Abstract
Decomposition of 1,4-dichlorobenzene (1,4-DCB) in different gas mixtures [e.g. air, N2, 1.027%NO/N2 (N2 as balance gas)] in an electron beam generated plasma reactor was investigated to gain insight into the base gases influence on 1,4-DCB decomposition. Decomposition efficiency of 1,4-DCB increased with the absorbed dose increasing, the order of 1,4-DCB to be easily decomposed in different gas mixtures was: \(\hbox{N}_{2}\,>\,\hbox{air}\,> 1.027\%\hbox{NO}/\hbox{N}_{2}\), the reason for this was discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chlorinated organic compounds, which are emitted from fossil–fuel power stations, waste incinerators, chemical factories, etc. are very harmful to the environment and human health. Recent studies reported that chlorinated benzenes or phenols are suspected as precursors for dioxins emission. In Poland, dioxins emission from some medical waste incinerators was very high, up to \(983.67\,\upmu\hbox{g}\)-TEQ/ton [1] [Total toxic equivalency (TEQ), it is a term which describes all the products summed to give a single 2,3,7,8-TCDD equivalent]. Dioxins are relatively chemical inert and demonstrate long life time in environment, therefore they are called persistent organic pollutants (POPs). They are transported by air and are deposited on the surfaces of the soil, water and plants. They can accumulate in the fat of food-producing animals and are transferred into meat, eggs, and diary products. Dioxins has been detected in the milk of breastfeeding mothers [2].
Chlorinated volatile organic compounds (Cl-VOCs) destruction from gas phase, contaminated soil and groundwater has been intensively studied in the recent years. Different technologies have been applied [3, 4]. Electron beam (EB) process is a promising technology for removal of low concentration Cl-VOC contained in gas mixture. When fast electrons from electron beam produced by an accelerator are absorbed in a carrier gas which contains Cl-VOCs, these fast electrons cause ionization and excitation processes of nitrogen, oxygen, H2O etc. molecules in the carrier gas and generate plasma. Primary species (such as excited-state molecules, neutral radicals and ion pairs formed by direct dissociation) and secondary electrons are formed. The secondary electrons are thermalized fast within 1 ns in air at 1 atmospheric pressure. These primary species and thermalized secondary electrons cause Cl-VOCs decomposition. Chlorinated methane and ethylene have been extensively studied using this technology [5–8] and promising results were achieved.
Dioxin’s treatment using EB technology was reported [9, 10], over 90% dioxins was removed from the waste off gases.
The dechlorination of chlorobenzene from solution and gas phase has been studied. Xu et al. [3] studied 1,4-dichlorobenzene (1,4-DCB) dechlorination over Pd/Fe catalysts solution, they found that p-DCB was reduced to chlorobenzene as an intermediate product and to benzene as a final product. Hirota et al. [11] studied dechlorination of monochlorobenzene in an air mixture, aliphatic acids and aerosols were identified as dechlorination products.
Study on 1,4-DCB decomposition in gas phase was not reported by other authors. In our previous work [12], we examined removal efficiency of 1,4-DCB in air mixture using EB-irradiation and promising results were obtained.
Since the Stockholm Convention on POPs entered into force on 17 May 2004, dioxins emission from off-gases were monitored and their concentration should be lower than 0.1 ng-TEQ/m3. In this work, we studied destruction of 1,4-DCB in various conditions in the aim of obtain better knowledge to diminish dioxins formation in the waste incineration process using this technology.
Experimental
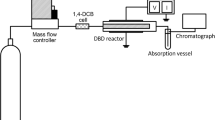
Set-up for preparation of the model gas containing 1,4-DCB
Figure 1 illustrates set-up for preparation of model gas containing 1,4-dichlorobenzene.
