Abstract
Liquid-phase degradation of chlorobenzene (CB), induced by contact glow discharge electrolysis under various reaction conditions, such as, the initial solution pH, current intensity, volume of solution and iron salts was investigated. Experimental results indicated that, in the absence of catalysts, the depletion of CB followed first-order kinetics, where the observed value of the first-order rate constant ‘k’ is directly proportional to the applied current intensity and inversely proportional to the solution volume. Initial solution pH had little effect on the value of k. HPLC and IC analyses showed that the major intermediate products were chlorophenols, phenol, organic acids and chloride ions. During the treatment, a lot of hydrogen peroxide was formed. Role of Fenton’s reaction was examined. A reaction pathway is proposed based on the degradation kinetics and the distribution of intermediate products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, plasma based technologies have been extensively investigated as novel kind of advanced oxidation processes for removal of organic pollutants from water because of their high removal efficiency and environmental compatibility [1–3]. Contact glow discharge electrolysis (CGDE) is a unique plasma process where the plasma is sustained between a pointed electrode and an aqueous solution in contact with it [4, 5]. When an anodic CGDE occurs, the positive species in the plasma are accelerated by the cathode-fall and enter the solution, leading to the formation of hydroxyl radicals and hydrogen peroxide with yields much higher than those expected on the basis of Faraday’s law [4, 5]. Some researchers [6–13] have utilized CGDE for decomposing aqueous organic pollutants. Experimental results indicate that many organic pollutants can be destroyed by CGDE and the final products are either non-toxic or less toxic compounds, such as, carbon dioxide, water, inorganic salts, etc. These findings suggest that CGDE is a useful tool for the treatment of wastewater contaminated with organic compounds.
Chlorinated aromatics are very persistent in the environment and EPA lists many of them as priority pollutants. Especially decontamination of chlorobenzene(CB) is most desirable as it pollutes the water during production of phenol, dinitrochlorobenzene, dynamite, intermediate products for dyes and medicine, etc. Therefore, CB is chosen as a model pollutant in the present investigation. The study is also focused on the reaction mechanism of the CB in the plasma environment and kinetics.
Experimental
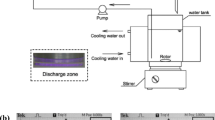
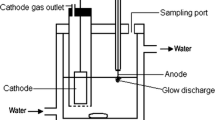
The experimental apparatus consisted of a DC high-voltage power supply (variable voltage 0–600 V and current of 0–600 mA) and a reactor as shown in Fig. 1. The anode, from which the discharge emits, was a pointed platinum wire (id = 0.6 mm) sealed into a glass tube and the cathode was a stainless steal plate placed in another glass tube and separated from the anodic compartment by a glass frit of medium porosity. The solution in a jacked reaction vessel was maintained at 298 ± 2 K by circulating water. The solution for treatment was prepared by dissolving a known amount of CB in a sodium sulfate solution (conductivity: 6.4 mS/cm). A desired portion of the solution was poured into the reaction vessel for treatment. DC voltage of 500 V was applied across the electrodes through a high-voltage power source to start the reaction. The depth of the anode immersed into solution was carefully adjusted to adjust the current to the desired value. The current deviation was less than ±4%. The current is proportional to the depth of the anode immersed (about 100 mA per 1.2 mm).
The solution was gently stirred with a magnetic stirrer. A small portion of the solution was periodically withdrawn from the reactor for HPLC (Angilent 1100) analysis to determine the CB concentration and its degraded products. A Spherisorb C18 reversed-phase column was employed in HPLC and H2O:CH3CN:H3PO4 = 50:50:0.2 was employed as a mobile phase at a flow rate of 0.8 ml/min. The organic acids and chloride ions were determined by ion-chromatography(IC, Dionex Dx-500) equipped with an Ion Pac AS-14 column. An aqueous solution of Na2CO3 was used as the mobile phase at a flow rate of 0.25 ml/min. Hydrogen peroxide in the treated solution was determined by colorimetric method [14]. All the measurements were repeated thrice and averaged readings were recorded.
Results and Discussion
CB Degradation
Figure 2a shows the variation of CB concentration during the discharge treatment. The CGDE gradually decomposed the CB, leaving less than 50% of CB after 40 min., less than 95% in 120 min. and below detection limits of the HPLC after 240 min.
The degradation curve in Fig. 2a seems to be exponential. Therefore, an exponential equation (Eq. 1), representing first-order reaction kinetics, was fitted to the data as shown in Fig. 1b.
where C 0 is initial concentration of CB, Ct is the concentration of CB at time t, k is apparent first order rate constant and t is the reaction time.
