Abstract
The present study investigates the characteristics of nanocomposites comprising polyvinyl alcohol (PVA), tin dioxide (SnO2), and chromium trioxide (Cr2O3) with respect to their synthesis, structural, electrical, and optical properties. Nanocomposite samples with flexibility were expeditiously synthesized using the cast method. Optical microscope (OM) images elucidated that an increase in the concentration of nanoparticles results in the formation of network pathways within the polymeric matrix, which function as charge carriers. FTIR analysis has demonstrated the existence of physical interactions between the (PVA) and the nanoparticles. The AC electrical characteristics of nanocomposites, specifically the dielectric constant and dielectric loss, exhibit a decrease as the frequency of the applied electric field increases. Conversely, an increase in concentration of SnO2/Cr2O3 nanoparticles results in an increase in these properties. AC electrical conductivity of the nanocomposites increases with both increasing frequency and concentration of SnO2/Cr2O3 NPs. Based on the analysis of its structural and electrical properties, it can be inferred that the PVA/SnO2/Cr2O3 nanocomposite exhibits potential for application in diverse electronic devices. The optical characteristics of PVA/SnO2/ Cr2O3 nanocomposites indicated that these materials exhibit greater absorbance in the ultraviolet region. This behavior suggests that the nanocomposites have potential for utilization in optoelectronics applications. The optical properties including absorbance, absorption coefficient, extinction coefficient, refractive index, real and imaginary dielectric constant, and optical conductivity, exhibit an upward trend with increasing concentrations of SnO2/Cr2O3 nanoparticles. At a concentration of 6 wt.%, the allowed and forbidden indirect transitions exhibit a reduction in their optical energy gap from 4.38 to 2.53 eV and from 3.67 to 1.4 eV, respectively. This nanostructured composition tested the first time for the optoelectrical applications. The results showed that the PVA/SnO2/Cr2O3 nanostructures can be considered as promising nanostructures for optics, photonics and electronics nanodevices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polymer nanocomposites were suggested as a viable option for a wide range of applications, including but not limited to car protection and developed optoelectronic devices. Comprehending the influence of the nanofillers on the physical characteristics of composites is imperative for the triumph of aforementioned applications (Naeimirad et al. 2016). As a result, numerous research teams are currently dedicated to formulating a comprehensive framework for forecasting, or at the very least, comprehending the interplay between the chemistry and morphological concepts of the matrix of polymers and the surface chemistry, size, and shape of a nanoscale filler in order to establish mechanical properties. The fundamental mechanisms underlying this framework are situated at the intersection of various disciplines, including chemistry, physics, materials science, and continuous mechanics. Hence, the scientists engaged in this crucial field of study exhibit a diverse range of backgrounds and methodologies (Habeeb 2011; Kim et al. 2006). Nanomaterials exhibit distinct physical characteristics in the field of nanotechnology as compared to conventional chemicals. Examples of such nanomaterials include silver Nano, carbon nanotubes, fullerenes, and carbon Nanos (Mahdi and Habeeb 2023; Hayder et al. 2020). Polyvinyl alcohol (PVA) exhibits promising characteristics such as elevated dielectric strength, favorable charge storage capacity, and electrical and optical properties that are contingent upon the dopant utilized. The molecule possesses a carbon chain as its backbone that is accompanied by hydroxyl groups that are affixed to the methane carbons. These hydroxyl groups have the potential to serve as a source of hydrogen bonding, thereby facilitating the creation of polymer complexes (Lee et al. 2004; Jebur et al. 2020). Poly(vinyl alcohol) (PVA) has garnered attention due to its favorable characteristics and diverse applications. The extensive practical applications of PVA resins, including their use in clothing and industrial fibers, adhesives and binders, films, membranes, drug delivery systems, and cancer cell-killing embolic materials, can be attributed to their exceptional chemical resistance, physical properties, and biocompatibility (Makhlouf et al. 2013; Habeeb et al. 2020a). The semiconductor Cr2O3 is considered intrinsic and exhibits conductance that remains unaffected by oxygen partial pressure when subjected to high temperatures (> 1273 K). However, at lower temperatures, the oxide behaves as an extrinsic p-type semiconductor. The utilization of nanoparticles of chromium oxide (Cr2O3) has garnered attention in various fields such as green pigments, thermal protection, wear resistance, chemical catalysts, and digital recording systems (Parthibavarman et al. 2010). Nanometric particles of SnO2 are a significant group of materials with technological relevance, exhibiting