Abstract
Crosslinking graft polymerization of poly acrylic/montmorillonite superabsorbent composite in aqueous solution was prepared by using glow-discharge electrolysis plasma, in which acrylic acid and acid-activated montmorillonite were used as starting materials, N,N-methylenebisacrylamide as a crosslinking agent. To optimize the synthetic conditions, seven important parameters of the graft-polymerization were examined in detail, such as the discharge voltage, discharge time, the neutralization of acrylic acid, post polymerization temperature, amounts of crosslinking agent, montmorillonite and acrylic acid used in this study. The structure, thermal stability and morphology of product were characterized by Fourier transform infrared spectroscopy, thermogravimetric analysis, scanning electron microscopy, respectively. The absorbency of poly acrylic/montmorillonite superabsorbent was examined to yield the following results: 1,834 g/g for distilled water, 116 g/g for 0.05 mol/L and 75 g/g for 0.15 mol/L sodium chloride solution. Such excellent character could be important to use in many fields, for example, in agricultural and horticultural applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The study of superabsorbent polymers (SAPs) has received a great deal of attention due to their application in many fields, such as agriculture and horticulture [1], sanitary goods [2], drug delivery [3, 4], artificial implant [5], waste-water treatment [6], biomedical area [7], and so on. The excellent ability to absorb water and/or heavy-metal ions is related to their three-dimensional crosslinked structure [8–10]. Thereby, various techniques are appearing for improving the properties of superabsorbents. Roughly, it may be classified into two categories, i.e., initiated polymerization by using chemicals [8, 11] and by physical techniques, for example, photo-polymerization [12], irradiation-polymerization [13], plasma-polymerization [14] and so on. Various physical methods have little pollution to environment, in which the GDEP has more advantages such as lower cost in set-up and easy operation in polymerization.
Glow-discharge electrolysis is a kind of non-equilibrium plasma, in which the glow-discharge occurs under aqueous solution. The energy of active species produced in solution such as •OH, •H, and HO2 • can initiate many unusual chemical reactions. For example, Sengupta et al. [15] found that the yield of •OH radical was 12 mol for the passage of each mole electron of electricity during glow-discharge electrolysis. That is to say, glow-discharge electrolysis could be considered as rich sources of free radicals in solution chemistry. Although the technique has been used widely in the field of wastewater treatment [16] and oxidative degradation [17–20], there have been few studies on synthetic chemistry. Early reports were concerning the synthesis of acrylamide polymerization [14], uracil and thymine [21], amino acids [22–24]. In recent, Gao and his co-workers have also synthesized successfully some polymers [25–27] by means of GDEP. All facts indicated that GDEP could be used in synthetic chemistry to initiate radical reactions.
The synthesis of new superabsorbents is still an interesting topic due to their application in many areas. Recently, clay has become a new focus for the preparation of superabsorbent composite in order to improve swelling properties, enhance gel strength and reduce production cost. Montmorillonite, which is a kind of mineral clays, has been more suitable as additives, and have often been used in the synthesis of superabsorbent composite materials [8, 25] because of their hydrophilic nature, chemical and mechanical stability, high specific surface area. In this paper, a novel acrylic acid/montmorillonite superabsorbent composite was synthesized by aqueous solution polymerization with GDEP. Some variables on the water-absorption property were also studied in detail.
Experimental Section
Reagents and Apparatus
Acrylic acid (AA) was distilled under reduced pressure before use. N,N-methylenebisacrylamide (MBA), sodium hydroxide and methanol were of analytical reagent grade. Montmorillonite (MMT, supplied by Xinjiang Tuokexun chemical technology general factory, China) was purified and firstly treated with 0.25 mol/L H2SO4 for 3 h, then washed with distilled water to SO4 2− ion-free, and dried at 105°C for getting a constant weight before the use [28, 29].
