Abstract
An investigation into the erosion–oxidation behavior of chromized–aluminized 9% chromium steel has been carried out in a fluidized-bed erosion–oxidation rig in air at temperatures of 550 °C to 700 °C for particle impact angles of 30° and 90°, at speeds of 7.0–9.2 m s−1. After exposure for 200 h, the mean-thickness changes were determined, and the specimens were examined and analyzed by scanning electron microscopy and X-ray diffraction.
The results show that the chromized–aluminized specimens experience only relatively small amounts of material loss for 30° particle impacts but greater amounts for 90° particle impacts; such angle dependence is typical of a brittle erosion process. Under both impact angles, the thickness losses increase with increase in speed, and also, in temperature up to 650 °C, but then decrease with further increase in temperature. This change in the behavior between 650 and 700 °C is due to an increase in ductility of the coating and a greater contribution from oxidation. The two coating layers (an outer Al13Cr2 and an inner α-chromium layer) show similar behavior, i.e., have lower material loss rates under 30° impacts than under 90° impacts. As the oxidation rate of the α-chromium layer is greater than that of the outer layer, the oxide scale plays a greater role in the erosion process for the former, particularly under 30° impacts. These observations are discussed in terms of the thickness changes and the morphologies of the damaged surfaces, with the emphasis being put on the relative roles of growth of the oxide scales and removal of such scales (and the underlying coating) by the impacting particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is known that 9–12% chromium ferritic steels are candidate materials for heat exchangers in future power plants. The mechanical properties of these steels have been designed for steam temperatures up to 650 °C [1], but their high-temperature oxidation and erosion–oxidation resistances are limited; they are on the borderline in terms of resistance to oxidation at 650 °C [2]. Austenitic steels and nickel-base alloys generally show better oxidation and erosion–oxidation resistance, but they are more expensive than ferritic steels, and have higher coefficients of thermal expansion and lower heat conductivities; these are significant drawbacks for their use as heat exchanger tubes. Therefore, if oxidation and erosion–oxidation resistance of ferritic steels could be enhanced by suitable coatings, the service temperature of such steels could be increased up to that given by their mechanical limitation.
High-temperature coatings generally consist of elements, such as aluminium, chromium or silicon, that form a protective oxide scale on the surface in oxidizing environments [3, 4]. Aluminized coatings are the most widely used diffusion coatings for high-temperature applications, due to the slow rate of oxide-scale growth, and subsequently, excellent oxidation resistance under various conditions [5–8]. However, such coatings usually degrade in two ways: by loss of near-surface aluminium because of oxidation of the coating surface to form the oxide scale and by interdiffusion with the underlying substrate [9–11]. A chromized diffusion coating underneath the aluminized coating can act as a diffusion barrier against the inward diffusion of aluminium into the steel substrate. Moreover, such a layer has been reported to be beneficial in terms of adherence of the aluminized coating and provision of additional protection, since chromium is also able to develop a protective and stable oxide scale [12].
Previously, such two-step chromized-aluminized diffusion coatings have been reported to decrease the mass gain of 9% chromium steel by about one order of magnitude in a simulated coal-fired environment containing 14 vol.% CO2, 10 vol.% H2O, 1 vol.% O2, 0.1 vol.% SO2, 0.01 vol.% HCl in N2 after 1,000 h at 650 °C [13]. Moreover, chromized–aluminized steels have shown excellent resistance against metal dusting in an atmosphere containing 25% CO, 73% H2 and 2% H2O at 400, 620 and 700 °C [12].
Although chromized–aluminized steels are relatively resistant to both oxidation and metal dusting at elevated temperatures, their erosion–oxidation behavior has received little attention. However, a fundamental understanding of interactions between the erosion and the oxidation processes for such materials and of the coating degradation mechanisms under these conditions is of key importance if they are to be used as heat exchanger materials for future power plants. The present paper presents some results from a study of the erosion–oxidation behavior of chromized–aluminized 9% chromium ferritic steels, coated by a two-step pack-cementation process, under fluidized-bed conditions.