1,4-dichlorobenzene model gas was prepared by bubbling base gas into solid particles bed of 1,4-DCB (1,4-DCB, Purity >99.0%, from Sigma-Aldrich company, Germany). Applied bases gas were prepared as follows: synthesized air (≥99.995% purity; 21% O2, N2 as balance gas;\(\hbox{CO}_{2}\leq 1\,\hbox{ppm}\)); N2 (99.995% purity) and 1.027%NO/N2 (N2 as balance gas). They were delivered by Praxiar company, Poland. The concentration of 1,4-DCB in the model gas was adjusted by controlling of the base gas flow rate and of the same dilution gas flow by means of valve and rotameters. The model gas of 1,4-DCB was introduced into four connected Pyrex glass vessels by parallel connection. When concentration in glass reactors became constant, the glass vessels were sealed with the stopcocks. The water concentrations in the model gas mixture were measured by a HM141 humidity and temperature indicator (Vaisala company, Finland). Water concentration in air and N2 mixture was 170, 30 ppm, respectively. Experiments were carried out at ambient temperature condition and 1 bar atmospheric pressure.
An accelerator applied for generating plasma reactor
A pulsed electron beam accelerator ILU-6 (2.0 MeV max, 20 kW max) was used to generate plasma. Irradiation set up was illustrated in Fig. 2. Pyrex glass vessels were put under scan horn of the accelerator for irradiation. Irradiation conditions were described as follows: 2 Hz (pulse repetition rate), 2 MeV (energy) and 54 mA (pulse current). The absorbed dose rate inside glass vessel was 10.835 kGy/min (1 kGy = 1 kJ/kg) measured by N2O gas dosimeter. Total absorbed dose was adjusted by changing irradiation time of the Pyrex glass vessels.
Analytical methods
Concentrations of 1,4-DCB before and after irradiation were measured by a gas-chromatography (Perkin Elmer 8700) equipped with a flame ionizing detector (GC-FID). A capillary column (\(\hbox{SPB-5},\,30\,\hbox{m}\,\times\,0.32\,\hbox{mm}\,\times\,0.25\,\upmu\hbox{m}\), Supelco Company, USA) was used. Stock solutions of 1,4-DCB (\(200\,\upmu\hbox{g}/\hbox{ml}\) in methanol) were used (Supelco company, USA). Analytical conditions of 1,4-DCB were given as follows: column temperature: 60–150°C at 4°C/min; flow rate: 18 psig He; Injector temperature: 200°C, Detector temperature: 340°C; sample size: 0.3–1.0 ml relative error of the data measured in the experiment was below 5%.
A GC-MS (Hewlett Packard-5972 GC-MSD System, USA) with a HP-1 column (\(30\,\hbox{m}\,\times\,0.25\,\hbox{mm}\,\times\,0.25\,\upmu\hbox{m}\), HP Company, USA) was used to identify byproducts. The organic compounds contained in the gas samples (before and after irradiation) were adsorbed by coconut charcoal adsorbents and XAD-2 adsorbents and were desorbed by 1 ml ethyl acetate as extractant from these adsorbents. Analytical condition: column temperature was 70–300°C, at 5°C/min; \(1\,\upmu\hbox{l}\) solution was injected into the GC-MS.
Dose dependence of removal efficiency/decomposition efficiency (R)
In electron beam process, it is very important to consider energy consumption for degradation of pollutants, how much energy (unit: kJ) is consumed/absorbed to decompose amount of pollutants in the base gas (unit: kg). Energy absorbed by per amount of gas is defined as a term of dose, unit is kGy. \(1\,\hbox{kGy}\,=\,1\,\hbox{kJ}\,\cdot\,\hbox{kg}^{-1}\).
Removal efficiency or decomposition efficiency (R) of organic pollutants is defined as:
where: C 0: initial concentration of organic pollutants, unit: ppm (v/v), C i : concentration of organic pollutants at i kGy absorbed dose, unit: ppm (v/v)
Results and discussion
1,4-DCB decomposition in air mixture versus dose under EB-irradiation
Decomposition of 1,4-DCB at different initial concentration versus dose in air mixture was studied, water vapor concentration in air mixture was 170 ppm. The results are presented in Fig. 3. It can be noticed that concentration of 1,4-DCB is decreased with the absorbed dose increasing for the initial concentration of 1,4-DCB being 32.0 ppm ∼90.0 ppm, over 57.7% 1,4-DCB is decomposed at 57.9 kGy for the initial concentration of 1,4-DCB being 90.0 ppm.