The experimental apparatus, which is similar to the one described in our previous works [25–27], contains a high voltage power supply and a reactor. The power supply was a Model of LW100J1 DC power supply (Liyou, Shanghai, China) providing the voltage of 0–1,000 V and the current of 0–1 A. The reactor was a 250 mL three-necked flask equipped a reflux condenser, a platinum wire anode with a diameter of 0.5 mm sealed into a glass tube to generate glow-discharge plasma and a graphite cathode with a diameter of 10 mm. Both electrodes were immersed in the solution and the distance between each other was about 20 mm. There was a magnetic stirring bar at the bottom of the flask to keep the solution mixed well.
Preparation of Superabsorbent Composite
All reactions were conducted in a three-necked flask equipped with a stirrer, a condenser, a platinum wire anode and a graphite cathode. All parameters that affect the characteristic of superabsorbent, such as the discharge voltage, discharge time, the neutralization of acrylic acid, post polymerization temperature, amounts of crosslinking agent, montmorillonite and acrylic acid used in this study, are examined in detail. The general procedure sequence gives below. A weighed amount of MMT, along with a cross-linking agent (MBA) dissolved in distilled water, was firstly placed in the reaction vessel, and stirred for 40 min, followed by adding a desired amount of monomer into the above mixture solution with stirring another 20 min to mix well. Then, the discharge was lasting for about 10 min. Stop the discharge, the reaction mixture was warmed (ca. 80°C) on an oil bath for the post polymerization for 3 h, following by cooling the resulting product to room temperature. The solid product was cut into small pieces (diameter of ca. 5 mm), and then, treated with 1 mol/L sodium hydroxide (58.3–105 mL) to neutralize in part (50–90%) the carboxylic groups of the grafted poly (acrylic acid) composite. Finally, the resulting product was washed several times with distilled water. After removing water with methanol, the product was dried in vacuum oven at 65°C to a constant weight, then, milled and screened. The PAA/MMT superabsorbent composite was obtained.
Measurements of Water Absorbency
An accurately weighed sample (0.10 g) was immersed in excess distilled water or saline solution at room temperature to reach swelling equilibrium. Swollen samples were then filtered through a 120-mesh screen. Water absorbency in distilled water of the superabsorbent composite, \( Q_{{{\text{H}}_{2} {\text{O}}}} \), was calculated using the following Equation:
where m 1 and m 2 are the weights of the dry sample and the swollen sample, respectively. \( Q_{{{\text{H}}_{2} {\text{O}}}} \) is expressed in g/g for pure water or brine.
Characterization
The infrared spectra of superabsorbent composite were recorded on a DIGILAB FTS 3000 FT-IR spectrophotometer (USA) with a KBr pellet in the range of 4,000–400 cm−1. SEM observation was performed in a JSM 5600 LV scanning electron microscope (Japan), using an acceleration voltage of 20 kV. Before SEM observation, all of the samples were coated with gold. Thermogravimetric analysis (TGA) was carried out in a PETG/DTA 6300 instrument (USA), over a temperature range of 20–800°C, with a heating rate of 10°C/min and under a nitrogen flow rate of 50 mL/min.
Results and Discussion
Infrared Spectra Analysis
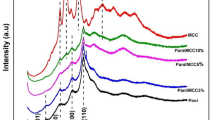
The FTIR spectra of montmorillonite (a), acrylic (b) and their corresponding superabsorbent composite (c) are shown in Fig. 1, respectively. For montmorillonite, the absorption peaks 3,614 and 3,437 cm−1 are attributed to the stretching bands of OH groups, absorption bands at 1,636, 1,040, 466 cm−1 to the H2O stretching vibration, the Si–O stretching vibrations within the layer and the Si–O–Al vibration, respectively. For acrylic, the characteristic absorption peak at 1,703 and 1,635 cm−1 are corresponding to the –COOH and C=C stretching vibration in acrylic, respectively. As can be seen, for superabsorbent composite, the intensities of absorption bands at 3,614, 1,040 cm−1 ascribed to –OH, Si–O of MMT are disappeared. A series of new absorption bands at 2,930, 1,700, 1,455 and 1,409 cm−1 ascribed to C–H stretching, –COOH stretching, symmetric –COO− stretching and C–H bending appeared after the reaction with AA. The information from montmorillonite and acrylic in Fig. 1 may indicate a bridge between MMT and AA has been built by the –OH group of MMT through the graft reaction.
