Abstract
The influence of solvent type on splat formation and coating microstructure using the Solution Precursor Plasma Spray process was studied. Droplet with a high surface tension and high boiling point solvent experiences incomplete solvent evaporation process in the plasma jet leading to a porous coating. Droplet created from a low surface tension and low boiling point solvent undergoes rapid solvent evaporation, solute precipitation, pyrolysis, melting process in the plasma jet and forms splat upon impacting on the substrate; the build-up of splats results in a dense coating.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solution Precursor Plasma Spray (SPPS) process has been successfully developed to deposit YSZ-based TBCs with excellent durability as well as to deposit dense coatings [1–7]. In the SPPS process, an aqueous solution precursor, instead of the conventional powder feedstock, is injected into the plasma jet. The solution droplets undergo a series of physical and chemical reactions prior to deposition on the substrate as a coating. It has been recognized that the SPPS process for the deposition of ceramic coatings offers several advantages over the conventional plasma-spray method, such as circumvention of powder-feedstock preparation, improved deposit chemistry control, the ability to deposit compositionally graded coatings, and the ability to deposit much finer spat structures.
It has been studied that the precursor and processing parameters have significant effect on the SPPS coating microstructure [8, 9]. Recent studies indicated that the solution precursor concentration has a significant effect on the splat formation and coating microstructure. Low concentration precursors experience surface precipitation that leads to shell formation. The deposits consist of semi-pyrolyzed material and result in a soft, porous coating. High concentration solutions are beneficial for volume precipitation within droplets. Solid particles are formed, melted and form splats on contact with the substrate. The build-up of dense splats results in a dense coating. In the suspension plasma spray, which is a process similar to the solution precursor plasma spray, Fauchais et al. [10] studied the effects of water and ethanol solvent on the zirconia splat formation and found under the same plasma and processing conditions, it takes longer time for water droplet evaporation than that of ethanol droplet evaporation and the zirconia particles contained in ethanol are melted while those in water are very poorly melted.
To explore one aspect of the solution precursor and microstructure relationship in the SPPS process, here we use TiO2 as an example to study the influence of solvent type on splat formation and coating microstructure.
Experimental Procedures
Two TiO2 solution precursors were prepared by dissolving titanium isopropoxide in triethanolamine and ethanol solvents, respectively. The physical properties of triethanolamine and ethanol solvents are shown in Table 1. The as-made two precursors have the same molar concentration of 1.5 M. The average droplets sizes under the same atomization conditions were measured by Phase Doppler Particle Analyzer (PDPA) system (reference [11] provides the detailed procedure).
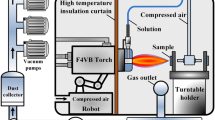
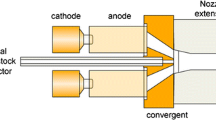
The coatings were deposited using the direct current (DC) plasma torch (Metco 9 MB, Sulzer Metco, Westbury, NY, USA), which was attached to a six-axis robotic arm. Argon and hydrogen are used as the primary and the secondary plasma gases, respectively. An atomizing nozzle attached to the plasma torch is used to inject solution precursor mist into the plasma jet. Nitrogen is used as the solution-precursor atomizing gas. The schematic of the solution precursor plasma spray process is shown in previous publications [1]. The coatings from the two different precursors were deposited using the identical processing parameters (such as standoff distance, liquid flow rate, atomizing gas pressure, plasma power, etc.) on type 304 stainless steel substrates (disks 25 mm diameter, 3 mm thickness), surfaces of which were previously roughened by grit blasting (Al2O3 grit of #30 mesh size). The detailed coating deposition parameters are listed in Table 2.
The crystalline phase composition of all the samples was determined using X-ray diffraction (Cu Kα radiation; D5005, Bruker AXS, Karlsruhe, Germany). The XRD patterns were collected in a 2θ range from 20° to 80° with a scanning rate of 2°/min. An environment scanning electron microscope (ESEM 2020, Philips Electron Optics, Eindhoven, The Netherlands) and a JEOL JSM-6335F field emission scanning electron microscope (FESEM, Tokyo Japan) were used to characterize the coating microstructure.
Experimental Results
Atomizing Droplet Size
The droplet size distributions from precursors with triethanolamine and ethanol as solvents under the same atomization conditions measured by PDPA are shown in Fig. 1a, b, respectively. It can be seen that the droplets from both the precursors have a wide size distribution and the largest droplets can reach ~ 140 μm. The average droplet sizes for precursors using triethanolamine and ethanol as solvents are ∼31 and 23 μm, respectively. The average droplet size using triethanolamine as solvent is slightly larger than that using ethanol as solvent.
Influence of Solvents on Splat Formation
To reveal the effect of solvent type on the splat formation, single scan experiments were carried out on the two solution precursors. In single scan experiments, the plasma jet is scanned rapidly (~1.0 m/s) across the substrate to deposit individual particles.