a diverse array of applications in gas sensors, transparent conducting electrodes, and transistors. The optical conductivity of SnO2 films has led to the expansion of its utilization in solar cells. Semiconductors in the form of nano crystals, such as SnO2, have been studied extensively (Habeeb and Mahdi 2019). In recent times, significant attention has been directed towards the synthesis of thin films or particles of SnO2 and the investigation of their unique properties. Several techniques, including chemical vapor deposition (CVD), magnetron sputtering, and thermal evaporation, have been employed (Gu et al. 2003). The technological advancements in stretchable and flexible-type organ electronic, optoelectronic, and energy harvesting storage devices have led to the establishment of polymer nanocomposites (PNCs) as multifunctional materials. The concentration of nanofillers has been found to have an impact on the structural, dielectric, and optical properties of PNCs, making them a smart choice for such technological applications (Habeeb and Hamza 2018). Wei, et al. (Wei and jinliang He et al. 2011) studied the influence of Cr2O3 on the microstructure, nonlinear J–E characteristics and surge current of SnO2-based varistor. They obtained the residual voltage ratio is 1.68, when the content of Cr2O3 is 0.03 mol%. Hu, et al. (Guangliang et al. xxxx) studied effect of Cr2O3 addition on the microstructure and electrical properties of SnO2. They found the highest nonlinear coefficient value of 27 and lowest leakage current density of 9 μA/cm2. Zang, et al. (Zang et al. 2014) studied dielectric properties of Cr2O3 doped SnO2–Zn2SnO4 composite. They obtained the dielectric properties increase with increasing of Cr2O3 content. Prashanth, et al. (Prashanth et al. xxxx) They found the addition of Cr2O3 nanoparticles creates the interfacial interaction between the surface of nanoparticles and PVA. Gao, et al. (Xin Gao et al. 2019) They found improvement the gas sensing performances for the Cr2O3 nanoparticles coated SnO2 nanfibers. The efficiency of composites’ production is significantly impacted by the usage of these nanoparticles, which enhance the chemical processes inside them. There have not been many researches, nevertheless, on how nanomaterials affect the performance characteristics of an enhanced composite. To our knowledge, no attempt has been made to synthesize PVA/SnO2/Cr2O nanocomposites.
2 Experimental part
(PVA/SnO2/Cr2O3) nanocomposites were created by dissolving 1gm of PVA in 30 ml of distilled water for 30 min under continuous stirring using a magnetic stirrer at 70 °C to achieve a more homogeneous solution. SnO2/Cr2O3 nanoparticles were added to the PVA in various ratio (0, 2, 4 and 6) wt.%. The structural characteristics of (PVA/SnO2/Cr2O3) nanocomposites tested by the optical microscope (OM) provided by Olympus (Top View, type Nikon-73346) and Fourier transformation infrared ray (FTIR) (Bruker company type vertex-70, German origin) with range wavenumber (500–4000) cm−1. The dielectric characteristics were studied in the range (f = 100 Hz to 5 × 106 Hz) by LCR meter (HIOKI 3532–50 LCR HI TESTER). The optical characteristics of (PVA/SnO2/Cr2O3) nanocompsites were tested using spectrophotometer (UV-1800 0A-Shimadzu).
The dielectric constant (ε′) of (PVA/SnO2/Cr2O3) nanocomposites is given by the following equation (Mohammed and Habeeb 2023a)
where, \(C_{p}\) is capacitance and Co is a vacuum capacitance.
The dielectric loss (ε˝) of (PVA/SnO2/Cr2O3) nanocomposites is given by following equation (Al-Sharifi and Habeeb 2023a)
where, D is the displacement.
The A.C electrical conductivity is determined by Mahdi and Habeeb (2022)
where,\(\omega\) is the angular frequency.
The absorption coefficient (α) is given as (Du et al. 2002)
where t: thickness of the samples, A: represent the optical absorbance. The energy gap is given by Habeeb and Abdul Hamza (2018)
where B is constant, hυ is the photon energy, Eg is the energy gap, m = 2 and 3 to indirect transition of allowed and forbidden.
The index of refractive (n) is defined by Habeeb et al. (2023)
Which R is the reflection, the coefficient of extinction (k) is determined by Chen et al. (2021)
Which λ is the wavelength. The dielectric constant parts: real (ε1), and imaginary (ε2) are given by Habeeb et al. 2020b
The optical conductivity (σop) is defined by Hadi (2021)
where c is velocity of light.
3 Results and discussion
The optical microscopy images presented in Fig. 1 depicts PVA/SnO2/Cr2O3 nanocomposites with various ratio of SnO2/Cr2O3 NPs, observed at a magnification power of 10x. Image A (PVA) exhibits remarkable miscibility, as evidenced by its refined structure and uniform, seamless surface. Conversely, images B, C, D, and E demonstrate that SnO2/Cr2O3 nanoparticles are evenly dispersed on the polymer’s surface. The images reveal that the SnO2/Cr2O3 nanoparticles have formed clusters in the shape of chains that extend along the surface of the films. This unique property of the SnO2/Cr2O3 nanoparticles (Rezvanpour et al. 2018), enhances not only charge transport within polymer matrices but also the percentage of SnO2/Cr2O3 nanoparticles.