Thermal Analysis
The thermogravimetric analysis (TGA) of MMT (a) and PAA/MMT (b) are given in Fig. 2. A small weight loss before 200°C for two products means that the moisture is present in the samples. For MMT, the TGA curve has only a little decline (1.5% at 124.7°C and 1.4% at 228.1°C respectively), implying a good stability with heating. Thus, adding MMT into PAA would also increase the stability of PAA/MMT composite, just as shown in Fig. 2b, before 258.9°C the lost weight of 7.4% was found. The major lost-weight for PAA/MMT superabsorbent composite was found at 364.7°C (18.3%) and 603.5°C(26.7%), imply that the decomposition of carboxyl groups of PAA. Over 800°C, the final residue of PAA/MMT left about 29.4%. That is to say, the thermal stability of poly acrylic/montmorillonite superabsorbent was improved significantly.
Morphology Analysis
The scanning electron micrographs of PAA/MMT composites containing 1% (a), and 3% (b) of MMT are showed in Fig. 3. It can be seen that the surface PAA/MMT composite containing 1% of MMT is relatively flat and not too many porous, however, when the 3% of MMT is introduced, the surface becomes more rough and porous. The rough surface structure with many pores may be one of reasons to have large swelling capability.
Optimization of the Grafting Conditions
Effects of Discharge Voltage and Discharge Time on Water Absorbency
An excellent superabsorbent must have a reasonable three-dimensional network structure. The property of superabsorbent, generally speaking, not only depends on swelling capability, but also the gel strength. As the lower discharge voltage is used, it is not feasible to form a well-ordered three-dimensional network structure, at too high discharge voltage the swelling capability would be decreased. For this purpose, the polymerization conditions should be optimized first. Figure 4 shows the relationship between the discharge voltage used and water absorbency, results indicated that lower voltage (less than 640 V) is not benefit to polymerization completed due to the amount of free radical being not enough; over 640 V an excess of free radical could also destroy the polymer. Similarly, the discharge time should be also controlled about 10 min (see Fig. 5), less or more this threshold value the water absorbency of product would be decreased [25–27].
Effect of Monomer AA Content on Water Absorbency
The relationship between the monomer AA content and water absorbency values was studied by varying the AA content from 22 to 31% (see Fig. 6). At the beginning, the absorbency increased with increasing the AA content and the maximum water absorbency reached at 29% of AA, implying that a three-dimensional network polymer was formed gradually. Further increasing the AA amount used in polymerization, the water absorbency decreased. The reason may be that the higher monomer content could be attributed to increase the homopolymerization reaction and the chance of chain transfer to AA [30, 31], decreasing the swelling ability of polymer.
Effect of MMT Content on Water Absorbency
MMT powder as a cross-linking point plays an important role in the formation of composite superabsorbent. Its effect on water absorbency of the PAA/MMT superabsorbent composite was showed in Fig. 7. It is noticeable that the water absorbency of composite increases with increasing the MMT content <3%. This tend is attributed to the fact that the –OH on the surface of MMT could react with acrylic acid to improve the polymeric network and to enhance the water absorbency. However, >3% of MMT used in polymerization would produce more crosslink points in the polymeric network to decrease the elasticity of polymer. Additionally, the excess of MMT would also decrease the hydrophilicity as well as the osmotic pressure difference, resulting in shrinkage of the composite [32, 33].