Figure 2b shows a linear relationship between \( \ln {(C_{0} } \mathord{\left/ {\vphantom {{(C_{0} } {Ct)}}} \right. \kern-\nulldelimiterspace} {Ct)} \) and t, with R 2 value close to 1, proving that the degradation of CB follows a first-order kinetics. The experimental results of this study show that under the whole range of experimental conditions (namely pH, 3–9, C 0, 50–400 mg/l, current 0–400 mA and solution volume 150–1,000 ml) the linear relationship between \( \ln ({C_{0} } \mathord{\left/ {\vphantom {{C_{0} } {Ct}}} \right. \kern-\nulldelimiterspace} {Ct}) \) and t persists. However, the value of k is dependent on the reaction conditions. The dependences of k on the reaction conditions are discussed in the following sections.
Effect of Current on the First-order Rate Constant of CB Removal
The k is the slope of the linear curve between \( \ln ({C_{0} } \mathord{\left/ {\vphantom {{C_{0} } {Ct}}} \right. \kern-\nulldelimiterspace} {Ct}) \) and t. Figure 3 shows the dependences of k on current. It can be seen in Fig. 3 that k linearly increases with increase in current. The current was increased by increasing the depth of the anode resulting in increase in the plasma zone in contact with the solution. The increase in the plasma zone in contact with the solution explains the increase in the rate of CB degradation.
Effect of Initial pH on the First-order Rate Constant of CB Removal
The pH is an important factor in water treatment. The effect of pH on the first-order rate constant for CB degradation is shown in Fig. 4. It can be seen from Fig. 4 that the pH had little effect on the value of the rate constant. Our results do not agree with the results reported in [12] where degradation of auramine was studied under CGDE. The difference of our results with the results reported in [12] can be explained on the basis of difference in the chemical structure of CB and auramine. Although the oxidation potential of hydroxyl radical decreases with the increase in the solution pH, it is still high enough to rapidly oxidize CB, while in the case of auramine it may not be so. Therefore, the removal rate of CB is not influenced by pH. In practice, the pH of wastewater often varies in a large range. The present results show that the water contaminated with CB does not need pH adjustment before being subject to CGDE treatment.
Effect of the Solution Volume on the First-order Rate Constant of CB Removal
In general, a decrease in the rate of reaction is expected with increase in solution volume. Therefore the rate constant k was plotted against \( 1 \mathord{\left/ {\vphantom {1 V}} \right. \kern-\nulldelimiterspace} V \) and linear curve was fitted to the data as shown in Fig. 5. A straight line curve between k and \( 1 \mathord{\left/ {\vphantom {1 V}} \right. \kern-\nulldelimiterspace} V \) fitted almost perfectly to the data with R 2 value close to one. This result proves that the rate of degradation of CB is inversely proportional to the solution volume. The results can be explained on the basis decrease in the ratio of plasma surface in contact with the solution to the bulk volume of the solution. In other words the results demonstrated that the degradation takes place mainly at the surface of plasma exposed to the solution and the reaction in the bulk solution was negligible.
On the basis of the above discussions, Eq. 1 describing the removal of CB by CGDE in the absence of any catalyst can be modified as:
where a is a constant, I the current intensity (A) and V the volume of liquid (l). When the values of k, I, V in Figs. 2–5 were substituted in Eq. 2, the average value of a was found to be 2.93 × 10−2 l/A/min.
Hydrogen Peroxide Formation and the Effect of Iron Salts on CB Removal
The hydrogen peroxide formation by CGDE in the absence of organic pollutants has been extensively studied [4, 5]. However, the hydrogen peroxide formation behavior in the presence of organic pollutants has been rarely reported. The hydrogen peroxide formation under different initial CB concentration is shown in Fig. 6. The concentration of hydrogen peroxide increased with increase in treatment time but the rate of increase of the hydrogen peroxide concentration was lower when the CB concentration was higher in the solution.
Since a lot of hydrogen peroxide is formed in the presence of CB, it is desirable to add iron salts to enhance the degradation rate of CB through Fenton’s reactions (Eqs. 3 and 4).
Effect of addition of ferric as well as ferrous ions was studied. Changes in the concentration of CB after 6 min of CGDE treatment in the presence of iron salts is shown in Fig. 7.
Figure 7 shows that both Fe2+ and Fe3+ had remarkable catalytic effects on CB removal. Interestingly, the catalytic effect of Fe3+ is better than that of Fe2+, which is contradictory to the principles of the Fenton reaction. The rate constant of ferric ion with hydrogen peroxide (0.01/M/s) is almost four orders of magnitude smaller than that of ferrous ion with hydrogen peroxide (76/M/s).
The better catalytic effect of Fe3+ in the present study can be explained by the conversion of Fe3+ to Fe2+ by the following reaction with by-products of CB, i.e., hydroxychlorocyclohexadienyl radicals [15–17]:


Then the produced Fe2+ reacts with hydrogen peroxide in the solution to produce more hydroxyl radicals (Eq. 3). It can also be observed from Fig. 7 that the optimum concentration of ferric ions (about 20 mg/l) is much lower than that of ferrous ions (about 30 mg/l) for CB removal, which is very important in practice; higher concentration of ferrous ions lead to the decrease in CB removal rate due to the following reaction (Eq. 7):
It is noted that the role of Fenton’s reaction in pulsed corona reactor have been investigated [18]. The novelty of present investigation is that the catalytic effect of ferric ions is better than that of ferrous ion, which was not reported in pulsed corona reactor.