Experimental Procedures
Materials
Deposition of chromium and aluminium diffusion coatings on the 9% chromium ferritic steel, P91, supplied by Vallourec and Mannessmann Tubes Corporation, Germany, and of nominal composition: 9 wt.% Cr, 0.93 wt.% Mo, 0.55 wt.% Mn, 0.4 wt.% Si, 0.36 wt.% Ni, 0.22 wt.% V, 0.093 wt.% C, 0.07 wt.% Nb, 0.056 wt.% N, 0.006 wt.% Al and Fe (balance), was carried out by a two-step pack-cementation process. In the first step, chromium deposition from a pack containing 30 wt.% chromium powder, 5 wt.% ammonium chloride activator and balance alumina as an inert filler was combined with a heat treatment of the steel; specimens were held at an austenitizing temperature of 1,000 °C for 2 h, followed by rapid cooling in argon inside the pack cementation reactor. In the second step, aluminium was deposited from a pack containing 20 wt.% aluminium powder, 1 wt.% ammonium chloride and balance alumina, for 1 h at 650 °C. In both steps, the substrate specimens, ground to 120 grit finish and cleaned ultrasonically in ethanol, and the powder mixture were placed in an alumina crucible, covered with an alumina lid; the crucible was inserted into a furnace with flowing Ar-10% H2. After each step, the pack was cooled to room temperature, and the specimens were removed and ultrasonically cleaned in ethanol to remove any loosely embedded pack material.
Erosion–Oxidation Tests
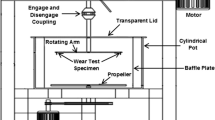
The erosion–oxidation experiments were carried out in a fluidized-bed rig (Fig. 1), consisting of a fluidized bed containing 40 vol.% particles, a heating system and a specimen-holder assembly. The heating system has three independent heating zones, which were held at the same temperature in these tests; air is preheated before it enters the bed of particles, heated by the bed heater, while the temperature of the specimens is maintained by the body heater. During operation, air is pumped through the spiral flow tube in the preheater, enabling it to attain the necessary acceleration as well as the desired temperature before entering the bed. After fluidizing the particles, the air enters the coolers and cyclones and, finally, recirculates through the system. The specimen arm is rotated through the fluidized bed by a drive motor; it is designed to allow the attached specimens to be completely immersed in the bed at the lowest point and then to move through the space above the bed. The specimens, 35 mm by 7 mm by 4.5 mm, were fastened in the holders, located at the two ends of the specimen arm, and oriented so that they impacted the bed of particles at angles of 30° and 90°. The back side and the edges of each specimen were protected from the environment by the specimen holder, exposing only the front of the specimens to the impacting particles. By adjusting the rotational speed of the drive motor, the linear speed of the samples relative to the bed particles could be set at the desired value. Here, the rotational speed was set to give linear speeds ranging from 7.0 to 9.2 m s−1 along the length of each specimen.
In the present study, angular silica sand particles of average diameter of 200 μm (Fig. 2) were used as the bed particles. New particles were used in each test, with the duration being 200 h in each case. The tests were conducted at temperatures of 550, 600, 650 and 700 °C.
Characterization Methods
Thickness measurements were made before and after the tests, enabling the average specimen thickness changes to be determined, using a micrometer, at twelve locations along the specimen length, with each location representing a particular linear speed between the specimen and the erodent particles. The thickness at each location was measured at five points across the specimen and ten measurements were taken at each point. The average values and standard deviations for the fifty measurements were then calculated. In all cases, the standard deviation was relatively low; less than 1.5 μm for the 4.5 mm thick specimens. However, it was usually not possible to locate the micrometer head in some of the narrow pits in the surface, so these may have penetrated much more deeply than suggested by the measured mean values and standard deviations.
Amray 1810 and Philips XL30 scanning electron microscopes (SEM), equipped with ISIS and EDAX energy dispersive spectrometer (EDS) systems, respectively, were used to examine the sand particles before the tests and the coated specimens before and after the tests, to reveal the morphologies and microstructures of the surfaces. Moreover, unexposed and exposed specimens, gold-coated and metallographically polished, were studied by SEM in cross section, to determine the thicknesses of, and the elemental distributions in, the coatings. A Philips PW 3710 X-ray diffractometer (XRD) with CuKα radiation was used to characterize the phase structures of surfaces of the unexposed and exposed specimens. The XRD data of the exposed specimens was obtained from areas that had been exposed to speeds ranging from 7.0 to 7.8 m s−1.
