Abstract
The transition behavior of an Al-rich amorphous oxide layer to an external Al2O3 layer on Fe–(4, 24)Cr–(6, 10)Al (at.%) alloys was investigated during heating to 1000 °C at a heating rate of 50 °C/min, by means of in situ high-temperature X-ray diffraction measurement and TEM observation. In the alloy containing 6Al, internal amorphous Al2O3 was initially developed below the Al-rich amorphous surface layer. The amorphous internal precipitates transformed to be crystalline and grew laterally with time. The internal precipitates subsequently connected with each other to form a continuous α-Al2O3 scale. In the case of 10Al alloy, an Al-rich amorphous layer transitioned to a crystalline α-Al2O3 layer from the interface between transient/amorphous layers during heating. The Al2O3 scale developed on high Al alloys contained Fe and Cr with relatively higher contents, but that formed on low Al alloy contained low Fe and Cr. The effect of Cr on promoting an external Al2O3 scale formation was found to be weaker for alloys with higher Al content compared to the alloys with lower Al content, if Al2O3 scale was directly transitioned from the amorphous layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the initial oxidation stage at higher temperatures, high-temperature oxidation resistant alloys usually form various transient oxides, such as Fe-, Ni-, Al-, Cr-rich oxides. The oxidation stage then transitions to a steady-state stage where a protective oxide scale such as Cr2O3 or Al2O3 forms below the transient oxides by selective oxidation of Cr or Al. In the steady-state oxidation stage, the alloys are protected from high-temperature oxidizing environments by the stable protective oxide scale.
The oxidation behavior of the alloys in the transient oxidation stage has been widely reported. In the case of the Al2O3 scale-forming alloys, the transient oxidation behavior contains various phenomena such as initial transient oxide formation and metastable to stable Al2O3 phase transformation. Golightly et al. [1] investigated the early stage of development of an Al2O3 scale on Fe–Cr–Al alloys at 1000 and 1200 °C. They reported that a steady-state oxide α-Al2O3 was established on Fe–14Cr–4wt%Al and Fe–27Cr–4wt%Al alloys after only a few minutes of oxidation at 1000 °C, and transient oxides rich in Fe and Cr were initially formed prior to α-Al2O3 scale formation. The important finding in their study is that the steady-state oxidation stage was established more rapidly for alloys with higher Cr contents and at higher temperatures. However, Kear et al. [2] reported that the transition to Al2O3 takes about 40 min on Ni–15Cr–6wt%Al at 1000 °C. Regarding the phase transformation of Al2O3 scale, Brumm et al. [3] reported that Cr in Ni–Al–Cr alloys accelerates the phase transformation from metastable θ-Al2O3 to α-Al2O3, and Cr2O3 nuclei serve as nucleation sites for α-Al2O3. Kitajima et al. [4] reported that Fe or Cr coating on Fe-50Al alloy accelerates the phase transformation from metastable θ-Al2O3 to α-Al2O3. These results suggest that the initial transient oxidation affects the subsequent phase transformation of Al2O3 scale.