Effect of MBA Content on Water Absorbency
The effect of crosslinking agent on water absorbency was showed in Fig. 8. It is well known that the amount of crosslinking agent determined the crosslinking density of the hydrogel network. According to the theory of Flory, at the lower amount of MBA, the absorbency of superabsorbent would be decreased due to the lower crosslinking density, and the stability of product will also reduce. Contrarily, the excess of MBA will cause the elasticity enhanced and the space of polymer three-dimensional network decreased,such results would not be beneficial to the swelling of product.
Effect of Degree of Neutralization on Water Absorbency
Because the hydrophilicity of carboxylate group (COO−) is better than carboxylic group (COOH), the degree of neutralization of acrylic acid in the composite had significant effect on the water absorbency (see Fig. 9). According to the Flory theory, at the lower degree of neutralization, the carboxylate group is protonated, the ion mass fraction of polymer network structure would decrease to cause a lower absorbency. At the higher degree of neutralization, the ion mass fraction of the network increases and the excess of Na+ ion would reduce the electrostatic repulsion by the negative charges of carboxylate groups.
Effect of Post Polymerization Temperature on Water Absorbency
Figure 10 shows the effect of post polymerization temperature on water absorbency. As we know, post polymerization temperature has a major impact on the free radical reactions, which affect water absorbency. The activity of free radical increases with increasing temperature before 80°C; in contrast, the higher of temperature will lead to polymerization rate and self-crosslinking degree increased. That is to say, raising the temperature can speed the polymerization rate, benefiting the formation of network polymer. This trendy is in agreement with the commonly chemical polymerization. However, the burst-polymerization at higher temperature could cause the chain termination and chain transfer reaction appeared, which result in the increase of polymerization rate and the decline in the absorbency of hydrogel.
Water Retention
Water retention of the swollen superabsorbent composite is determined by centrifuging technique with 4,000 rpm. Results showed that superabsorbent composite had higher water retention ability and could keep >70% of distilled water for 120 min.
Influence of the Concentration of Brine on the Swelling Capability of Hydrogel
Figure 11 shows the relationship of the swelling capability of hydrogel and the concentration of brine solution. It is well known that the swelling capability of hydrogel decreased with an increase in the concentration of brine solution. This could be attributed to the reduction in the osmotic pressure difference between the superabsorbent composite and the external saline solutions with increasing ionic strength. Moreover, the penetration of Na+ into the network results in a more evident screening effect of the anionic groups (–COO−), decreasing the salt absorbency of the hydrogel.
Effect of Temperature on the Swelling Capability of Hydrogel
The influence of temperature on the swelling behavior of hydrogels is showed in Fig. 12. The decrease of water absorbency with an increase of ambient temperature, because the water molecules have more kinetic energy and higher collision to each other, which may decrease its chance from outside going into the hydrogel network, resulting in the decline of absorbency.
Conclusion
In this study, a polyacrylic grafted montmorillonite superabsorbent has been synthesized successfully by using glow discharge electrolysis plasma. The optimum conditions are below: discharge voltage 640 V, discharge time 10 min, post polymerization temperature 80°C, degree of neutralization 70%, crosslinker content 0.6%, MMT content 3%, monomer content 29%, and post-polymerization time 3 h. The highest water absorbency of product is about 1,834 g/g for distilled water, and 116 g/g for 0.05 mol/L NaCl solution, 75 g/g for 0.15 mol/L NaCl solution, respectively. Meanwhile, owning to the proposed superabsorbent composite with excellent water retention, it can be expected to find the use in agricultural and horticultural applications, especially, in dry and desert regions.