Results
Unexposed Specimens
The deposited diffusion coatings were relatively dense and contained two layers (Fig. 3a, b). The outer layer, thickness ranging from 13 to 19 μm, comprised the Al13Cr2 phase, average composition of 82 at.% Al and 18 at.% Cr, with an underlying 2–5 μm thick essentially α-chromium layer, containing some Al and Fe. Chromium enrichment was detected in the substrate, to a total depth of 55 to 60 μm from the surface (Fig. 3c), consistent with earlier studies [14, 15]. Moreover, pores were observed in the ferritic substrate immediately below the coating layers, up to a depth of 10 μm from the inner edge of the α-chromium layer, probably due to the Kirkendall effect [16]. The XRD spectrum also revealed the presence of the Cr23C6 phase (Fig. 3d). Previously, in the case of chromized steels, chromium carbides have been reported to form within the α-chromium layer or the zone of chromium enrichment in the substrate [17–19]. Here, EDS linescan results (Fig. 3c) are consistent with such observations.
Microstructure of the specimens. (a) SEM micrograph, showing the surface of the coating; (b) Cross-sectional BSE micrograph of the specimen, with a bar indicating the EDS linescan; (c) EDS linescan results. The numbers on the y-axis refer to the maximum signal intensity; (d) XRD spectum in plan for the specimen
Thickness Changes Following Erosion–Oxidation Tests
Figure 4 shows the mean thickness changes for the coated specimens measured after the tests as a function of speed at the four temperatures. At 550 °C (Fig. 4a) for 30° impacts, apart from at one location, where a thickness increase of +4 μm was recorded, the mean values varied between 0 μm and −19 μm, giving an average of −7 μm. Moreover, there was a trend for the thickness loss to increase slightly with increase in speed: although at most locations the losses were smaller than the thickness of the outermost Al13Cr2 layer, occasionally, at the highest speeds, the losses were greater than the thickness of both the Al13Cr2 and the α-chromium layers. For 90° impacts, thickness losses were recorded at all speeds, average: −24 μm, with the loss increasing in an almost linear manner with increase in speed. Thus, the maximum loss, −32 μm, was observed at the highest speed. Here, at the lowest speeds, the losses were usually greater than the thickness of the outermost Al13Cr2 layer but smaller than the thickness of both the Al13Cr2 and the α-chromium layers, while, at the highest speeds, the losses were generally greater than the thickness of the two layers.
At 600 °C (Fig. 4b) for an impact angle 30°, small thickness losses, between −9 and −19 μm, were recorded at all speeds, giving an average value of −14 μm. Although there was a slight trend for the loss to increase with increase in speed, it was less pronounced than at 550 °C. Thus, apart from the highest speeds, the losses remained smaller than the thickness of the outermost Al13Cr2 layer. Similarly, for an angle of 90°, thickness losses were experienced at all speeds, with an average value of −24 μm, and the loss increased slightly with increase in speed. Thus, the maximum loss, −29 μm, was recorded at the highest speed. Here, the losses were generally greater than the thickness of both the Al13Cr2 and the α-chromium layers.
At 650 °C (Fig. 4c) for 30° impacts, thickness losses between −8 and −20 μm were observed, giving an average value of −15 μm. There was a slight trend for the thickness loss to increase with increase in speed, with the extent of loss, at the lowest speeds, generally being smaller than the thickness of the outermost Al13Cr2 layer but, at the highest speeds, greater than that. Moreover, at the highest speeds, the losses at some locations were greater than the thickness of both the Al13Cr2 and the α-chromium layers. At 90°, the extent of material loss increased more significantly with increase in speed, to −71 μm at the highest speed, with an overall average value being −44 μm. Here, at all speeds, the losses were greater than the thickness of the two coating layers.