In our previous studies [5, 6], we investigated the initial oxidation behavior by using Fe–xCr–6at.% Al (x = 0–24) alloys, which are relatively lower Al alloys comparing to commercial Fe–Cr–Al alloys such as Kanthal AF and MA956, containing about 10 at.%Al, during heating in order to clarify the effect of Cr on an external Al2O3 scale formation, and discussed the effect of Cr on promoting an external Al2O3 scale formation by in situ high-temperature X-ray diffraction analysis and TEM observation. Figure 1 shows a schematic of the effect of Cr on oxidation behavior of binary Fe–Al and ternary Fe–Cr–Al alloys during heating to 1000 °C. In this schematic, Fe–Cr alloy is also included in order to indicate the effect of Al. We found (1) An Al-rich amorphous oxide layer was found to develop below the outer Fe-rich transient oxide scale on Fe–Al and Fe–Cr–Al alloys at lower temperature. This Al-rich amorphous layer was found to act as a protective layer during heating up to about 800 °C until a protective Al2O3 scale was formed. Therefore, initial oxidation mass gain of Fe–Al alloy is much lower than that of Fe–Cr, and (2) Cr improved the long-term oxidation resistance of this amorphous layer by delaying the time for breakdown [6]. After heating to 900 °C, Al was internally oxidized below the amorphous layer in Fe–xCr–6Al alloy regardless of alloy Cr content, and (3) the volume fraction of internal Al2O3 increased with increasing alloy Cr content which results in higher mass gain of alloys with higher Cr content, but may promote rapid transition to external Al2O3 scale. Finally, an external Al2O3 scale was developed and oxidation continued with a slower oxidation rate. The transition from Al-rich amorphous layer accompanied by internal Al2O3 to a protective Al2O3 scale formation occurred at temperatures between 900 and 1000 °C; However, the detail of transition behavior from internal to external Al2O3 scale was not clearly understood. Moreover, the effect of Cr on this transition phenomenon has not been clearly understood. Therefore, in this study, we investigated the detailed transition behavior by observing the change in the structure of internal precipitate until establishment of an external Al2O3 scale in order to understand the effect of Cr on Al2O3 scale formation in more detail. Additionally, we also investigated the direct transformation from an Al-rich amorphous to an external Al2O3 scale by using the alloys with higher Al content, ~10at.%.
The purpose of this study is to investigate the process of the establishment of the protective Al2O3 scale and understand the effect of Cr and Al contents on Al2O3 scale formation by analysis of structural and microstructural developments of oxides during the heating stage, by means of in situ high-temperature X-ray diffraction measurement and TEM observation.
Experimental Procedures
Table 1 shows the alloy composition used in the present study. All alloys were prepared by argon arc melting using high-purity metals (99.99 at.%) as the starting constituents. The alloy ingots were then homogenized in vacuum at 1200 °C for 48 h. Approximately 1-mm-thick samples were cut from the homogenized ingots and ground to a 4000-grit finish using SiC abrasive paper. Samples were then polished using 3 μm diamond paste, followed by ultrasonic cleaning in acetone prior oxidation test.
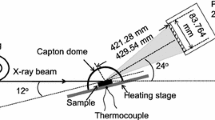
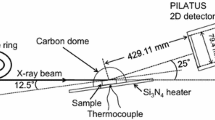
In situ high-temperature X-ray diffraction measurement was performed at the beam-line BL19B2 of the SPring-8 at Japan Synchrotron Radiation Research Institute. Detailed experimental setup for in situ measurement is explained elsewhere [5]. The camera length between the two-dimensional X-ray detector and the sample surface was set to 438.31 mm. After setting up the apparatus, the sample was heated to 1000 °C in air at a heating rate of 50 °C/min, followed by isothermal oxidation for 30 min in air. During heating, isothermal oxidation and cooling, the diffracted signals from the sample surface were collected every 10 s, with a collection time of 6 s.
After oxidation testing, surface morphology was observed by scanning electron microscopy (SEM) and cross sections of the oxide scale were observed by transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM). Element distributions were analyzed by an energy dispersive X-ray spectrometer (EDS) attached to the STEM.
Results
Oxidation Kinetics
Figure 2 shows the oxidation kinetics of Fe–6Al and Fe–Cr–Al alloys with different Al and Cr contents during heating to 1000 °C. The oxidation mass gains of Fe–6Al and Fe–xCr–6Al in Fig. 2 were superimposed from our previous study [5]. Cr effect on oxidation performance of Fe–6Al alloy was very clear as discussed in our previous study; 4%Cr addition significantly decreased the oxidation mass gain in the heating stage. Higher alloy Al content of 10% further decreased the oxidation mass gain in this initial oxidation stage. The oxidation mass gains of alloys containing 10Al were comparable to those of ternary alloys with 6Al up to 700 °C. The oxidation rates of Fe–Cr–6Al alloys started to increase with further heating, but those of 10Al alloys remained lower, resulting in relatively large difference in oxidation mass gains between the alloys with 6 and 10Al after heating to 1000 °C. Among the alloys with 6Al, oxidation mass gain after heating to 1000 °C was higher for 24Cr than for 4Cr, whereas no such difference in oxidation mass gain was observed between Fe–10Al alloys with different Cr contents.