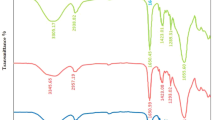
FTIR was employed to examine the atomic or ionic interactions within nanocomposites consisting of polyvinyl alcohol (PVA), tin dioxide (SnO2), and chromium trioxide (Cr2O3). The aforementioned interactions may entail alterations in the vibrational patterns exhibited by the nanocomposites. Figure 2 displays the transmission spectra of nanocomposite films composed of PVA, SnO2, and Cr2O3 nanoparticles, as measured by FTIR. The spectra correspond to films with varying ratios of SnO2/Cr2O3 nanoparticles, denoted as A, B, C, and D. (FTIR) technique provides insight into the nature of functional groups present in (PVA/SnO2/Cr2O) nanocomposites. The nanocomposites exhibit OH group frequencies at approximately 3259 cm − 1. The nanocomposites composed of PVA, SnO2, and Cr2O3 exhibit spectral peaks at 1084, 1416, 2908, and 3259 cm−1, which are associated with the C–O, C = C, and C–H functional groups present in the nanocomposites (Mahdi and M. A. Habeeb”, 2022a; Habeeb 2014).
The dielectric constant (έ) of (PVA/SnO2/Cr2O3) nanocomposites was determined using Eq. (1). The variation of dielectric constant with frequency for nanocomposites consisting of PVA, SnO2, and Cr2O3 is depicted in Fig. 3. The presented figure illustrates a negative correlation between frequency and dielectric constant values. As frequency increases, the polarization of space charge decreases, eventually leading to complete polarization. The primary form of polarization, as indicated is the polarization of the space charge at low frequencies (Bigg 1979).
The plot in Fig. 4 illustrates the relationship between the dielectric constant and the concentration of SnO2/Cr2O3 nanoparticles in the (PVA/SnO2/Cr2O3) nanocomposites, specifically at a frequency of 100 Hz. The augmentation of SnO2/Cr2O3 nanoparticles results in an associated rise in the dielectric constant of (PVA/ SnO2/Cr2O3) nanocomposites. The observed phenomenon can be attributed not only to the effects of atomic, ionic, and electronic influences, but also to the influence of space charge (Mahdi and M. A. Habeeb”, 2022b).
The calculation of the dielectric loss (ε″) of nanocomposites was performed using Eq. (2). The frequency-dependent dielectric loss of nanocomposites consisting of (PVA/ SnO2/Cr2O3) is illustrated in Fig. 5. The figure illustrates that the dielectric loss exhibits a notable increase at lower applied frequencies, while it exhibits a decrease as the applied frequencies increase. The aforementioned phenomenon can be ascribed to the fact that with an increase in frequency, the extent of the space charge polarization component diminishes, as stated in reference (Hadi and M. A. Habeeb”, 2021; Vidyalaya 2009).
The impact of SnO2/Cr2O3 nanoparticles ratio on the dielectric loss of PVA/ SnO2/Cr2O3 nanocomposites at a frequency of 100 Hz is illustrated in Fig. 6. The concentration in SnO2/Cr2O3 nanoparticles has a direct correlation with the dielectric loss of (PVA/SnO2/Cr2O3) nanocomposites, indicating a rise in the dielectric loss as the ratio of nanoparticles increases. The aforementioned increments are attributed to the rise in the quantity of charge carriers. in the case of (PVA/SnO2/Cr2O3) nanocomposites, it has been observed that at low ratio, the SnO2/Cr2O3 nanoparticles tend to aggregate and form clusters. The aforementioned study indicates that at a nanoparticle concentration of 6 wt. %, a path network is formed within the (PVA/SnO2/Cr2O3) nanocomposite (Abdul Hamza and Habeeb, 2023).
The electrical conductivity in alternating current (A.C) was computed using Eq. (3). Figure 7 illustrates the frequency-dependent alteration of A.C electrical conductivity in (PVA/SnO2/Cr2O3) nanocomposites at ambient temperature. The electrical conductivity under alternating current conditions exhibits a positive correlation with the frequency of the applied electric field. The observed phenomenon can be rationalized by the polarization effect and hopping mechanism. Specifically, the polarization effect arises due to the limited concentration of SnO2/Cr2O3, which is inadequate to establish an extensive network. Consequently, there is a polarization effect between these finite networks or clusters, and the electron hopping occurs between adjacent states that are randomly distributed within these finite networks (Hadi and M. A. Habeeb”, 2021).
The graphical representation depicted in Fig. 8 illustrates the fluctuation in A.C electrical conductivity of (PVA/SnO2/Cr2O3) nanocomposites in relation to the varying concentrations of SnO2/Cr2O3 nanoparticles, specifically at a frequency of 100 Hz. It can be observed from the figure that the alternating current electrical conductivity of the nanocomposites exhibits an increase with an increase in the concentrations of SnO2/Cr2O3 nanoparticles. The observed increase in conductivity can be attributed to the rise in the number of charge carriers resulting from the presence of dopant nanoparticles in the composition of the nanocomposite. This, in turn, leads to a gradual reduction in resistance and a subsequent increase in A.C electrical conductivity. Moreover, it has been observed that the SnO2/Cr2O3 nanoparticles establish a network of pathways within the nanocomposites, particularly when the nanoparticle concentration is at 6 wt.%, in the case of (PVA/SnO2/Cr2O3) nanocomposites (Hashim et al. 2020). The dielectric constant, dielectric loss, and A.C. electrical conductivity values of (PVA/SnO2/Cr2O3) nanocomposites at 100 Hz are presented in Table 1.