At 700 °C (Fig. 4d), for 30° impacts, the thickness changes were very small, varying between a slight thickness gain, maximum +1 μm, and a slight thickness loss, maximum −6 μm, with an average value of −4 μm. Thus, at all speeds, the losses remained smaller than the thickness of the outermost Al13Cr2 layer. For 90° impacts, thickness losses were recorded at all speeds, with an average value of −14 μm; these increased in an almost linear manner with increase in speed, to a maximum loss, −20 μm, at the highest speed. Here, at the lowest speeds, the losses were generally smaller than the thickness of the outermost Al13Cr2 layer, while, at the highest speeds, the losses were greater than that and, at some locations, even greater than the thickness of both the Al13Cr2 and the α-chromium layers.
The thickness change data for the three speeds, of 7, 8 and 9 m s−1, are plotted as a function of temperature in Fig. 5. For an angle of 30° (Fig. 5a), the trend of thickness loss with temperature depended slightly on speed. At 7 m s−1, the thickness loss increased with increase in temperature from 550 to 600 °C, but then slightly decreased with further increase in temperature. At 8 and 9 m s−1, there was a progressive increase in thickness loss with increase in temperature up to 650 °C, and again, a decrease with further increase in temperature. At 90° (Fig. 5b), the maximum thickness loss at all speeds was recorded at 650 °C. At 7 m s−1, the thickness loss increased in a linear manner with increase in temperature up to 650 °C, although, at the higher speeds, a slight decrease in thickness loss was recorded between 550 and 600 °C. At all speeds, relatively small thickness losses were recorded at the highest temperature. It is emphasized that, in general, the largest thickness losses occurred at the highest speed and at 650 °C, while the smallest thickness losses occurred at the lowest speed and at 700 °C, for both impact angles.
Exposed Specimens
30° Impacts
After 200 h exposure at 30° (Fig. 6), some coating was retained on the surface at all temperatures. However, under all conditions, the outermost Al13Cr2 layer was, at least partly, worn away, with the extent of damage depending on temperature and speed. At 550 °C, where only small thickness losses were generally recorded, the surface was relatively uneven in appearance, with some impact craters being apparent. Here, a thin layer of the Al13Cr2 phase was still retained over much of the surface. Nevertheless, in a few locations, both the Al13Cr2 and the α-chromium layers were worn away, particularly at the base of the impact craters and at the highest speeds. Thus, occasionally, greater material losses were evident from SEM surface studies than from thickness change data. Moreover, in some cases, thicker regions of coating were detected at the edges of the craters, possibly due to material extrusion and lip formation as a result of a cutting type of wear. At 600 and 650 °C, the surfaces contained numerous craters and some indications of cutting type wear by chip formation. At 600 °C, some of the Al13Cr2 phase was retained on the surface at the lowest speeds, while, at 650 °C, the Al13Cr2 phase was only detected at the edges of the craters (Fig. 7). At both temperatures, the α-chromium phase was exposed on much of the surface, covering it almost completely at the highest speeds at 600 °C and at almost all speeds at 650 °C, with thicker regions being detected at the edges of the impact craters (Figs. 6c and 7a); such observations are in agreement with the thickness change data. At 700 °C, where the thickness changes were small, the surface morphology was slightly different from that at the lower temperatures: there were some surface irregularities, probably related to the original coating morphology, and numerous indications of ductile cutting type of wear by chip formation. Moreover, both the Al13Cr2 and the α-chromium phases were detected on the surface at all temperatures; this is consistent with the thickness loss data.
SEM micrographs in plan (a, c, e, g) of, and corresponding EDS results (b, d, f, h) for, the specimens exposed to 30° impacts at 7 m s−1 for 200 h. (a), (b) 550 °C. (c), (d) 600 °C. (e), (f) 650 °C. (g), (h) 700 °C. Quantitative EDS analyses show average values for three measurements. The arrows point out the most important surface features: impact craters and indications of cutting type of wear
At all temperatures, oxides were present on the specimen surfaces, with significant levels of oxygen being detected by EDS analyses (Figs. 6 and 7). Generally, larger amounts of oxygen were detected on the surface of the exposed α-chromium layer than on that of the Al13Cr2 layer. Moreover, deposition and embedment of remnants of the sand particles took place, irrespective of temperature and speed, since silicon was also detected in the EDS analyses. Furthermore, under all conditions, small loose particles, of either coating or the coating plus substrate, were found on the surface: analyses of such debris detected oxygen, indicating that the particles were debris of coating plus oxide scale and, occasionally, substrate.