This tendency of Cr effect on oxidation mass gain is clearly seen in Fig. 3a–c. After heating to 650 °C, the oxidation mass gain of 6Al alloys was drastically decreased by only 4%Cr addition and gradually decreased with further Cr addition. However, after heating to higher temperatures, 900 and 1000 °C, higher Cr addition resulted in an increase in the oxidation mass gain. In the case of 10Al alloys, the oxidation mass gain tended to decrease with higher Cr addition at all temperatures.
In Situ High-Temperature X-Ray Diffraction Measurement
Figure 4 shows the in situ high-temperature X-ray diffraction patterns of Fe–4Cr–6Al, Fe–24Cr–6Al, Fe–4Cr–10Al and Fe–24Cr–10Al during heating to 1000 °C, followed by isothermal oxidation for 20 min. The diffraction patterns of Fe–4Cr–6Al and Fe–24Cr–6Al are also shown from our previous study [5] for further discussion of the development of Al2O3 scale. Fe-rich transient oxide, Fe2O3, was always observed initially even on the alloys with higher Al content (see Fig. 4e–h). As we previously discussed [5], the peak position of this transient Fe2O3 formed on Fe–4Cr–6Al alloy shifted toward lower 2θ position during heating, whereas it shifted opposite direction, i.e., toward higher angles on Fe–24Cr–6Al. In the case of alloys containing higher Al content, the peak position of the transient Fe2O3 shifted to higher 2θ position regardless of Cr content. The diffracted signal from α-Al2O3 was observed on all alloys during heating. Intensity of signals from Fe2O3 suddenly increased when the signals α-Al2O3 were observed.
Figure 5 shows the change in signal intensity of different oxides formed during heating to 1000 °C followed by isothermal oxidation for 20 min. The diffracted signals from α-Al2O3 were initially observed on all alloys when the sample temperature reached about 900 °C. The time to observation of the signals from α-Al2O3 tended to be earlier for the alloys with higher Al content, suggesting higher Al content is beneficial for rapid establishment of α-Al2O3. The intensity of the signals from Fe2O3 suddenly increased concurrently with the formation of α-Al2O3 on the alloys with 6Al, and then it gradually decreased after longer oxidation time due to the formation and growth of (Fe,Cr)2O3 solid solution. However, the signals from transient oxides formed on 10Al alloys disappeared shortly after Al2O3 formation. On the alloys with higher Al content, intensity of the signal from α-Al2O3 gradually increased with temperature and/or time. Additional signals from α-Al2O3 which have different 2-theta angles were observed on all alloys except Fe–4Cr–6Al during isothermal oxidation.
Change in intensity of different oxide phases on a Fe–4Cr–6Al, b Fe–24Cr–6Al, c Fe–4Cr–10Al and d Fe–24Cr–10Al with temperature and/or oxidation time obtained from the XRD patterns in Fig. 4
Change in the d-spacing and the full width at half maximum, FWHM, obtained from the signal of Al2O3 (104) are shown in Fig. 6. The d-spacing of Al2O3, which formed initially on alloys with low Al content, was similar and close to that of pure Al2O3 [7, 8] (Fig. 6a). However, on the alloys with higher Al content, the d-spacing of initially formed Al2O3 was larger than that of pure Al2O3 and gradually decreased with oxidation time. The secondary Al2O3 formed on Fe–24Cr–6Al alloy had larger d-spacing, but that formed on 10Al alloys was smaller and similar to pure Al2O3. The FWHM of Al2O3 was higher when the Al2O3 has larger d-spacing.