1,4-DCB decomposition in N2 versus dose under EB-irradiation
Different base gas mixtures influence on the organic pollutants decomposition was reported by some authors. Won et al. [13] studied trichloroethelene (TCE) decomposition in different gas mixtures and found that the order of decomposition efficiency of TCE in different base gases was:\(\hbox{O}_{2}>\,\hbox{air}\,>\,\hbox{H}_{2}>\,\hbox{He}\). Decomposition efficiency of TCE in O2 is higher than that in air. It can be explained by chemical mechanism of TCE decomposition. Similar to 1,1-dichloroethylene decomposition [7], TCE decomposition is mainly caused by Cl− dissociated secondary electron attachment, followed by peroxyl radical reaction pathway. Kim [14] studied toluene decomposition in different gas mixtures and found that the order of decomposition efficiency of toluene in different base gases was:\(\hbox{N}_{2}>\,\hbox{air}\,>\,\hbox{O}_{2}>\,\hbox{He}\).
In order to obtain the better removal efficiency of 1,4-DCB, its decomposition in N2 was examined and the results are illustrates in Fig. 4. Similar to 1,4-DCB decomposition in air mixture, removal efficiency of 1,4-DCB in N2 increased with the absorbed dose increasing. About 80% 1,4-DCB was removed at initial concentration of 1,4-DCB in N2 being 50.9–101.4 ppm.
1,4-DCB decomposition in 1.027%\(\hbox{NO}{-}\hbox{N}_{2}\) (N2 as balance gas) versus dose under EB-irradiation
In the waste incineration process, NO is formed. NO influence on the removal efficiency of 1,4-DCB is not clear. In this work, we studied 1,4-DCB decomposition in a 1.027%\(\hbox{NO}{-}\hbox{N}_{2}\) (N2 as balance gas) gas mixture, the results are illustrated in Fig. 5. Compared with 1,4-DCB decomposition in air or N2 mixture, removal efficiency of 1,4-DCB in 1.027%\(\hbox{NO}{-}\hbox{N}_{2}\) is much lower (Fig. 6).
Organic compounds in 1,4-DCB/1.027%NO/N2 system before and after irradiation were identified by GC/MS. The total gas chromatograms are shown in Fig. 7a, b, respectively. Aside from 1,4-DCB, 1,3-diisocyanato-2-methyl-benzene (\(\hbox{C}_{9}\hbox{H}_{6}\hbox{N}_{2}\hbox{O}_{2}\),

) and 2,4-diisocyanate-toluene (\(\hbox{C}_{9}\hbox{H}_{6}\hbox{N}_{2}\hbox{O}_{2}\),

) were found at retention time (RT) of 17.039 and 17.168 min respectively in 1,4-DCB/1.027%NO/N2 system before irradiation. Mass spectrums for these two compounds were given in Fig. 8a, b, respectively. They are isomers and very toxic. It is not clear how these two compounds were formed.
Two compounds (A and B) were found as products for 1,4-DCB/1.027%NO/N2 irradiation (Fig. 9a, b) at retention time of 2.607 and 11.620 min. Possible candidate structure for compound A is\((\hbox{C}_{6}\hbox{H}_{4}\hbox{ClO})_{2}\). Because lacking of actual standards for compounds A and B, it is very difficult to predict their chemical structures.
Mechanism
As mentioned in introduction part, when fast electrons are absorbed in carrier gas, they cause excitation and ionization of base gas, secondary electrons, positive ions, excited species are formed. These species can cause 1,4-DCB decomposition.