XRD analyses of the surfaces after the tests involving 30° impacts (Fig. 8) revealed the presence of the Al13Cr2 phase, elemental chromium and the Cr23C6 phase, all arising from the coating, and the Fe–Cr steel substrate, at all temperatures. However, the intensities of the peaks arising from the α-Cr layer and from the substrate were greater after exposure at 650 °C than at the other temperatures, indicating greater contribution of these structures to the measured signal.
Examination of the exposed surfaces in cross section (Figs. 9 and 10) revealed that the outermost Al13Cr2 layer had been lost, with only remnants being detected at 550 and 700 °C but none at the two intermediate temperatures. In each case, some α-chromium layer was retained, with a minimum thickness of 2 μm, irrespective of temperature, and maximum thickness of 3–5 μm, depending on temperature. Such observations agree relatively well with the thickness change data. However, it is emphasized that some of the outermost coating layers might have been lost during preparation of the cross-sectional specimens (the dark region between the cold coating and mounting and the specimen is a crack); this explains why some of the Al13Cr2 layer could only be detected at 550 and 700 °C, although, in surface studies, it was observed in some regions at all temperatures. Moreover, it is worth noting that the microstructures of the exposed specimens were very similar to that of the as-coated specimens, with no phase transformations or significant diffusion between the coating layers being observed.
SEM micrographs of the specimens exposed to 30° impacts for 200 h at 9 m s−1. (a) Cross-sectional BSE micrograph, 550 °C; (b) Cross-sectional BSE micrograph, 600 °C; (c) EDS linescan results along the bar marked on (a); (d) EDS linescan results along the bar marked on (b). The numbers on the y-axis refer to the maximum signal intensity
SEM micrographs of the specimens exposed to 30° impacts for 200 h at 9 m s−1. (a) Cross-sectional BSE micrograph, 650 °C; (b) Cross-sectional BSE micrograph, 700 °C; (c) EDS linescan results along the bar marked on (a); (d) EDS linescan results along the bar marked on (b). The numbers on the y-axis refer to the maximum signal intensity
90° Impacts
Following erosion–oxidation tests at 90° impacts (Figs. 11, 12), remnants of the two coating layers could be detected on the specimen at 550, 600 and 700 °C, but not at 650 °C. At 550 and 600 °C, where moderate mean thickness losses were recorded, remnants of the Al13Cr2 layer were apparent only at the edges of individual impact craters, whereas those of the α-chromium layer were observed at the edges of individual impact craters, irrespective of speed, and, at 550 °C, over much of the surface at the lowest speeds, consistent with the thickness loss data. Elsewhere, a porous and cracked oxide scale was observed on the exposed substrate, with the extent of porosity and cracking apparently increasing with increase in speed. At 650 °C, where relatively large thickness losses were measured at all speeds, the coating layers had been lost completely and a porous, cracked and continuous oxide scale was detected on the substrate surface. At 700 °C, remnants of the Al13Cr2 layer were again detected at the edges of impact craters, particularly at the lowest speeds, while those of the α-chromium layer were distributed over much of the surface at almost all speeds, consistent with the thickness loss data. Here, some plastic deformation was observed within the chromium layer, particularly at the lowest speeds. However, at the highest speeds, the remnants of the α-chromium layer were relatively thin and discontinuous, since a porous and cracked iron-rich oxide scale could be detected on many areas of the surface.
At all temperatures, oxides were present on the surfaces. However, in each case, they were iron-rich oxides and, therefore, on the exposed substrate. In all tests, remnants of sand particles were also evident in the surface. In addition, debris particles, containing iron, chromium, aluminium and oxygen, were occasionally encountered in the surface, evidently due to removal of oxide scale from the substrate.