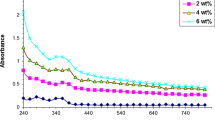
The graphical representation depicted in Fig. 9 illustrates the correlation between absorption and wavelength in (PVA/SnO2/Cr2O3) nanocomposites. The observed high absorption of all samples in the ultraviolet region can be due to the sufficient energy of photons to interact with atoms, leading to the excitation of electrons from lower to higher energy levels. The nanocomposite exhibits low absorption in the near-infrared region, which can be attributed to the limited energy of the incident photons that are insufficient for contact with the atoms (Habeeb and Kadhim 2014). The phenomenon of film absorbing exhibits a higher magnitude at shorter wavelengths, followed by a gradual decline as the wavelength increases. The phenomenon of absorption is observed to exhibit a positive correlation with the concentration of SnO2 and Cr2O3 nanoparticles, as well as with the corresponding increase in charge carriers. The observed phenomenon can be attributed to the density of the positional levels that arise from the presence of impurity atoms situated between the conduction and valence band. The absorption process takes place when electrons, which receive photons with an energy level lower than the optical energy gap of the incident photons, are transferred to auxiliary levels (Yagci et al. 2001).
The transmittance spectrum of films comprising (PVA/SnO2/Cr2O3) has been depicted in Fig. 10, as a function of the wavelength of incident light. The spectrum has been plotted for varying concentrations of (SnO2/Cr2O3) nanoparticles. As depicted in the graph, the increase in concentration of SnO2/Cr2O3 leads to a decrease in transmittance. The observed phenomenon can be attributed to the capacity of SnO2/Cr2O3 electrons in their valence shells to assimilate the electromagnetic energy of the incoming light and transition to greater energy states (Habeeb and Jaber, 2022). As a consequence of the occupied energy locations within each band by the travelling electron, the substance is capable of absorbing any incoming light, thereby precluding its transmission (Mahdi1 and Habeeb 2023).
Formula (4) is employed for the computation of the absorption coefficient. The difference of the absorption coefficient of (PVA/SnO2/Cr2O3) nanocomposites with respect to photon energy is illustrated in Fig. 11. The absorption coefficients exhibit a decrease at higher wavelengths and lower energies, as visually discernible. This suggests that the probability of electron displacement due to low energy photons (hν < Egoρ) is minimal, as stated in reference (Yu et al. 2001). Due to the pronounced absorption at elevated energies, there exists a greater probability for electron migration. The incident photon possesses adequate energy to facilitate the transition from the valence bands to the conduction bands, indicating that the energy gap is less than that of the photon. The present study demonstrates the impact of the absorption coefficient on the electron transition mechanism. Specifically, it is observed that at high energies, the absorption coefficient is expected to be significantly high, exceeding 104 cm−1. This indicates that direct electron transitions take place, with both electrons and photons conserving their energy and momentum. On the other hand, at low energies, the absorption coefficient is approximately 104 cm−1, indicating the occurrence of indirect transitions, where the electric momentum is strengthened by the phonons (Dwech et al. 2022). The nanocomposites comprising of PVA, SnO2, and Cr2O3 exhibit a coefficient of absorption that is less than 104 cm−1. This observation, along with other findings, indicates the occurrence of an indirect electron transition.
The extinction coefficient pertains to the degree of reduction in the intensity of an electromagnetic wave as it traverses a medium. The similarity between the attenuation coefficient curves and the absorption coefficient curves can be attributed to their inherent relationship in Eq. (7). The graphical representation in Fig. 12 illustrates the relationship between the extinction coefficient and the wavelength. It was observed from the figure that there is a positive correlation between the concentrations of SnO2/Cr2O3 nanoparticles and the values of extinction coefficient. The heightened absorbance of the prepared films can be attributed to the density of levels generated by the impurities present within the energy gap. The nanocomposites exhibit high extinction coefficients in the ultraviolet region owing to their significant absorbance characteristics. The extinction coefficient of nanocomposites exhibits an increase as the wavelength increases within the visible and near-infrared regions. This phenomenon can be attributed to the nearly constant absorption coefficient of the nanocomposites within these regions, as reported in reference (Mohammed and Habeeb 2023b).
The calculation of refractive index (n) is performed through the utilization of Eq. (6). The refractive index as a function of the wavelength is depicted in Fig. 13. The figure demonstrates a positive correlation between the SnO2/Cr2O3 nanoparticles and the refractive index. The rationale behind this phenomenon is that the introduction of doping agents results in the creation of additional energy levels through the inversion of the incident rays by a greater magnitude. This, in turn, leads to an augmentation in the intensity of the reflected rays, thereby elevating the refractive index. High values of the refractive index are observed in the ultraviolet (U.V) region owing to the limited transmittance (T) in this spectral range. Conversely, in the visible and near-infrared (IR) regions, the refractive index values are low due to the high transmittance (T) in these regions, as reported in reference (Al-Sharifi and Habeeb 2023b).