1,4-DCB decomposition in air mixture
Positive charge transfer with/without dissociation reactions
When carrier gas air is ionized under electron beam,\(\hbox{N}_{2}^{+},\,\hbox{O}_{2}^{+}\), etc are formed. They react with 1,4-DCB (ionization energy: 8.92 eV) by charge transfer reaction with/without dissociation reactions. The relevant reactions are given as follows:
Marotta et al. [15] studied positive atmospheric pressure chemical ionization (APCI) spectrum of chlorobenzene, they observed that \(\hbox{C}_{6}\hbox{H}_{5}\hbox{Cl}^{+},\,\hbox{C}_{6}\hbox{H}_{5}^{+}\,\hbox{and}\,\hbox{C}_{4}\hbox{H}_{3}^{+}\) are formed. The possible reactions are written as follows:
Analogy to this, author propose following such reactions besides reactions listed above:
Nomura et al. [16] studied difluorobenzene ionization by using Ar +8 , they also found that fragment ions, such as \(\hbox{C}_{2}\hbox{Hx}^{+},\,\hbox{C}_{3}\hbox{Hx}^{+},\,\hbox{C}_{5}\hbox{Hx}^{+},\,\hbox{C}_{5}\hbox{HxF}^{+}\), are formed.
Negative ions transfer reactions
Nakagawa [17] stated that chlorinated phenols react with electrons and Cl− is formed. Marotta et al. [15] studied negative spectrum of chlorobenzene and found that Cl− is formed. Besides this and the complexes of M with the background ions, the oxygenated species [M–Cl+O]−(m/z 93), [M–Cl+O2]−(m/z 109) [M–H+O]−(m/z 127) were detected.
Based on Nakagawa [17] and Marotta et al.’s work [15], 1,4-DCB reactions with thermalized electrons and negative ions could be written as:
Marotta et al. [15] also observed that H is released from chlorobenzene, for 1,4-DCB, because the C–Cl dissociation energy is smaller than C–H dissociation energy, so Cl− is released.
Negative ions, such as \(\hbox{O}_{2}^{-}(\hbox{C}_{6}\hbox{H}_{4}\hbox{Cl}_{2})\,\hbox{and}\,\hbox{O}_{3}^{-}(\hbox{C}_{6}\hbox{H}_{4}\hbox{Cl}_{2})\), would go through neutralization reactions with positive ions, such as \(\hbox{O}_{2}^{+},\,\hbox{O}_{3}^{+},\,\hbox{C}_{6}\hbox{H}_{4}\hbox{Cl}_{2}^{+}\) et al.:
Other decomposition pathway
Besides positive and negative charge transfer reactions, OH radical reaction with 1,4-DCB cause 1,4-DCB degradation.
[18]
1,4-DCB/N2 mixture decomposition under EB-irradiation
Decomposition efficiency of 1,4-DCB degradation in N2 is higher than that in air mixture (Fig. 6). The similar phenomenon was observed by Kim [14] during toluene decomposition under EB-irradiation.
1,4-DCB decomposition efficiency in N2 mixture is higher than that in air mixture, it is perhaps caused by the reactions of \(\hbox{O}_{2}^{-}(\hbox{C}_{6}\hbox{H}_{4}\hbox{Cl}_{2})\), which is formed by the reaction of 1,4-DCB with O −2 (see R13), and positive ions O +2 to release 1,4-DCB (as R19 shown). The relative reaction is shown below:
In pure nitrogen base gas, no reactions mentioned above happened. Ionization energy of nitrogen is higher than that of oxygen, N +2 can cause 1,4-DCB dissociation reactions besides positive charge transfer reaction, which accelerate 1,4-DCB decomposition.
1,4-DCB/(1.027% \(\hbox{NO}{-}\hbox{N}_{2}\)) mixture decomposition under EB-irradiation
Efficiency of degradation of 1,4-DCB/(1.027% \(\hbox{NO}{-}\hbox{N}_{2}\)) mixture under EB-irradiation is shown in Fig. 5. It is found that degradation efficiency of 1,4-DCB in 1.027% \(\hbox{NO}{-}\hbox{N}_{2}\) (N2 as balance gas) mixture is much smaller than that in air or nitrogen mixture (Fig. 6).