References
Ibrahim SM, Salmawi KM-El, Zahran AH (2007) J Appl Polym Sci 104:2003–2008
Lokhande HT, Gotmare VD (1999) Bioresour Technol 68:283–286
Kikuchi A, Okano T (2002) Adv Drug Deliv Rev 54:53–77
Dorkoosh FA, Brussee J, Verhoef JC, Borchard G, Rafiee-Tehrani M, Junginger HE (2000) Polymer 41:8213–8220
Rosiak JM, Ulanski P, Pajewski LA, Yoshii F, Makuuchi K (1995) Radiat Phys chem 46:161–168
Davies LC, Novais JM, Martins-Dias S (2004) Bioresour Technol 95:259–268
Murthy PSK, Mohan MY, Varaprasad K, Sreedhar B, Raju MK (2008) J Colloid Interface Sci 318:217–224
Zhang JP, Wang AQ (2007) React Funct Polym 67:737–745
Li P, Kim NH, Siddaramaiah OKP, Lee JH (2009) Compos B 40:275–283
Bulut Y, Akcay G, Elma D, Serhatli IE (2009) J Hazard Mater 171:717–723
Tong QY, Zhang GW (2005) Carbohydr Polym 62:74–79
Wan T, Wang XQ, Yuan Y, He WQ (2006) Polym Int 55:1413–1419
Bardajee GR, Pourjavadi A, Soleyman R, Sheikh N (2008) Nucl Instr Meth Phys Res B 266:3932–3938
Sengupta SK, Sandhir U, Miara N (2001) J Polym Sci A: Polym Chem 39:1584–1588
Sengupta SK, Singh R, Srivastava AK (1998) Indian J Chem 37:558–560
Gao JZ (2006) Pak J Biol Sci 9:323–329
Tezuka M, Iwasaki M (2001) Thin Solid Films 386:204–207
Kokufuta E, Fujii S, Ishibashi H, Yokoi H, Harada K, Nakamura I (1980) Polym Bull 3:173–178
Gao JZ, Hu ZA, Wang XY, Hou JG, Lu XQ, Kang JW (2001) Thin Solid Films 390:154–158
Lu QF, Yu J, Gao JZ (2006) J Hazard Mater B 136:526–531
Harada K, Terasawa J, Suzuki S (1978) Nature 65:259
Harada K, Suzuki S (1977) Nature 64:484
Suzuki S, Tamura M, Terasawa J, Harada K (1978) Bioorg Chem 7:111–113
Harada K, Nomoto MM, Gunji H (1981) Tetrahedron Lett 22:769–772
Gao JZ, Wang AX, Li Y, Fu Y, Wu JL, Wang YD, Wang YJ (2008) React Funct Polym 68:1377–1383
Wang AX, Gao JZ, Yuan Y, Yang W (2009) Plasma Chem Plasma Process 29:387–398
Gao JZ, Wang YD, Yang W, Li Y (2010) Bull Korean Chem Soc 31:406–414
Bhattacharyya KG, Gupta SS (2007) J Colloid Interface Sci 310:411–424
Bhattacharyya KG, Gupta SS (2006) Colloids Surf A Physicochem Eng Asp 277:191–200
Pourjavadi A, Kurdtabar M, Mahdavinia GR, Hosseinzadeh H (2006) Polym Bull 57:813–824
Pourjavadi A, Bardajee GR, Soleyman R (2009) J Appl Polym Sci 112:2625–2633
Wu L, Liu MZ (2007) Ind Eng Chem Res 46:6494–6500
Zhang JP, Wang Q, Wang AQ (2007) Carbohydr Polym 68:367–374
Acknowledgments
This work was supported in part by the Key Project of Science and Technology of Education Ministry (00250), the Natural Science Foundation of Gansu Province (3ZS041-A25-028 and 096RJ2A120), the Project of KJCXGC-01, NWNU, and Gansu Key Lab of Polymer Materials, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Ma, D., Lu, Q. et al. Synthesis and Characterization of Montmorillonite-Graft-Acrylic Acid Superabsorbent by Using Glow-Discharge Electrolysis Plasma. Plasma Chem Plasma Process 30, 873–883 (2010). https://doi.org/10.1007/s11090-010-9251-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-010-9251-6