After exposure at 90° impacts, XRD analyses (Fig. 13) indicated the presence of the steel substrate and hematite, Fe2O3, plus the SiO2 phase from the remnants of sand particles in all cases. Here, the intensities of the peaks at 65° and 82° at 2θ scale, arising from the substrate, were lower after exposure at 650 °C than at the other temperatures, indicating a slightly smaller contribution of the substrate to the measured signal, even if the highest-intensity peak was related to the substrate, similarly to other temperatures. Moreover, the phases in the coating could not be detected by XRD analyses; this indicates that, if present on the substrate, the coating layers were extremely thin and discontinuous. Indeed, no such layers could be observed by SEM examination of the exposed surfaces in cross section.
Discussion
The mean thickness change data and the microstructural study of the damaged surfaces clearly show that impact angle and temperature have significant effects on the interaction between the erosion and oxidation processes; often, speed influences the interaction. For 30° impacts, the specimens experienced relatively little damage; at 550–650 °C, the thickness losses were small but increased slightly with increases in speed and temperature, while, at 700 °C, the losses were even smaller and independent of speed. For 90° impacts, the thickness losses were greater than at 30° under all conditions, with the extent of damage increasing with increases in speed and, up to 650 °C, in temperature. At 700 °C, the losses also increased slightly with increase in speed, but the trend was less pronounced and the values were less than at the lower temperatures. This relationship between extent of damage and impact angle, with small amounts of material loss for 30° impacts and greater amounts for 90° impacts, is typical of a brittle erosion process [20, 21] and consistent with the brittle nature of aluminized [11, 22] and chromized [19, 22] surface layers. Moreover, it is apparent that there was a small change in behavior of the coating between 650 and 700 °C.
For 30° impacts at temperatures to 650 °C, some of the outer layer of Al13Cr2 was still retained in some areas after 200 h, particularly at the lowest speeds and around the impact craters, while the underlying α-chromium layer was exposed in other areas, enabling the damage to both phases to be studied. In each case, irrespective of the prevailing coating phase, the damage at some locations was typical of brittle erosion, with formation of impact craters and no associated plastic deformation, typical of brittle fracture; in other areas, impact craters and evidence for a cutting type of wear, with some associated plastic deformation (Fig. 6c, e, g), were observed. The proportion of damage by brittle fracture was highest at 550 °C and at the highest speeds, with the proportion of damage by plastic deformation increasing slightly with increase in temperature, i.e., ductility, and decrease in speed, i.e., impact energy. Therefore, it is apparent that, the erosion conditions were relatively severe and the Al13Cr2 and the chromium layers behave in a similar manner; each is relatively brittle and its ductility increases with increase in temperature.
For 30° impacts, not only did material removal by erosion occur, but oxide scales were observed also on the surfaces, since significant amounts of oxygen were detected on all specimens exposed at 550–650 °C and on the debris deposited in them. However, it is known that the two coating layers oxidize at different rates, with the outer Al13Cr2 layer having a lower rate than the inner α-chromium layer [23]. It is also apparent from microstructural studies and thickness change data that the rate of growth of the oxide scale on the Al13Cr2 phase is relatively low compared to that of material removal by erosion, and thus, such scale has little effect on the erosion process; it is evidently removed by the impacting particles, together with the Al13Cr2 layer. Nevertheless, as it is easily worn away (thereby exposing new surface for oxidation and particle impacts), the oxide scale does contribute to material wastage. During 200 h exposure, at least at the higher speeds, the Al13Cr2 layer was removed almost completely, with only residues being detected around brittle impact craters. Under these conditions, where thickness losses increase with increase in temperature and the impacting particles remove both oxide scale and underlying coating, the prevailing erosion–oxidation mode is essentially oxidation-affected erosion [21, 24, 25].
Once the α-chromium layer is exposed, the oxidation rate is faster, and oxide scales play a greater role in the erosion process. As shown in Fig. 4b, c, following removal of the outer coating layer, there was relatively little effect of speed on the extent of thickness change. Moreover, microstructural studies on specimens in cross section (Figs. 9, 10) demonstrated that the α-chromium layer was retained on the substrate under 30° impacts at all temperatures and the material removal rate was relatively low. Thus, since both coating layers are relatively brittle, it may be concluded that the lower rate of damage that accompanies exposure of the chromium layer is due to a greater contribution from oxidation. Under these conditions, where a thin oxide scale forms on it, the scale is able to absorb some energy of the particles, although the particles apparently interact also with the substrate: the oxide scales provide some protection, although they are unable to prevent completely brittle fracture and deformation of the underlying coating surface. Further, microstructural studies on specimens in cross section (Figs. 9, 10) show that there is no significant diffusion of either aluminium or chromium into the substrate, making the chromium layer a good diffusion barrier.
