.5%, the other 3.5% part may be catechol, which is beyond the detection limit.
The fraction of hydroxylated derivatives can be defined as:
Then the concentration of ·OH can then be calculated by Eq. 3.
C Total, Hydroxylated derivatives concentration formed during sampling (mol/l); C SALr, the total concentration of the products of the reaction between SAL and ·OH, which was calculated by all SAL loss (mol/l); V L, scrubbing solution volume after sampling (l); N, Avogadro’s number (6.02 × 1023); F Total, fraction of main hydroxylated derivatives in the total products (which is 96.5%, the other 3.5% part may be catechol, but it is beyond the detection limit); F g, air flow rate (ml/min); t, sampling period (min); η, extraction efficiency (%); α, loss rate of ·OH passing the sampling films, which was set to zero in this study.
The toluene removal efficiency could be defined as below:
Preparation of Catalyst
Catalyst of manganese oxide/alumina/nickel foam (MnO x ) was prepared by an incipient wetness impregnation method, with a mass ratio of 50%:50%:100%. First a sol of alumina was loaded on nickel foam until the mass ratio was up to 50%:100%, then precursors of Mn(NO3)2 were deposited on the support. After that, it was dried at 393 K for 2 h, and then calcined at 723 K for 4 h.
Results and Discussion
Separation and Identification of Species
The separation of SAL and hydroxylated products was achieved with a reversed-phase C18 column under the elution conditions described previously. The UV detector was coupled with the system to monitor products at 300 nm. Chromatograms of hydroxylated derivatives in an experiment are shown in Fig. 3. To identify these species, the retention time were compared with standards. As shown Fig. 3, these species were well separated within 20 min. Peaks 1–3 agreed well with 2,5-DHBA, 2,3-DHBA and SAL. Their retention time was 5.604, 7.525, and 17.686 min, respectively.
From Fig. 3, it can be seen that only 2,3-DHBA and 2,5-DHBA were found as the hydroxylated derivatives and the quantity of 2,3-DHBA was larger than that of 2,5-DHBA. The possible reason could be that the generation of 2,5-DHBA and 2,3-DHBA was affected by the distribution of the electron cloud on the benzene ring, due to the presence of a carboxyl and a hydroxyl group. Moreover, the activity of site 3 was higher than that of site 5 for the conjugative effect of the electrons.
Calibration Plots and Reproducibilities for the Hydroxylated Derivatives
In order to test the applicability of HPLC method for quantitative determination of hydroxylated derivatives and SAL, calibration plots were determined for these species over the concentration ranges summarized in Table 1. They were analyzed under optimal conditions by UV detectors. The repeatability was examined by injecting 20-μl volumes of each sample in triplicate. Table 1 clearly shows that there was a linear relationship between peak area and injected quantity.
Sampling Time to Trap the Primary Hydroxylated Derivatives
An ideal analytical technique should be able to complete sample preparation and analysis quickly. Although an excess of SAL was added, the sampling time should be investigated to obtain sufficient amounts of derivatives for quantitative determination. The films were placed in the plasma area and the sampling time was set at 1, 2, 3, 4, 5 and 6 h. Then samples were taken for HPLC analysis. The total concentration of ·OH could be obtained from the sum of hydroxylated products. Figure 4 shows the quantities of ·OH for various trapping times. It can be seen that the yield of ·OH increased quickly in the first 3 h in the DBD system, but it slowed sharply over the next 3 h. Hence, a 3 h trapping reaction was selected in this study.
The possible reason was that when the sampling time was too long, the collected hydroxylated products would react with other species under plasma area and decomposed. Or just as Chang et al. [19] reported that ·OH reacts with the hydroxylated products, but it happened only that the hydroxylated produced run up to a certain amount. This was consistent with the phenomenon observed in the experiment: After sampling for more than 4 h, some yellow solids could be seen on the filter paper. But it is not possible to detect the products of their reaction with ·OH, maleyl pyruvate and malic acid, for it is too little to be determined.
Effect of Specific Input Energy (SIE) on the Amount of ·OH
To understand the effect of ·OH on the removal of toluene, the quantities of ·OH with/without toluene were studied. Meanwhile, the removal efficiencies of toluene with/without films impregnated with SAL to trap ·OH in the DBD reactor were investigated by means of GC.
In condition of Fig. 5, it was the quantities of ·OH with/without toluene, the amount of ·OH increased a little when 400 mg/m3 of toluene was added. On the one hand, toluene consumed a little amount of ·OH, for almost all the ·OH would react with SAL when both SAL and toluene in the reactor. This is probably due to that the rate constant of ·OH with SAL (2.2 × 1010 cm3 s−1) is much higher than that of ·OH with toluene (5.2 × 10−12 cm3 s−1) [20]. Meanwhile, on the other hand, ·OH can be generated by extraction of ·H from toluene by O atoms or O3 and the rate constant of ·OH formation is a little higher than that of ·OH depletion, for example, the rate constant of O3 and ·H is 1.4 × 10−10 cm3 s−1 [20].