The calculation of the energy gap of nanocomposites is performed through the utilization of Eq. (5). It has been observed that the energy gap exhibited by nanocomposites is comparatively lower than that of pure polymers. Specifically, an increase in doping levels has been found to correspond with a decrease in energy gap. Figures 14, 15 depict the energy gaps pertaining to permissible and impermissible indirect transitions, respectively, as a function of the energy of the incident photon. The allowed energy gap can be obtained by drawing a straight line from the highest point of the curve towards the x-axis at (αhv)1/2 = 0. On the other hand, the forbidden energy gap can be determined when the value (αhν)1/3 = 0 is reached. The concentration of SnO2/Cr2O3 nanoparticles has been observed to have a decreasing effect on the energy gap values. This decrease is attributed to the doping process, which creates levels within the energy gap. The transition of electrons occurs in two stages, namely from the valence band to the local levels within the energy gap, and subsequently to the conduction band (Manhas et al. 2019). These results are agree with other researchers results (Mondal et al. 2023; Habeeb and Mohammed 2023).
The dielectric constant is a measure of a material’s polarizability in response to electromagnetic radiation. It is typically characterized by two distinct components: the real dielectric constant (ε1) and the imaginary dielectric constant (ε2). The aforementioned categories were determined through the utilization of Eqs. (8) and (9), correspondingly. The real and imaginary dielectric constant variations with wavelength for (PVA/SnO2/Cr2O3) nanocomposites are depicted in Figs. 16, 17, respectively. The presented data illustrates that the actual and imaginary dielectric constants exhibit an upward trend as the ratio of SnO2/Cr2O3 nanoparticles increases. This phenomenon can be due to the heightened electric polarization induced by the nanoparticles. The rise in electric polarization results in a higher density of dipoles, which in turn leads to an increase in the dielectric constant. The curves of the real dielectric constant depicted in Fig. 16 exhibit comparable behavior to the refractive index curves, owing to their interconnectedness. This similarity can be attributed to the fact that the real dielectric constant is contingent upon the values of (n) to a greater extent than the value of (k), given that the values of (k) are considerably smaller in comparison to the refractive index, particularly after being squared (Atta et al. 2021). The graph depicted in Fig. 17 demonstrates the variation of the imaginary dielectric constant with wavelength. It is observed that the imaginary component of the dielectric constant is influenced by the damping coefficient, particularly in the infrared and visible regions. In these regions, the refractive index remains relatively constant, while the extinction coefficients exhibit an increasing trend with increasing wavelength, as reported in reference (Habeeb and Rahdi 2023).
The formula (10) was utilized to compute the optical conductivity. The optical conductivity of the (PVA/SnO2/Cr2O3) nanocomposites is depicted in Fig. 18, exhibiting a wavelength-dependent trend. The presented figure illustrates a decline in optical conductivity as the wavelength raises across all nanocomposite specimens, while exhibiting an increase at lower wavelengths. The heightened optical conductivity observed at shorter photon wavelengths can be attributed to the heightened absorption of all nanocomposite samples in this spectral range. This, in turn, leads to a rise in charge transfer excitations. The spectra indicate a notable degree of transmittance in the visible and near-infrared regions. Additionally, it was observed that the optical conductivity escalates in tandem with the concentration of SnO2/Cr2O3 nanoparticles. This phenomenon can be attributed to the emergence of localized levels of energy inside the energy gap (Kumar et al. 2022).
4 Conclusion
In this work, the solution casting technique was used to prepare (PVA/SnO2/Cr2O3) NCs films. The optical microscope images revealed a uniform dispersion of the nanoparticles inside the polymeric matrix, and the emergence of network pathways as the concentration of SnO2/Cr2O3 nanoparticles increases. FTIR measures indicate a physical superposition between the polymer and the nanoparticles upon their mixture. The frequency-dependent behavior of (PVA/SnO2/Cr2O3) NCs is characterized by a decrease in the dielectric constant and dielectric loss, and an increase in A.C electrical conductivity. The concentration of SnO2/Cr2O3 nanoparticles has been observed to have a significant impact on the dielectric constant, dielectric loss, and A.C electrical conductivity of (PVA/SnO2/Cr2O3) NCs. Specifically, an increase in concentration results in a raise in the aforementioned properties. The dielectric constant increasing from 3 to 45, the dielectric loss increasing from 1 to 25, and the A.C electrical conductivity increasing from 5 × 10−11 to 1 × 10−9 S/cm. These characteristics can be applied to films in a variety of electrical applications. The optical properties of the (PVA/SnO2/Cr2O3) nanocomposite, including absorbance, absorption coefficient, extinction coefficient, refractive index, real and imaginary dielectric constants, and optical conductivity, exhibit an upward trend with an increase in the concentration of SnO2/Cr2O3 nanoparticles. Conversely, transmittance demonstrates a decreasing trend. At 8 wt.% concentration, the permitted and forbidden indirect transitions experienced a reduction in their optical energy gap, decreasing from 4.19 eV to 2.69 eV and from 3.5 eV to 1.45 eV, respectively. The decrease in the energy gap is observed with an increase in the concentrations of SnO2/Cr2O3 nanoparticles. The decrease in optical energy gap and increase in refractive index brought on by the inclusion of PVA/SnO2/Cr2O3 NCs point to the possibility of their usage in optoelectronic devices.
Data availability
Available.