Figure 2a, b show the typical SEM microstructures of the single scan deposits on room temperature substrates from precursors with triethanolamine and ethanol as solvents, respectively. There are no splats observed using triethanolamine as solvent. Instead, it is mainly composed of cracked mud-like film, few ruptured bubbles and a small volume fraction of solid spheres (Fig. 2a). These results indicated there are a lot of unpyrolyzed precursors arriving at the substrate. The deposits from solution precursor with ethanol as solvent are mainly composed of overlapped splats and a small amount of unmelted solid spheres (<0.5 μm). The splats average diameter ranges from 0.5 to 2 μm (Fig. 2b).
Microstructure and Phase Composition of the As-sprayed Coatings
In the SPPS process, 25 coating scans were carried out for both solution precursors. Figure 3a, b show typical polished SPPS TiO2 coating cross-section microstructures from solutions with triethanolamine and ethanol as solvents, respectively. The coating from precursor with triethanolamine as solvent is discontinuous and very porous (Fig. 3a). The coating from solution precursor with ethanol as solvent is quite dense (Fig. 3b). Due to the porous microstructure, the coating sprayed using triethanolamine as solvent is thicker than that sprayed using ethanol as solvent.
The effects of solvent type on the coating surface microstructures are shown in Fig. 4. The coating microstructure from precursor using triethanolamine as solvent is totally different from that of single scan deposits. The coating is mainly composed of cauliflower-like aggregates with diameters of ~1 μm (Fig. 4a); high magnification shows the aggregates are composed of nanograins with size of ~20 nm (Fig. 4b). The microstructure of coating from precursor using ethanol as solvent is similar to that of single scan deposits, and consists of ultrafine splats (Fig. 4c).
X-ray diffraction patterns of the coatings from precursors using triethanolamine and ethanol as solvents are illustrated in Fig. 5. Both coatings are composed exclusively of the rutile phase; there is no anatase phase.
Discussion
The coating microstructure was profoundly altered from a porous structure using the triethanolamine as solvent to a dense splat structure using the ethanol as solvent. Since thermal spray deposition experiments were carried out on both the TiO2 precursors using exactly the same process parameters such as plasma power, standoff distance, liquid flow rate, and primary and secondary gas flows. The only difference is the precursor solvent type. Therefore, the solvent plays the major role for the splat formation and the resultant coating microstructure. The effect of solvent on the splat formation and coating microstructure can be attributed to two physical properties of solvent: (1) surface tension and (2) boiling point. The surface tension and boiling point will affect the droplet breakup and evaporation behaviors, respectively. The SPPS coating deposition mechanisms investigated by Xie et al. [11, 12] indicated that the droplet experiences rapid solvent evaporation, breakup, solute precipitation, pyrolysis, sintering and melting process in the plasma jet. Among them, the solvent evaporation and breakup are the initial steps for the droplets to undergo further physical and chemical changes. Experimental modeling also indicated that larger droplets will result in poor pyrolysis and porous coating microstructure [13]. As shown in Table 1, the surface tension of triethanolamine (45.95 mN/m) is about two times larger than that of ethanol (22.39 mN/m). Therefore, it is expected the average droplet size using high surface tension triethanolamine as solvent will be larger than that using ethanol as solvent, which is confirmed by the PDPA measurements (Fig. 1a, b). Moreover, when the droplets are injected into the plasma jet, secondary droplet breakup is induced [8]; the droplet breakup in the plasma jet using high surface tension triethanolamine as solvent will be more difficult than that using ethanol as solvent. As a result, there will be more large droplets in the plasma jet using triethanolamine as solvent than that using ethanol as solvent. The larger droplet will result in poor pyrolysis and lead to porous coatings (Figs. 2a, 3a). Another physical property which has a significant effect on the splat formation and coating microstructure is the solvent boiling point. The boiling point for triethanolamine and ethanol is 335 and 78°C, respectively. The heat of vaporization for triethanolamine and ethanol is 67.7 and 38.6 kJ/mol, respectively. The droplet with high boiling point triethanolamine as solvent will be much more difficult to completely evaporate for a very short residence time in the plasma jet; the droplet containing significant amount of triethanolamine solvent will spread to form the film upon impacting on the substrate (Fig. 2a). In contrast, since the ethanol has a lower boiling point and requires less vaporization heat, the droplet with ethanol as solvent will be much easier to evaporate, thus followed by solute precipitation, pyrolysis, melting and splat formation (Fig. 2b).
It is noted that the coating surface microstructures using triethanolamine as solvent are different from the single scan deposits morphologies. The smooth film and semi-pyrolyzed hollow shell structures disappear in the as-sprayed coatings. The coating is mainly composed of cauliflower-like aggregates with nanosized grains (Fig. 4a, b). Because the high temperature plasma jet will repeatedly scan over the substrate during the plasma spray process, it has a strong heat treatment effect on the coating surface, the film-like deposits which contain significant triethanolamine solvent and the semi-pyrolyzed hollow-shell structures will in situ evaporate, pyrolyze on the substrate. As a result, cauliflower-like aggregates and porous coating are formed.