Figure 5 also shows that the concentration of ·OH increased with SIE. It was possible that an increase in SIE would lead to an increase in the high-energy electron density, the electron mean energy as well as a yield increase of oxidative radicals, which was beneficial to the degradation of pollutants as could be seen in Fig. 6. ·OH was mainly produced by electron–molecule collision process. The reaction was as follows [21]:
The concentrations of ·OH during the sampling were in the range of (5.9–23.6) × 1013 radicals/cm3.
Figure 6 shows that there was a huge gap of toluene efficiencies with/without films in the reactor, which was likely a consequence of the important role of ·OH. With the ·OH trapped by films impregnated with SAL, there was still some toluene degradated. It should be a consequence of the reactivity of active species with toluene, such as the oxygen atom (O), the various other radicals and molecules (O3) created in the discharge. However, reactions with ·OH were more efficient than the reaction with O3 or O atom, which may due to the differences of their rate constants with toluene. The rate constant of O3 with toluene was 1.5 × 10−22 cm3 s−1, and that of O atom with toluene is 8.4 × 10−14 cm3 s−1, which is much lower than that of ·OH 5.2 × 10−12 cm3 s−1 [20].
Figures 5 and 6 shows that there was a consistent trend between the ·OH densities and the margin of toluene removal efficiency with/without SAL in the reactor.
Determination of Hydroxyl Radicals as a Function of Relative Humidity
It is well known that the formation of ·OH can be affected by the humidity of gas mixture. In order to investigate the generation of ·OH during DBD of toluene at various RH, reactions were carried out in DBD reactor with an air mixture, which had been adjusted to RH of 0, 20, 40, 60 or 80% by bubbling water vapor.
Figure 7 shows the concentration of ·OH after a 3 h trapping reaction at various RH. It can be seen that the ·OH yield increased as the RH increased to 20%, but decreased when the humidity was greater than 20%. Under the optimal RH of 20% and with an applied voltage of 320 J/L, the yield of ·OH reached up to 2.36 × 1014 radicals/cm3. This was consistent with our previous report [16], which indicated that the ·OH yield increased within a narrow range of humidity.
As can be seen from Fig. 7, without H2O in the mixture, a small amount of ·OH could also be produced, probably due to H-abstraction from toluene by O atom. With the addition of water vapor, reaction (5) takes place. Therefore, with a water vapor content increasing, the probability of collisions among water molecules and electrons or reaction with excited state oxygen atoms increased, resulting in more ·OH. However, with RH increasing, ·OH yield reduced,so was the toluene removal efficiency. A possible reason was the collisions between active species and water molecules, the electron mean energy decreased [22]. Though the 2,5-DHBA, 2,3-DHBA and SAL were water-soluble, they would not dissolve into bubbled water vapor in the gas flow during discharge even at higher RH. Moreover, the water vapor would also attach to the film and be trapped, so the less ·OH yield should not be due to the loss but the less production (which can be verified by the residual SAL and the hydroxylated products).
The toluene removal efficiency, which was affected by ·OH yield, was significantly affected by the water content in DBD system. As can be seen from Fig. 8, with a SIE of 225 J/L, when the humidity was increased, the degradation of toluene increased up to 52.5% at RH 20%. When the system reached a RH of 80%, the toluene removal efficiency decreased significantly, just 22.0%.
Effect of Catalyst on the Formation of ·OH
As non-thermal gas-phase plasma treatment usually cannot achieve both high conversion and satisfactory product selectivity, catalysts, which could broaden the application of this technology, were added to the system. MnO x was used to investigated the effect of catalyst on the formation of ·OH and toluene removal efficiency.
From Figs. 9 and 10, it can be seen that there was a consistent trend between the ·OH density and toluene removal efficiency without catalyst in air gas stream, the highest ·OH yield and toluene removal efficiency were achieved with a RH of 20% without catalyst. However, when water was added to the DBD reactor with catalyst in situ, there was no direct relationship between the quantities of ·OH in the gas stream and the toluene removal efficiency. That is, with MnO x catalyst, the toluene removal efficiency was nearly two times than that of without catalyst, but the ·OH yield in gas stream was only one-sixth of that without catalyst at a SIE of 180 J/L. The highest ·OH yield was also obtained at a RH of 20%, but there were fine distinctions of ·OH yields among various humidities. With catalyst, the toluene removal efficiency decreased when water vapor increased. It is possible that water covered on the surface of the catalyst and results in a decrease in the reaction probability.