The possible explanation for this is that: some positive ions \(\hbox{N}_{2}^{+},\,\hbox{O}_{2}^{+}\) would transfer charge to NO to form \(\hbox{NO}^{+},\,\hbox{NO}^{+}\) react with 1,4-DCB to form ions cluster, this ion-cluster has backward reaction to hinder 1,4-DCB decomposition. The relevant reactions can be written as follows:
In 1,4-DCB/air mixture, NO concentration is very low, reactions (R23, R24) can be ignored.
By GC-MS analysis, a speculated compound \((\hbox{C}_{6}\hbox{H}_{4}\hbox{ClO})_{2}\) is formed. It might be formed by following reactions:
\(\hbox{C}_{6}\hbox{H}_{4}\hbox{Cl}(\hbox{OH})\) is formed though OH radical with \(\hbox{C}_{6}\hbox{H}_{4}\hbox{Cl}_{2}\) reaction (as shown in reaction R18). R26 is a chain termination reaction, it will slow down 1,4-DCB decomposition.
Conclusions
1,4-DCB could be removed from gas phase in an accelerator generated plasma reactor. Different base gas mixtures have influence on the 1,4-DCB decomposition. Removal efficiency of 1,4-DCB in different base gas mixtures in such order as follows (from higher to lower): \(\hbox{N}_{2}>\,\hbox{air}\,>1.027\% \hbox{NO}{-}\hbox{N}_{2}\). The reason for this might be explained by that ion cluster formed by O2 or NO has backward reaction to release 1,4-DCB and thus hinder 1,4-DCB degradation. Charge transfer reactions play very important role for 1,4-DCB degradation. Products for 1,4-DCB/1.027%NO/N2 system before and after irradiation are very complicated, it needs further investigation.
References
Jindrich P, Deti Z (2001) http://www.otzo.most.org.pl/en/docs/POPs_Poland.pdf, 1
Głuszyński P (2002) http://www.otzo.most.org.pl/en/docs/pops_milk-poland.pdf, 1
Xu XH, Zhou HY, He P, Wang DH (2005) Chemosphere 58:1135
Mohseni M (2005) Chemosphere 59:335
Petrović ZL, Wang WC, Lee LC (1989) J Chem Phys 90(6):3145
Hakoda T, Zhang G, Hashimoto S (2001) Radiat Phys Chem 62:243
Sun Y, Hakoda T, Chmielewski AG, Hashimoto S, Zimek Z, Bułka S, Ostapczuk A, Nichipor H (2001) Radiat Phys Chem 62:353
Sun Y, Hakoda T, Chmielewski AG, Hashimoto S (2003) Radiochim Acta 91:295
Hirota H, Hakoda T, Taguchi M, Takigami M, Kim H, Kojima T (2003) Environ Sci Technol 37:3164
Paur H-R, Baumann W, Mätzing H, Jay K (1998) Radiat Phys Chem 52(1–6):355
Hirota K, Hakoda T, Arai H, Hashimoto S (2000) Radiat Phys Chem 57:63
Chmielewski AG, Sun Y-X, Bułka S, Zimek Z (2004) Radiat Phys Chem 71:435
Won Y-S, Han D-H, Stuchinskaya T, Park W-S, Lee H-S (2002) Radiat Phys Chem 63(2):165
Kim J-C (2002) Radiat Phys Chem 65(4–5):429
Marotta E, Scorrano G, Paradisi C (2005) Plasma Process Polym 2(3):209
Nomura M, Veshapidze G, Shiromaru H, Achiba Y, Kobayashi N (2004) Int J Mass Spectrometry 235:43
Nakagawa S (2004) Int J Mass Spectrometry 232:265
Arnts RR, Seila RL, Bufalini JJ (1989) J Air Pollut Control Assoc 39:453
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Y., Chmielewski, A.G., Bułka, S. et al. Influence of Base Gas Mixture on Decomposition of 1,4-Dichlorobenzene in an Electron Beam Generated Plasma Reactor. Plasma Chem Plasma Process 26, 347–359 (2006). https://doi.org/10.1007/s11090-006-9029-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-006-9029-z