Byproducts Formation and Proposed Mechanism of CB Degradation
HPLC analysis was performed in order to explore the possible degradation mechanism. Three isomeric chlorophenols were identified during the discharge treatment, and the changes in their concentration with increase in treatment time are shown in Fig. 8. The chlorophenol intermediates were found to be in the follows the order: p-chlorophenol > o-chlorophenol > m-chlorophenol. These results can be explained on the basis of ortho- and para- directing nature of chlorine substituent, which is because of the donation of lone pair of electrons on chlorine to the ortho- and para- positions of the benzene ring by conjugation. The hydroxyl radicals attaching the benzene ring are electrophilic in nature and prefer to react at ortho- and para position. These intermediates increased to the maximum at about 40 minutes and decreased gradually with longer discharge time. Phenol was also detected, but its concentration was too low to be analyzed quantitatively.
Chloride ions, formic acid, oxalic acid and acetic acid were identified as the main intermediates and the variation of their concentration during the CGDE of CB are shown in Fig. 9. It can be observed from Fig. 9 that nearly 90% of the chlorine atoms can be eliminated from CB when the discharge time was prolonged. The distribution of organic acids follows the following order: oxalic acid > acetic acid > formic acid. Other organic acids such as malonic acid, malic acid, etc. were not observed by IC. The acid intermediates eventually disappeared with longer discharge time.
Based on the distribution of intermediates, the degradation of CB by means of CGDE can be described by the mechanism illustrated in Fig. 10.
It can be seen from Fig. 10 that the CB degradation in CGDE can be divided into many steps. The first step is the hydroxylation of benzene ring, where chlorophenols, phenol and chloride ions were produced. Further hydroxylation opens the benzene ring resulting in the production of various unstable intermediates. These short-lived intermediates would be rapidly oxidized by hydroxyl radicals or hydrogen peroxide to produce the organic acids and chloride ions. The organic acids are relatively stable and can be analyzed by IC. The final step is the oxidation of these organic acids to carbon dioxide and water.
Conclusions
The following conclusions can be drawn from the present study:
-
1.
CB can be efficiently decomposed by CGDE.
-
2.
The degradation of CB is first-order, where the rate constant is proportional to the applied current intensity and inversely proportional to the solution volume. However the pH showed no evident influence on the rate constant.
-
3.
The major intermediates of CB decomposition were three isomeric chlorophenols, phenol, carboxylic acids and chloride ions.
-
4.
During the degradation of CB, a lot of hydrogen peroxide was formed. Both the ferrous and ferric ions showed remarkable catalytic effect on CB removal. The catalytic effect of ferric ions was better than the ferrous ions.
References
Clements JS, Sato M, Davis RH (1987) IEEE Trans Ind Appl IA–23:224–235
Sharma AK, Locke BR, Arce P, Finney WC (1993) Hazard Waste Hazard Mater 41:209–219
Malik MA, Ghaffar A, Malik SA (2001) Plasma Sources Sci Technol 10:82–91
Hickling A (1971) In: Bockris JO’M, Conway BE (eds) Modern aspects of electrochemistry, vol 6. Butterworths, London, p 329
Susanta KS, Rajeshwar S, Ashok KS (1998) J Electrochem Soc 145(7):2209–2213
Tezuka M, Iwasaki M (1997) Denki Kagaku 65:1057–1058
Gao J, Liu Y, Yang W, Pu L, Yu J, Lu Q (2003) Plasma Sources Sci Technol 12:533–538
Tezuka M, Iwasaki M (1998) Thin Solid Films 316:123–126
Tezuka M, Iwasaki M (2001) Thin Solid Films 386:204–207
Gao J, Wang X, Hu Z, Deng H, Hou J, Lu X, Kang J (2003) Water Res 37:267–272
Tomizuwa S. Tezuka M (2006) Plasma Chem Plasma Proces 26:43–52
Gai K, Dong Y (2005) Plasma Sources Sci Technol 14:589–593
Liu Y, Jiang X (2005) Environ Sci Technol 39:8512–8517
Eisenberg GM (1943) I&EC Anal Ed 15:327–328
Eberhardt MK (1975) J Phys Chem 79(18):1913–1916
Wang L, Jiang X, Liu Y (2007) Plasma Chem Plasma Proces 27:504–515
Liu Y, Jiang X, Wang L (2007) Plasma Chem Plasma Proces 27:496–503
Grymonpre DR, Sharma AK, Finney WC, Locke BR (2001) Chem Eng J 82:189–207
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Jiang, X. Plasma-induced Degradation of Chlorobenzene in Aqueous Solution. Plasma Chem Plasma Process 28, 15–24 (2008). https://doi.org/10.1007/s11090-007-9110-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-007-9110-2