References
Abdul Hamza, R.S., Habeeb, M.A.: Synthesis and tuning the structural, morphological and dielectric characteristics of PVA-CMC-SiO2–Cr2O3 hybrid nanostructures for nanoelectronics devices. Opt. Quant. Electr. 55(8), 705, 1–18 (2023). https://doi.org/10.1007/s11082-023-04995-3
Al-Sharifi, N.K., Habeeb, M.A.: Improvement structural and dielectric properties of PS/SiC/Sb2O3 nanostructures for nanoelectronicss devices. East European J. Phys. 2, 341–347 (2023a). https://doi.org/10.26565/2312-4334-2023-2-40
Al-Sharifi, N.K., Habeeb, M.A.: Synthesis and exploring structural and optical properties of ternary PS/SiC/Sb2O3 nanocomposites for optoelectronic and antimicrobial applications. SILICON, 1–11 (2023b). https://doi.org/10.1007/s12633-023-02418-2
Atta, A., Abdelhamied, M.M., Abdelreheem, A.M., Berber, M.R.: Flexible methyl cellulose/polyaniline/silver composite films with enhanced linear and nonlinear optical properties. Polymers (basel) 13(8), 1–16 (2021). https://doi.org/10.3390/polym13081225
Bigg, D.M.: Mechanical, thermal, and electrical properties of metal fiber-filled polymer composites. Polym. Eng. Sci. 19(16), 1188–1192 (1979). https://doi.org/10.1002/pen.760191610
Chen, N.F., Liao, Y.-H., Lin, P.-Y., Chen, W.-F., Wen, Z.-H., Hsieh, S.: Investigation of the characteristics and antibacterial activity of polymer-modified copper oxide nanoparticles. Int. J. Mol. Sci. 22(23), 12913, 1–17 (2021)
Du, H., Xu, G.Q., Chin, W.S., Huang, L., Ji, W.: Synthesis, characterization, and nonlinear optical properties of hybridized CdS-polystyrene nanocomposites. Chem. Mater. 14(10), 4473–4479 (2002). https://doi.org/10.1021/cm010622z
Dwech, M.H., Habeeb, M.A., Mohammed, A.H.: Fabrication and evaluation of optical characterstic of (PVA-MnO2–ZrO2) nanocomposites for nanodevices in optics and photonics. Ukr J Phys 67(10), 757–762 (2022). https://doi.org/10.15407/ujpe67.10.757
Gu, F., Wang, S.F., Song, C.F., Lü, M.K., Qi, Y.X., Zhou, G.J., Yuan, D.R.: Synthesis and luminescence properties of SnO2 nanoparticles. Chem. Phys. Lett. 372(3–4), 451–454 (2003). https://doi.org/10.1016/S0009-2614(03)00440-8
Habeeb, M.A.: Effect of rate of deposition on the optical parameters of GaAs films. Eur. J. Sci. Res. 57(3), 478–484 (2011)
Habeeb, M.A.: Dielectric and optical properties of (PVAc-PEG-Ber) biocomposites. J. Eng. Appl. Sci. 9(4), 102–108 (2014). https://doi.org/10.36478/jeasci.2014.102.108
Habeeb, M.A., Abdul Hamza, R.S.: Novel of (biopolymer blend-MgO) nanocomposites: fabrication and characterization for humidity sensors. J. Bionano. 12(3), 328–335 (2018). https://doi.org/10.1166/jbns.2018.1535
Habeeb, M.A., Hamza, R.S.A.: Synthesis of (polymer blend –MgO) nanocomposites and studying electrical properties for piezoelectric application. Indonesian J. Electr. Eng. Inform. 6(4), 428–435 (2018). https://doi.org/10.11591/ijeei.v6i1.511
Habeeb, M.A., Jaber, Z.S.: Enhancement of structural and optical properties of CMC/PAA blend by addition of zirconium carbide nanoparticles for optics and photonics applications. East European J. Phys. 4, 176–182 (2022). https://doi.org/10.26565/2312-4334-2022-4-18
Habeeb, M.A., Kadhim, W.K.: Study the optical properties of (PVA-PVAC-Ti) nanocomposites. J. Eng. Appl. Sci. 9(4), 109–113 (2014). https://doi.org/10.36478/jeasci.2014.109.113
Habeeb, M.A., Mahdi, W.S.: Characterization of (CMC-PVP-Fe2O3) nanocomposites for gamma shielding application. Int. J. Emerg. Trends Eng. Res. 7(9), 247–255 (2019). https://doi.org/10.30534/ijeter/2019/06792019
Habeeb, M.A., Mohammed, A.H.: Fabrication and tailored optical and electrical characteristics of Co2O3/SiC nanostructures doped PVA for multifunctional technological applications. Opt. Quant. Electron. 55(9), 791, 1–17 (2023). https://doi.org/10.1007/s11082-023-05061-8
Habeeb, M.A., Rahdi, W.H.: Titanium carbide nanoparticles filled PVA-PAAm nanocomposites, structural and electrical characteristics for application in energy storage. Opt. Quant. Electron. 55(4), 334, 1–18 (2023). https://doi.org/10.1007/s11082-023-04639-6