At 700 °C, where the thickness loss was relatively small and the exposed surface was mainly Al13Cr2 after 200 h, the main damage mode involved cutting type of wear, with clearly observable plastic deformation. Here, in contrast to lower temperatures, thickness losses were almost independent of speed. Moreover, thin and relatively continuous oxide scales were detected on the exposed surface. The results indicate that the coating is more resistant to erosion damage than at the lower temperatures because of both an increase in surface ductility of the Al13Cr2 layer and a higher rate of oxide scale development on it. Thus, oxide scales play a more significant role in the erosion process, although they are unable to prevent considerable deformation of the underlying coating surface and some material loss.
For 90° impacts, at temperatures to 650 °C, the extent of coating damage increased with increase in speed and temperature, with the coating being almost completely lost under all conditions. At the two lowest temperatures, some remnants of the Al13Cr2 and chromium layers were detected at the lowest speeds, particularly at the edges of some individual impact craters, while, at 650 °C, no such remnants were observed at all. As the rate of loss of coating is greater at 90° than at 30° impacts, which is typical of a brittle erosion process, it is evident that oxidation has less opportunity to influence the erosion–oxidation process. Thus, damage to the coating layers occurs principally by a brittle-erosion mechanism. Once the coating has been removed, to expose the substrate, oxidation is able to play a more significant role, due to apparent higher oxidation rate of the substrate than of the coating. Then, erosion of the substrate and, particularly, the oxide scale on it contribute to the overall thickness losses under these conditions, leading to relatively high rates of material removal that increase with increase in temperature.
For 90° impacts at 700 °C, the extent of coating loss was less than that experienced at the lower temperatures. Here, remnants of the Al13Cr2 layer were detected at the edges of some individual impact craters at all speeds and some of the α-chromium layer was retained on the substrate at almost all speeds. Significant oxide scales were present on the α-chromium layer. However, at the highest speeds, the residual scales contained much less chromium than at the lowest speeds. In fact, a porous and cracked iron-rich oxide scale was detected in many areas of the surface, indicating that the remnants of the α-chromium layer were relatively thin and discontinuous, particularly at the highest speeds. Moreover, where α-chromium layer was retained, the surface had undergone some plastic deformation, showing that the oxide scale was not thick enough to prevent deformation of the underlying coating. It is also evident that, at 700 °C, the ductility of (at least) the α-chromium layer was increased compared to that at lower temperatures, making it possible for the layer to be retained on the surface for a longer period at this temperature.
Conclusions
-
(1)
Chromized–aluminized diffusion coatings, deposited in a two-step pack-cementation process, contain two layers, an outer Al13Cr2 layer, with an underlying layer of essentially elemental α-chromium. There is some enrichment of chromium in the adjacent substrate, with Cr23C6 precipitates and some Kirkendall pores.
-
(2)
When exposed to angular sand particles in a fluidized-bed rig at 550–700 °C and speeds of 7.0 to 9.2 m s−1, the coated steel specimens experience some material loss for 30° particle impacts, but greater amounts for 90° particle impacts; consistent with a brittle erosion process.
-
(3)
Under both impact angles, the thickness losses increase with increase in temperature up to 650 °C and then decrease with further increase in temperature. This change in the behavior between 650 and 700 °C is due to an increase in ductility of the coating and a greater contribution from oxidation.
-
(4)
Under almost all conditions, thickness losses increase with increase in speed, i.e., in impact energy. Only at 700 °C under the shallow impact angle are the coating (and the oxide scale on it) sufficiently resistant to damage that the thickness losses are virtually independent of speed.