Though the coatings from precursors with triethanolamine and ethanol solvents have totally different microstructures, they have the same phase composition. To further reveal the phase evolution of the SPPS coatings from the two precursors, one pass coating deposition were carried out. Figure 6a, b shows the X-ray diffraction patterns of the one pass coatings from precursors using triethanolamine and ethanol as solvents, respectively. Both coatings are composed of anatase and rutile mixture phases, which are different from that of the 25 passes sprayed coatings. The results indicated that anatase to rutile phase transformation occurred during coating deposition. As mentioned above, the high temperature plasma jet will repeatedly scan over the coating surface during the plasma spray process; the coating surface temperature during deposition can reach over 700°C [5, 11], which is higher than anatase to rutile phase transformation temperature. Therefore, both coatings are composed exclusively of rutile phases.
The results indicate that to make dense coatings using the SPPS process, low surface tension and low boiling point solvent is desirable. This is because easy droplet breakup and solvent evaporation leads to full pyrolysis, melting and dense coating formation; while large droplet and difficult evaporation leads to incomplete evaporation, pyrolysis and the formation of a porous friable coating.
Conclusions
The influence of solvent type on splat formation and coating microstructure was studied. Solvent type has a significant effect on splat formation and coating microstructure. Droplet with a high surface tension and high boiling point solvent experiences incomplete solvent evaporation in the plasma jet and forms mud-like cracked film upon impacting the substrate. Under the repeated scan of the high temperature plasma jet, the mud-like film in situ evaporates, pyrolyzes and crystallizes on the substrate and results in a porous coating. Droplet created from a low surface tension and low boiling point solvent undergoes rapid solvent evaporation, solute precipitation, pyrolysis, melting process in the plasma jet leading to a fully melted splat microstructure and a high density coating.
References
Chen D, Jordan EH, Gell M, Ma X (2008) Dense alumina-zirconia coatings using the solution precursor plasma spray process. J Am Ceram Soc 91(2):359–365
Chen DY, Jordan EH, Gell M, Ma XQ (2008) Dense TiO2 coating using the solution precursor plasma spray process. J Am Ceram Soc 91(3):865–872
Gell M, Xie LD, Ma XQ, Jordan EH, Padture NP (2004) Highly durable thermal barrier coatings made by the solution precursor plasma spray process. Surf Coat Technol 177:97–102
Jordan EH, Xie L, Ma X, Gell M, Padture NP, Cetegen B, Ozturk A, Roth J, Xiao TD, Bryant PEC (2004) Superior thermal barrier coatings using solution precursor plasma spray. J Therm Spray Tech 13(1):57–65
Xie LD, Chen DY, Jordan EH, Ozturk A, Wu F, Ma XQ, Cetegen BM, Gell M (2006) Formation of vertical cracks in solution-precursor plasma-sprayed thermal barrier coatings. Surf Coat Technol 201(3–4):1058–1064
Chen DY, Gell M, Jordan EH, Cao E, Ma XQ (2007) Thermal stability of air plasma spray and solution precursor plasma spray thermal barrier coatings. J Am Ceram Soc 90(10):3160–3166
Chen D, Jordan EH, Gell M, Wei M (2008) Apatite formation on alkaline-treated dense TiO2 coatings deposited using the solution precursor plasma spray process. Acta Biomater 4(3):553–559
Chen D, Jordan EH, Gell M (2008) Effect of solution concentration on splat formation and coating microstructure using the solution precursor plasma spray process. Surf Coat Technol 202(10):2132–2138
Xie LD, Ma XQ, Ozturk A, Jordan EH, Padture NP, Cetegen BM, Xiao DT, Gell M (2004) Processing parameter effects on solution precursor plasma spray process spray patterns. Surf Coat Technol 183(1):51–61
Fauchais P, Etchart-Salas R, Rat V, Coudert JF, Caron N, Wittmann-Ténèze K (2008) Parameters Controlling Liquid Plasma Spraying: Solutions, Sols, or Suspensions. J Therm Spray Tech 17(1):31–59
Bhatia T, Ozturk A, Xie LD, Jordan EH, Cetegen BM, Gell M, Ma XQ, Padture NP (2002) Mechanisms of ceramic coating deposition in solution-precursor plasma spray. J Mater Res 17(9):2363–2372
Xie LD, Ma XQ, Jordan EH, Padture NP, Xiao DT, Gell M (2004) Deposition mechanisms of thermal barrier coatings in the solution precursor plasma spray process. Surf Coat Technol 177:103–107
Basu S, Cetegen BM (2007) Modeling of thermo-physical processes in liquid ceramic precursor droplets injected into a plasma jet. Int J Heat Mass Transf 50(17–18):3278–3290
Acknowledgments
This work is supported by the U.S. Office of Naval Research under Grant No. N00014-02-1-0171, managed by Dr. Lawrence Kabacoff.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, D., Jordan, E.H. & Gell, M. The Solution Precursor Plasma Spray Coatings: Influence of Solvent Type. Plasma Chem Plasma Process 30, 111–119 (2010). https://doi.org/10.1007/s11090-009-9200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-009-9200-4