Habeeb, M.A., Hashim, A., Hayder, N.: Fabrication of (PS-Cr2O3/ZnCoFe2O4) nanocomposites and studying their dielectric and fluorescence properties for IR sensors. Egyptian J. Chem. 63, 709–717 (2020a)
Habeeb, M.A., Hashim, A., Hayder, N.: Structural and optical properties of novel (PS-Cr2O3/ZnCoFe2O4) nanocomposites for UV and microwave shielding Egyptian. J. Chem 63, 697–708 (2020b)
Habeeb, M.A., Jaber, Z.S., Radi, W.H.: Synthesis and characterization of (PVA-CoO-ZrO2) nanostructures for nanooptoelectronic fields. East European J. Phys. 2, 228–233 (2023). https://doi.org/10.26565/2312-4334-2023-2-25
Hadi, A.H., Habeeb, M.A.: (2021) The dielectric properties of (PVA-PVP-CdS) nanocomposites for gamma shielding applications. J. Phys.: Conf. Series 1973(1), 012063 (2021)
Hadi, A.H., Habeeb, M.A.: Effect of CdS nanoparticles on the optical properties of (PVA-PVP) blends. J Mech. Eng. Res. Develop. 44(3), 265–327 (2021)
Hashim, A., Habeeb, M.A., Jebur, Q.M.: Structural, dielectric and optical properties for (Polyvinyl alcohol-polyethylene oxide manganese oxide) nanocomposites”. Egyptian J. Chem. 63, 735–749 (2020). https://doi.org/10.21608/ejchem.2019.14849.1901
Hayder, N., Habeeb, M.A., Hashim, A.: Structural, optical and dielectric properties of (PS-In2O3/ZnCoFe2O4) nanocomposites. Egypt. J. Chem. 63, 577–592 (2020). https://doi.org/10.21608/ejchem.2019.14646.1887
Hu, G., Zhu, J., Yang, H., Wang, F.: Effect of Cr 2 O 3 addition on the microstructure and electrical properties of SnO 2-based varistor. J. Mater. Sci.: Mater Electr. 24, 1735–1740 (2013)
Jebur, Q.M., Hashim, A., Habeeb, M.A.: Structural, A.C electrical and optical properties of (polyvinyl alcohol-polyethylene oxide-aluminum oxide) nanocomposites for piezoelectric devices”. Egyptian J. Chem. 63, 719–734 (2020). https://doi.org/10.21608/ejchem.2019.14847.1900
Kim, D., Jeong, S., Moon, J.: Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology 17(16), 4019–4024 (2006). https://doi.org/10.1088/0957-4484/17/16/004
Kumar, U., Padalia, D., Lendvai, L., Bhandari, P., Kumar, P., Ranakoti, L., Singh, T.: Fabrication of Europium-doped barium titanate/polystyrene polymer nanocomposites using ultrasonication-assisted method: structural and optical properties. Polymers (basel), 1–19 (2022). https://doi.org/10.3390/polym14214664
Lee, J.S., Ha Choi, K., DoGhim, H., Soo Kim, S., Hwan Chun, D., Yong Kim, H., Seok Lyoo, W.: Role of molecular weight of atactic poly(vinyl alcohol) (PVA) in the structure and properties of PVA nanofabric prepared by electrospinning”. J. Appl. Polym. Sci. 93(4), 1638–1646 (2004). https://doi.org/10.1002/app.20602
Mahdi, S.M., Habeeb, M.A.: Fabrication and tailored structural and dielectric characteristics of (SrTiO3/ NiO ) nanostructure doped (PEO/PVA) polymeric blend for electronics fields. Phys. Chem. Solid State 23(4), 785–792 (2022). https://doi.org/10.15330/pcss.23.4.785-792
Mahdi, S.M., Habeeb, M.A.: Evaluation of the influence of SrTiO3 and CoO nanofillers on the structural and electrical polymer blend characteristics for electronic devices”. Digest J. Nanomater. Biostruct 17(3), 941–948 (2022a). https://doi.org/10.15251/DJNB.2022.173.941
Mahdi, S.M., Habeeb, M.A.: Synthesis and augmented optical characteristics of PEO–PVA–SrTiO3–NiO hybrid nanocomposites for optoelectronics and antibacterial applications”. Opt. Quant. Electr. 54(12), 854, 1–17 (2022b). https://doi.org/10.1007/s11082-022-04267-6
Mahdi, S.M., Habeeb, M.A.: Tailoring the structural and optical features of (PEO–PVA)/(SrTiO3–CoO) polymeric nanocomposites for optical and biological applications. Polym. Bull., 1–20 (2023). https://doi.org/10.1007/s00289-023-04676-x
Mahdi, S.M., Habeeb, M.A.: Low-cost piezoelectric sensors and gamma ray attenuation fabricated from novel polymeric nanocomposites. AIMS Mater. Sci. 10(2), 288–300 (2023). https://doi.org/10.3934/matersci.2023015
Makhlouf, S.A., Bakr, Z.H., Al-Attar, H., Moustafa, M.S.: Structural, morphological and electrical properties of Cr2O3 nanoparticles. Mater. Sci. Eng.: B Solid-State Mater. Adv. Technol 178(6), 337–343 (2013). https://doi.org/10.1016/j.mseb.2013.01.012