-
(5)
Both coating layers behave in a similar manner, i.e., have lower material loss rates under 30° impacts than under 90° impacts. However, their oxidation rates are dissimilar, with the rate being higher for the chromium layer, so oxide scales play a greater role in the erosion process for this layer, particularly under 30° impacts.
References
R. Viswanathan, and W.T. Bakker, in Proceedings of International Joint Power Generation Conference 2000 Miami Beach, Florida, USA, (American Society of Mechanical Engineers, New Yours, USA, 2000) paper 15049.
J. P. T. Vossen, P. Gawenda, K. Rahts, M. Röhrig, M. Schorr, and M. Schütze, Materials at High Temperature 14, 387 (1997).
G. Wahl, Thin Solid Films 107, 417 (1983).
R. Mevrel, Materials Science and Engineering A120, 13 (1989).
F. J. Perez, F. Pedraza, M. P. Hierro, J. Balmain, and G. Bonnet, Oxidation of Metals 58, 563 (2002).
F. J. Perez, M. P. Hierro, F. Pedraza, M. C. Carpintero, C. Gomez, and R. Tarin, Surface and Coatings Technology 145, 1 (2001).
Y. Zhang, B. A. Pint, J. A. Haynes, and P. F. Tortorelli, Oxidation of Metals 62, 103 (2004).
K. Natesan, Materials Science and Engineering, A258, 126 (1998).
P. S. Liu, K. M. Liang, S. R. Gu, Surface and Coatings Technology 137, 60 (2001).
E. Huttunen-Saarivirta, F. H. Stott, V. Rohr, and M. Schütze, Materials at High Temperature 23, 1 (2006).
E. Huttunen-Saarivirta, F. H. Stott, V. Rohr, and M. Schütze, Corrosion Science 49, 2844 (2007).
C. Rosado, and M. Schütze, Materials and Corrosion 54, 831 (2003).
V. Rohr, Developpement de Revetements pour les Aciers D’Echangeurs Thermiques et Amelioration de leur Resistance a la Corrosion en Environnement Simulant les Fumees de Combustion de Charbon, PhD Thesis, Institut National Polytechnique de Toulouse (2005).
V. Rohr, M. Schütze, E. Fortuna, D. N. Tsipas, A. Milewska, and F. J. Perez, Materials and Corrosion 56, 874 (2005).
V. Rohr, and M. Schütze, Materials Science Forum 461, 401 (2004).
C. A. C. Sequeira, A. M. G. Pacheco, and C. M. G. S. Nunes, Surface Engineering 3, 247 (1987).
I. A. Menzies, and D. Mortimer, Corrosion Science 5, 539 (1965).
J.-W. Lee, and J. G. Duh, Surface and Coatings Technology 177–178, 525 (2004).
Y. Liu, and A. V. Levy, Surface and Coatings Technology 52, 119 (1992).
I. Finnie, in Corrosion-Erosion of Materials. K. Natesan, ed. (American Institute of Mining, Metallurgical and Petroleum Engineers, New York, USA) (1980).
R. Wellman, and J. R. Nicholls, Wear 256, 907 (2004)
A. Levy, and G. Hickey, Wear 108, 61 (1986).
M. Schütze in Corrosion and Environmental Degradation M. Schütze ed. Vol I. (Wiley-VCH Verlag GmbH, Weinheim Germany) (2000).
C. T. Kang, F. S. Pettit, and N. Birks, Metallurgical Transactions 18A, 1785 (1987).
M. M. Stack, F. H. Stott, and G. C. Wood, Wear 162(164), 706 (1993).
Acknowledgements
The authors wish to express their gratitude to the European Commission for the funding allocated to this study through a Research Training Network: SUNASPO (contract HPRN-CT-2001-00201). In addition, the Academy of Finland (decisions 105399 and 106160) and the Jenny and Antti Wihuri Foundation are acknowledged for further financial support to E. H.-S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huttunen-Saarivirta, E., Stott, F.H., Rohr, V. et al. Erosion–Oxidation Behavior of Chromized–Aluminized 9% Chromium Steel under Fluidized-Bed Conditions at Elevated Temperature. Oxid Met 68, 113–132 (2007). https://doi.org/10.1007/s11085-007-9064-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-007-9064-z