Abstract
A First-principles study was accomplish to examine the various characteristics of 2-D hetero-junction layer structure Molybdenum disulfide (MoS2) material such as structural, electronic, and optical properties through variation of external static pressure 0–100 GPa with a 4-steps escalations such as 0, 50, 75 100 GPa. Scheming bandgap tuning by the external static pressure engineering of 2-D layered MoS2 is a relatively new approach for tailoring different properties. The bandgap of MoS2 is found to be decreasing from 1.65 to 0.0 eV with increased external static pressure from 0 to 100 GPa. By increasing the external static pressure in MoS2 extra gamma states are produced into an energy band gap (Eg). The bandgap nature originated indirect moreover bandgap represented that material is an eminent semiconductor. MoS2 is a proficient photo-catalysis have a large surface area for light absorption and decreases the electron–hole pairs recombination rate and increases the charge transport. A significant decrement is found in the reflectivity due to the decrement in the bandgap. It is also examined that increment in energy adsorption peaks at 0 to 35 eV furthermore optical conductivity peaks shifts at larger energy band through increment the static pressure engineering. The optical properties significantly increased by the decrement of Eg. MoS2 has high energy absorption, optical conductivity, and refractive index and is an appropriate material for photocatalytic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Two-dimensional (2-D) MoS2 have a proficient structure and unique characteristics that can significantly play a role as photo-catalysts due to its volatile features. MoS2 sheets are not only used for high-quality 2-D photo-catalyst furthermore also used for a 2-D circuit board, with a smart potential to harness their perfect electrical characteristics(Tao et al. 2015). To enhance the photocatalytic activity of semiconductor photo-catalysts, there are three major points should be followed. Firstly the extension of excitation wavelength, secondly decrement of recombination of electron–hole pair, and lastly third point the increment of active sites around the surface edges for absorbance of the photon of light (Krishnan et al. 2019). The surface area can be increased by followed through strategies: Firstly by substitution with cations or anions secondly, by doping with metals and at third point by improving the structure of semiconductor photo-catalysts to enlarge their surface area or porosity to enhance the photocatalytic application. Owing to the astonishing characteristics, the researchers have newly paid attention to exploring Molybdenum disulfide (MoS2) as an efficient member for fundamental advances in the energy harvesting system, photocatalysis, aeronautics, and optoelectronic. Despite their pledge, in-depth experimental and theoretical studies of MoS2 are not explored systematically (Neupane and Adhikari 2020).
MoS2 is a proficient material for photocatalytic applications due to its electronic structure, optical conductivity, and bandgap can be tuned by external static 0, 50, 75, and 100 GPa. Due to the distinctive properties of the Molybdenum disulfide compound, they are more prominent for photocatalytic application. MoS2 is promising as a future ferroelectric applicant for photocatalytic activity due to its exceptionally high surface area, mobility of charge careers, and electronic and optical characteristics (Pan et al. 2010).
Among these MoS2 is a prominent material with immense potential to be used in wastewater treatment applications due to its efficient charge mobility, remarkably quantum confinement to direct band gap of 1.978 eV in single layer furthermore indirect band gap of 1.28 eV in multilayer. In the new decade, 2D potential materials such as WSe2, MoS2, WS2, and MoSe2 are attractive materials series as they have unique proficient, prominent properties such as TET, for photocatalytic application (Zhao et al. 2018). The bulk MoS2 has been recognized as a capable family of catalytic compounds for hydrogen (H2) evaluation. MoS2 shows a layered structure moreover with prominent covalent bonding with S-Mo-S atoms and weak Vander Waals contact among atomic structure crystals (Beqqali et al. 1997). Molybdenum disulfide compounds are also considered a prominent anode in lithium-ion batteries. These 2D materials are obtaining more interest than other oxides as lithium ions anode in lithium batteries. It is also examined that metal anion anode (M-X) bond polarity is one of the appropriate backbone reasons behind the polarization loss of these 2D compounds are less polar as compared to other metal sulfides (Mouhat and Coudert 2014).
2-D material Molybdenum disulfide (MoS2) architectures are also greatly attractive in the field of energy harvesting and environment. Photocatalysis is a good technique for photo-anode, dye degradation, water purification, and water splitting (Guan et al. 2017). 2-D material Molybdenum disulfide (MoS2) plays a significant role in the degradation of pharmaceuticals in industrial waste water due to its low bandgap, less toxicity, best dispersibility, etc. In the recent era, the dumping of pharmaceutical waste has become a significant concern worldwide. Separating various medications from wastewater effluents is often unsuccessful with convectional wastewater and biological treatments. However, pharmaceutical substances such as antibiotics, hormones, steroids, and other drugs can neither be separated from effluent nor be destroyed by biological treatment (Zan et al. 2015). As a result, heterogeneous photocatalysis has arisen as a promising strategy for reducing the adverse effects of industrial wastage. Sunlight-active materials used as catalysts for removing such pollutants are a realistic choice. Still, they produce harmful by-products, scale-up constraints, and difficulty with hetero-catalyst reduction and reuse keep them from being used in real-world applications(Agarwal and Talele 1985; Sikder et al. 2021).