Change in d-spacing and FWHM of Al2O3 on a, b Fe-4, 24Cr–6Al, c, d Fe-4, 24Cr–10Al with temperature and/or oxidation time obtained from the XRD patterns in Fig. 4
Change in Microstructure of Oxide Scale During Heating to 1000 °C
Figure 7 shows cross sections of Fe–24Cr–6Al after heating to 800, 900 and 1000 °C. As discussed in our previous study, the Al in low Al alloys oxidized internally, even though the Cr content was high, 24Cr. The internal oxide after heating to 800 °C was identified in this study as an Al2O3 by STEM–EDS as shown in Table 2 and was also confirmed to be amorphous due to the absence of diffracted signals from α-Al2O3 from both in situ high-temperature XRD measurement and TEM observation. The amorphous internal Al2O3 precipitates transitioned to crystalline Al2O3 at about 900 °C, and the crystalline Al2O3 precipitates grew larger with time and temperature during heating, finally connecting with each other to form an external Al2O3 scale below the transient oxide after heating to 1000 °C. STEM–EDS analysis revealed that Fe and Cr contents in the Al2O3 precipitates formed after heating to 1000 °C were very low as shown in Table 2. After the formation of internal Al2O3 precipitates at 800 °C, the Al-rich amorphous oxide layer disappeared (see Fig. 7a). Figure 8 shows cross sections of Fe–4Cr–6Al after heating to 800 and 900 °C. The volume fraction of Al2O3 internal precipitates in this alloy was smaller than that in Fe–24Cr–6Al.
TEM cross sections of Fe–24Cr–6Al after heating to a 800 °C, b 900 °C and c 1000 °C. Points in a and c are analysis points by EDS in Table 2
Figure 9 shows cross sections of Fe–4Cr–10Al after heating to 700, 900 and 1000 °C, and Fe–24Cr–10Al after heating to 1000 °C. Because the microstructures of oxide scale formed on Fe–4Cr–10Al and Fe–24Cr–10Al at 1000 °C are almost identical, as shown in Fig. 10c, d, Fe–4Cr–10Al alloy will be the focus of further discussion. A thin Al-rich amorphous oxide layer, which was seen as a bright contrast layer, was observed below the transient Fe2O3 layer after heating to 700 °C. Although the interface between outer crystalline transient Fe2O3 and inner amorphous Al-rich oxide layer was not clear, the region of crystallinity was clearly seen to increase in the outer part of the amorphous layer with increasing oxidation temperature. When the temperature reached 1000 °C, the Al-rich amorphous oxide layer had become completely crystalline. STEM–EDS analysis of oxide scales after heating to 1000 °C, shown in Figs. 10, 11 and Tables 3 and 4, revealed that the oxide scale formed on both alloys consisted of Al2O3 with much higher Fe and/or Cr contents in the outer part of the scale, and lower Fe and/or Cr contents in the inner part of the scale.
STEM–EDS mapping of Fe–4Cr–10Al after heating to 1000 °C. Points are analysis points by EDS in Table 3
STEM–EDS mapping of Fe–24Cr–10Al after heating to 1000 °C. Points are analysis points by EDS in Table 4
Discussion
Diffracted signals from Al2O3 were initially observed during heating on all alloys, and the time to detection of the signals from Al2O3 tended to be shorter for alloys with high Al content. An Al-rich amorphous oxide layer developed on all alloys and remained up to about 800 °C, but it broke down, and internal Al2O3 was formed in alloys with low Al content. However, the Al-rich amorphous layer transitioned to an external Al2O3 scale without internal precipitates on alloys containing higher Al content.
The mass gain during heating was small on all alloys regardless of composition of the transient oxide. This smaller mass gain was interpreted in our previous study to be due to suppression of outward diffusion flux of Fe and Cr by the Al-rich amorphous layer. Moreover, the difference in mass gain during heating before and after Al2O3 establishment was small, indicating that the transition from an Al-rich amorphous oxide layer at lower temperatures to formation of a protective Al2O3 scale proceeded quickly. However, different oxidation kinetics of Fe–xCr–6Al and Fe–xCr–10Al before and after formation of Al2O3 indicates that formation behavior of the external Al2O3 scale on alloys with different Al contents is not identical.
The Transition and Formation Behavior of the External Al2O3 Scale on Alloys with Low Al Content
As shown in Fig. 7, internal Al2O3 precipitates were developed below an Al-rich amorphous oxide layer in the low Al alloy. It was found that a continuous Al2O3 scale was established by connecting internal Al2O3 precipitates due to lateral growth of those precipitates with increasing temperature. The internal Al2O3 precipitates were amorphous when they formed initially. Although formation of the amorphous internal oxide has not been reported previously in the literature, it is reasonable to consider that an amorphous structure is favorable for reduction in interfacial energy at the interface between the precipitates and the substrate. With increase in temperature to about 900 °C, those internal Al2O3 precipitates became stable crystalline α-Al2O3, which has similar d-spacing to pure Al2O3. EDS analysis also revealed that Fe and/or Cr contents in the precipitates are very low. Additional peaks from Al2O3, which had larger d-spacing and FWHM, were detected during isothermal oxidation on Fe–24Cr–6Al. This result implies that the initially formed Al2O3 precipitates reacted with the outer Cr-rich transient oxide of (Cr, Fe)2O3 and/or Fe2O3 to form a solid-solution of (Al,Fe,Cr)2O3.