To confirm this hypothesis, the quantities of hydroxyl radicals both in the gas phase and on the surface of catalyst have been determined in the plasma-catalysis system. After the catalyst was prepared, salicylic acid solution was dripped evenly onto the surface of the catalyst. Then the same method as former was used to detect the quantities of ·OH on the surface of the catalyst. It was found that the amount of ·OH on the surface of catalyst was 62.14 × 1012 radicals/cm3, while that of 11.34 × 1012 radicals/cm3 existed in the gas stream. That is, the total amount of ·OH in the plasma-catalyst system 73.48 × 1012 radicals/cm3 was nearly equal to that without catalyst 73.94 × 1012 radicals/cm3, which can be seen in Table 2. Hence, the ·OH in the gas stream in the plasma-catalysis system did not play an important role, instead maybe it was the ·OH on the catalyst surface or the active site of catalyst that caused the higher toluene decomposition efficiency.
Conclusions
In this communication, HPLC, with ·OH trapped by SAL, has been shown to be a useful method for indirectly determining the concentrations of ·OH in DBD air plasma system at atmospheric pressure. The quantity of ·OH increased with an increase of SIE. The role of water molecules as the source of ·OH formation was identified. The RH of the gas mixture had a significant effect on ·OH yield and toluene removal efficiency. The highest ·OH yield was achieved with a RH of 20%. At a higher humidity, the concentration of ·OH declined because of an decrease in electron mean energy by water molecules. There was a consistent trend between the ·OH density and the toluene removal efficiency without catalyst. On the contrary, there was no direct relationship between them with catalyst: the concentration of ·OH in the gas stream with catalyst is much lower than that without catalyst. However, the total of ·OH in the gas stream and on the catalyst surface was nearly equal to that without catalyst, leading to conclude that the most important role in toluene decomposition is due to ·OH adsorbed on the catalyst.
References
Oda T (2003) J Electrostat 57:293–311
Bach RD, Dmitrenko O (2006) J Am Chem Soc 128:1474–1488
Jen JF, Leu MF, Yang TC (1998) J Chromatogr A 796:283–288
Freinbichler W, Bianchi L, Colivicchi MA, Ballini C, Tipton KF, Linert W, Corte LD (2008) J Inorg Biochem 102:1329–1333
Suh I, Zhang R (2002) Chem Phys Lett 363:454–462
Magne L, Pasquiers S, Simiand B (2007) J Phys D Appl Phys 40:3112–3127
Klamt A (1996) Chemosphere 32:717–726
Storch DG, Kushner MJ (1993) J Appl Phys 73:51–55
Miura Y, Ueda J, Ozawa T (1995) Inorg Chim Acta 234:169–171
Heard DE (2006) Annu Rev Phys Chem 57:191–216
Magne L, Pasquiers S (2005) C R Phys 6:908–917
Bektas B, Özyürek M (2008) Talanta 77:90–97
Campbell MJ, Hall BD, Sheppard JC, Utley PL, O’Brien RJ, Hard TM, George LA (1995) J Atmos Sci 52:3421–3427
Su ZZ, Ito K, Takashima K, Katsura S, Onda K, Mizuno A (2002) J Phys D Appl Phys 35:3192–3198
Rudolph R, Francke KP, Miessner H (2003) Plasmas Polym 8:153–161
Guo YF, Liao XB, Ye DQ (2008) J Environ Sci 20:1429–1432
Guo YF, Ye DQ, Chen KF, He JC, Chen WL (2006) J Mol Catal A Chem 245:93–100
Buxton GV, Greenstock CL, Helman WP (1988) J Phys Chem Ref Data 17:513–886
Chang CY, Hsieh YH, Hsieh LL, Yao KS, Cheng TC (2009) J Hazard Mater 166:897–903
Kohno H, Berezin AA, Chang J-S, Tamura M, Yamamoto T, Shibuya A, Honda S (1998) IEEE Trans Ind Appl 34:953–966
Futamura S, Zhang AH, Yamamoto T (1997) J Electrostat 42:51–62
Ono R, Oda T (2002) J Electrostat 55:333–342
Acknowledgments
This work was supported by the Doctoral Program of Higher Specialized Research Fund (20070561042); National High Technology Research and Development Program of China (No. 2006AA06A310), the National Natural Science Foundation of China (No. 50978103, No. 50708021), the Guangzhou University Xinmiao Project (No. gyf1-1001), and the Guangzhou University Scientific Research Startup Project (No. gyf1-1002). In addition, key laboratory of environmental protection and eco-remediation of Guangdong regular higher education institutions was gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liao, Xb., Guo, Yf., He, Jh. et al. Hydroxyl Radicals Formation in Dielectric Barrier Discharge During Decomposition of Toluene. Plasma Chem Plasma Process 30, 841–853 (2010). https://doi.org/10.1007/s11090-010-9253-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-010-9253-4