Manhas, S.S., Rehan, P., Kaur, A., Acharya, A.D., Sarwan, B.: Evaluation of optical properties of polypyrrole: Polystyrene nanocomposites. AIP Conf. Proc. 2100(4), 1–5 (2019). https://doi.org/10.1063/1.5098591
Mohammed, A.A., Habeeb, M.A.: Effect of Si3N4/TaC nanomaterials on the structural and electrical Characteristics of poly methyl methacrylate for electrical and electronics applications. East European J. Phys. 2, 157–164 (2023a). https://doi.org/10.26565/2312-4334-2023-2-15
Mohammed, A.A., Habeeb, M.A.: Modification and development of the structural, optical and antibacterial characteristics of PMMA/Si3N4/TaC nanostructures. SILICON, 1–12 (2023b). https://doi.org/10.1007/s12633-023-02426-2
Mondal, K.G., Jana, P.C., Saha, S.: Optical and structural properties of 2D transition metal dichalcogenides semiconductor MoS2”. Bull. Mater. Sci, 1–15 (2023). https://doi.org/10.1007/s12034-022-02852-9
Naeimirad, M., Zadhoush, A., Neisiany, R.E.: Fabrication and characterization of silicon carbide/epoxy nanocomposite using silicon carbide nanowhisker and nanoparticle reinforcements. J. Compos. Mater. 50(4), 435–446 (2016). https://doi.org/10.1177/0021998315576378
Parthibavarman, M., Hariharan, V., Sekar, C., Singh, V.N.: Effect of copper on structural, optical and electrochemical properties of SnO2 nanoparticles. J. Optoelectron. Adv. Mater. 12(9), 1894–1898 (2010)
Prashanth, K.S., Mahesh, S.S., Prakash, M.N., Munirathnamma, L.M., Ningaraju, S., Ravikumar, H.B., Nagabhushana, B.M.: Solution combustion synthesis of Cr2O3 nanoparticles and derived PVA/Cr2O3 nanocomposites-positron annihilation spectroscopic study. Mater. Today: Proceed. 3(10), 3646–3651 (2016)
Rezvanpour, M., Hasanzadeh, M., Azizi, D., Rezvanpour, A., Alizadeh, M.: Synthesis and characterization of micro-nanoencapsulated n-eicosane with PMMA shell as novel phase change materials for thermal energy storage. Mater. Chem. Phys. 215, 299–304 (2018). https://doi.org/10.1016/j.matchemphys.2018.05.044
Vidyalaya, K.M.: Analysis of Electrical Properties of Li 3 + ion Beam Irradiated Lexan Polycarbonate also act as catalyst to speed up the discoloration The formation of conjugated,. Asian J. Chem. 21(10), 43–46 (2009)
Wei, Q., He, J., Jun, H., Wang, Y.: Influence of Cr2O3 on the residual voltage ratio of SnO2-based varistor. J. American Ceram. Soc. 94(7), 1999–2002 (2011). https://doi.org/10.1111/j.1551-2916.2011.04620.x
Xin Gao, Qu., Zhou, Z.L., Lingna, Xu., Zhang, Q., Zeng, W.: Synthesis of Cr2O3 nanoparticle-coated SnO2 nanofibers and C2H2 sensing properties. Front. Mater. 6, 1–9 (2019). https://doi.org/10.3389/fmats.2019.00163
Yagci, Y., Yildirim, S., Onen, A.: A novel bifunctional addition-fragmentation agent for photoinitiated cationic polymerization. Macromol. Chem. Phys. 202(4), 527–531 (2001). https://doi.org/10.1002/1521-3935(20010201)202:4%3c527::AID-MACP527%3e3.0.CO;2-2
Yu, S.H., Yoshimura, M., Moreno, J.M.C., Fujiwara, T., Fujino, T., Teranishi, R.: In situ fabrication and optical properties of a novel polystyrene/semiconductor nanocomposite embedded with CdS nanowires by a soft solution processing route. Langmuir 17(5), 1700–1707 (2001). https://doi.org/10.1021/la000941p
Zang, G.-Z., Zhou, F.-Z., Cao, J.-X., Wang, X.-F., Wang, Z.-W., Li, L.-B., Li, G.-R.: Varistor and dielectric properties of Cr2O3 doped SnO2–Zn2SnO4 composite ceramics. Curr. Appl. Phys. 14(12), 1682–1686 (2014). https://doi.org/10.1016/j.cap.2014.07.002
Acknowledgements
Acknowledgments to University of Babylon
Funding
No funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MAH, NKAS and AAM. The first draft of the manuscript was written by MAH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Consent to participate
Consent.
Consent for publication
Consent.
Ethical approval
The Research is not involving the studies on human or their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Habeeb, M.A., Al-Sharifi, N.K. & Mohammed, A.A. Fabrication and exploring the structural, dielectric and optical features of PVA/SnO2/Cr2O3 nanostructures for optoelectronic applications. Opt Quant Electron 55, 1016 (2023). https://doi.org/10.1007/s11082-023-05261-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-05261-2