Figure 12a shows the change in thickness of both the outer oxide scale and internal oxidation zone during heating from 800 to 1000 °C on Fe–24Cr–6Al. The thickness of the outer oxide increased rapidly within this temperature range because of physical breakdown of the amorphous layer due to deformation of alloy substrate accompanied with the formation of internal Al2O3 precipitates [6]. However, the overall oxidation mass gain of this alloy was still much lower than that of Fe–6Al as shown in Fig. 2, indicating that the transition from internal Al2O3 to a continuous Al2O3 scale proceeds rapidly after destruction of the Al-rich amorphous layer. In comparison with the outer oxide scale, the increase in thickness of the internal oxidation zone from 800 to 900 °C is smaller, but the volume fraction of internal Al2O3 increased in this temperature range (Fig. 12c). Moreover, the number of internal Al2O3 precipitates per unit surface length decreased with increasing temperature. These results indicate that lateral growth of internal Al2O3 precipitates predominated, and this lateral growth of internal Al2O3 precipitates promoted the establishment of an external Al2O3 scale. Therefore, higher alloy Cr content and a higher volume fraction of internal Al2O3 precipitates are favorable for rapid establishment of an external Al2O3 scale.
The Transition and Formation Behavior of External Al2O3 Scale on the Alloy with High Al Content
As shown in Fig. 9, internal Al2O3 precipitates were not formed in the alloys with higher Al content. An Al-rich amorphous layer transformed to crystalline from the outer (transient oxide side) to the inner part of the amorphous layer during heating. Greater Al supply from the alloy substrate to the Al-rich amorphous layer in alloys with high Al content prevents formation of internal Al2O3 precipitates below the amorphous layer, resulting in a longer lifetime for the Al-rich amorphous layer. The transient oxide formed at the initial stage of oxidation was always of corundum-type (Fe,Cr)2O3, which is of similar crystallographic structure to α-Al2O3. Such initial transient oxide scale formation is known to promote the rapid transformation to α-Al2O3, or to suppress metastable Al2O3 formation [3, 9]. This “template effect” could also promote the amorphous to crystalline transformation.
The in situ high-temperature X-ray diffraction measurement clearly showed that the α-Al2O3, formed initially on alloys with higher Al content, had larger d-spacing and FWHM, because the α-Al2O3 contained higher Fe and/or Cr contents. This Al2O3, which has larger d-spacing, was reported on Fe–high Al binary alloys [10, 11]. Cr2O3 and Al2O3 form a solid-solution [12], but solubility of Fe2O3 in Al2O3 is relatively small, 5.3 mol% at 1000 °C [13], thus the d-spacing of initially formed α-Al2O3 on higher Cr alloys could be larger than that formed on a lower Cr alloy.
The Effect Cr and Al on the Transition Behavior of External Al2O3 Scale
Figure 13 shows schematics of the effect of Cr and Al on the transition to external Al2O3 scale. Cr content had little effect on the transition to an external Al2O3 scale on alloys with higher Al content, but Al content was found to strongly affect this transition. Internal Al2O3 precipitates were developed in 6%Al alloys regardless of Cr content; however, internal Al2O3 was not formed when Al was increased to 10%. This result suggests that the “true” critical Al content for external Al2O3 scale formation exists between 6–10% on Fe–Cr–Al at 1000 °C. Cr content seems not to significantly affect this critical Al content, but helps to promote establishment of an external Al2O3 scale when Al content is below the critical value.
An increase in volume fraction of internal Al2O3 resulted in fast establishment of Al2O3 scale, as presented in our previous paper [5]. Since the volume fraction of internal Al2O3 increases with increasing alloy Cr content, Cr should help to promote Al2O3 scale formation by accelerating the transition from internal to external scale formation; therefore, Cr is important mostly on the alloys with lower Al content. However, if the Al-rich amorphous layer directly transitions to an external Al2O3 scale, the Cr effect on external Al2O3 scale formation is greatly diminished.
Conclusions
The transition behavior from Al-rich amorphous oxide to external Al2O3 layer was investigated by structural and microstructural observation in the heating stage by means of in situ high-temperature X-ray diffraction measurement and TEM. The results obtained may be summarized as follows:
-
1.
Internal Al2O3 precipitates formed in 6Al alloys grew laterally and connected with each other to form an external Al2O3 scale. The Al2O3 scale developed by this pathway contained low Fe and Cr contents.
-
2.
Al-rich amorphous layer became crystalline from the outer toward the inner part of the layer with increasing temperature and time, and then external Al2O3 was finally developed on alloys with high Al content. Al2O3 scale formed by this pathway contained higher Fe and Cr content.
-
3.
Cr promoted the Al2O3 scale formation by increasing the volume fraction of internal Al2O3 on alloys with lower Al content; however, no significant effect of Cr on Al2O3 scale formation was observed on alloys with higher Al content, if the amorphous layer directly transitioned to Al2O3 scale.
References
F. A. Golightly, G. C. Wood and F. H. Stott, Oxidation of Metals 14, 217 (1980).
B. H. Kear, L. P. Lemaire, D. E. Fornwalt and F. S. Pettit, Oxidation of Metals 3, 557 (1971).
M. W. Brumm and H. J. Grabke, Corrosion Science 33, 1677 (1992).
Y. Kitajima, S. Hayashi, T. Nishimoto, T. Narita and S. Ukai, Oxidation of Metals 73, 375 (2010).
S. Yoneda, S. Hayashi, I. Saeki and S. Ukai, Oxidation of Metals 86, 357 (2016).
S. Yoneda, S. Hayashi and S. Ukai, Oxidation of Metals (2017). doi:10.1007/s11085-017-9761-1.
T. Fujimura and S.-I. Tanaka, Journal of Materials Science 34, 425 (1999).
I. E. Reimanis, K. P. Trumble, K. A. Rogers and B. J. Dalgleish, Journal of the American Ceramic Society 80, 424 (1997).
D. Renusch, M. Grimsditch, I. Koshelev, B. W. Veal and P. Y. Hou, Oxidation of Metals 48, 471 (1997).
S. Hayashi, I. Saeki, Y. Nishiyama, T. Doi, S. Kyo and M. Segawa, Materials Science Forum 696, 63 (2011).
S. Hayashi, Y. Takada, I. Saeki, A. Yamauchi, Y. Nishiyama, T. Doi, S. Kyo and M. Sato, Materials and Corrosion 63, 862 (2012).
M. Fujita, K. Inukai, S. Sakida, T. Nanba, J. Ommyoji, A. Yamaguchi and Y. Miura, Journal of the Society of Materials Science 56, 526 (2007).
F. A. Elrefaie and W. W. Smeltzer, Metallurgical Transactions B 14B, 85 (1983).
Acknowledgements
Part of this work was supported by “Research and development of oxide dispersion strengthened (ODS) ferritic steel fuel cladding for maintaining fuel integrity at high-temperature accident conditions” entrusted to Hokkaido University by the Ministry of Education, Culture, Sports, Science and Technology, MEXT, Japan and JSPS KAKENHI Grant Number JP16K14436. The synchrotron radiation experiments were performed at the BL19B2 of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal No. 2015A1727). The authors greatly appreciate Dr. M. Sato for his support for X-ray diffraction measurement at SPring-8. TEM characterization was conducted under the program of NIMS microstructural characterization platform as a program of “Nanotechnology Platform” of the MEXT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoneda, S., Hayashi, S. & Ukai, S. The Transition from Transient Oxide to Protective Al2O3 Scale on Fe–Cr–Al Alloys During Heating to 1000 °C. Oxid Met 89, 81–97 (2018). https://doi.org/10.1007/s11085-017-9778-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-017-9778-5