Two-dimensional MoS2 exhibited tremendous potential applications for removing heavy metals, dyes, and organic compounds in pharmaceutical wastewater. Photocatalysis could be considered a reliable, low-cost, feasible, and efficient green method for removing persistent antibiotics in wastewater because it has shown promising results. 2D materials, MoS2 exhibit a narrow indirect band-gap at the G point of the Brillion zone. Additionally, depiction of the structural and optical characteristics of MoS2 but there is a gap of knowledge of their electronic, and optical properties with the effect of static external pressure. Precise details of the electronic characteristics of MoS2 2D materials are essential since it plays a proficient role in determining different materials' properties. There is no theoretical and experimental research on the structural electronic and optical behavior of two-dimensional MoS2 with external pressure tuning in the available literature. As a result, the primary aim of the present work is to provide some significant additional information to the presented data on electronic, structural, and optical properties of MoS2 using density functional theory through CASTEP software simulation. MoS2 has high optical conductivity and are more consistent and appropriate surface area material for photocatalytic applications. 2-D MoS2 is used to determine appropriate and efficient photocatalytic applications. The results indicate that there is a decrement trend in the energy band gap from 1.65 to 0.0 eV with an increase in external static pressure. MoS2 has high optical conductivity is more consistent and has an appropriate surface area it enhances photocatalytic activity. MoS2 has high optical conductivity as well as absorbance are more consistent and appropriate material for photocatalytic applications.
1.1 Computational methodology
The layered crystal of Molybdenum disulfide (MoS2) material with space group P63/MMC (No.194) (Agarwal and Talele 1985) was used in the CASTEP program moreover before the analysis of the characteristics the geometry is optimized. CASTEP (Cambridge Serial Total Energy Package) based on DFT, is executed on the accomplishment of our first-principles calculations. The bandgap and electronic exchange correlation have been deliberated using GGA (Generalized Gradient Approximation) and PBE (Perdew Burke-Ernzerhof) parameterization method is used. The USP (ultra-soft pseudo-potential) is used to investigate the electrostatic interaction between ionic core and valance electrons. The atomic electronic configuration of Molybdenum (Mo) and sulfide (S−2) is \({4d}^{5}\) \({5s}^{1}\) and \({1s}^{2}{2s}^{2}{2p}^{6}{3s}^{2}{3p}^{6}\) respectively. The novel ionic states creation is the result of nuclei interaction with the internal core and outer side electrons. The outermost electrons in the 2-D MoS2 and core interact, then the ions-electron potential converges. Brillion zone integration is simulated through customized K-points, furthermore, these K-points meshes build by 4 × 4 × 1 on the Monkhorst pack grid for optimization of the crystal structure. During the complete optimization, the total energy keeps constant at 1 × 10–5 eV. Moreover, the convergence assessment of applied forces to the MoS2 atoms is set aside at 0.02 eV/ Ǻ. The geometry is optimized through the CASTEP algorithm with external static pressure increased in different escalations 0 to 100 GPa.
2 Results and discussion
2.1 Structural analysis
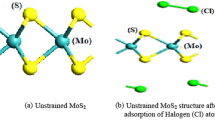
The super layer cell 4 × 4 × 1 of MoS2 is revealed in Fig. 1. The layer structure of MoS2 is with space group P63/MMC (No.194) (Agarwal and Talele 1985; Sikder et al. 2021). The layer 2D structure of MoS2 is oriented of Mo atoms with lattice parameters (a = 0.3333, b = 0.6667, c = 0.2500) and sulfur “S” with a = 0.3333, b = 0.6667and c = 0.6210 respectively. The energy of MoS2 of the overall crystal is maintained low level by estimating equilibrium lattice parameters with the help of the Murnagah energy state equation and results are obtained throughout a wide range of lattice parameter values. The total energy is a task of MoS2 unite volume of the equilibrium cell “Vo”. The decrement in lattice parameters values and angles with increasing pressure as result there is present a strong attraction between atoms. Furthermore, when the external static pressure rises from zero to 100 GPa, the supercell volume is 105.74 to 80. 75 Ǻ indicating that super-cell of 2-D material Molybdenum disulfide (MoS2) layer are prominent and remarkably shrink. There is no presented theoretical work with an external static high pressure. The energy formation of MoS2 material at 0 GPa is –1.978 eV which signifies that the material is stable.
2.2 Electronic band structures and density of states
The electronic band's structure shows the energy level whose electrons can exist (Energy band) as well as the energy level which is not convenient to occupy, there is the availability of an electron at zero (Band Gap). There are two types of the electron conduction band (CB) which is positioned at the exact Fermi level (EF) as well as VB (valance band) which is positioned below the EF.
Figure 2 shows the indirect band gap when the conduction band (CB) and valance band (VB) are not accurately aligned. In our current study, the direct band gap is reported at 0 GPa and 50 GPa, but an increase of external static pressure at 75 GPa and 100 GPa becomes indirect so, it is dependent on external static pressure. In Fig. 3 band structures are shown that are attained at different static pressures ranging from 0 GPa, 50 GPa, 75GPa, and 100 GPa. The minimum band gap of 0.0 eV is obtained at 100 GPa pressure. A decreasing trend in the band structures can be examined through increased external static pressure from 0 to 100 GPa as shown in Fig. 3. Due to a decrease in the lattice constants with increased pressure the CB moves beyond the Fermi level (EF) which is the main reason to reduction of the band gap of the MoS2 2D materials. The band gap structures revealed that 2D MoS2 layered material appears in semiconductors within indirect bandgap nature. These findings show that MoS2 is an appropriate 2D material for photocatalytic applications. The electronic band gap shows per unit energy is measured through TDS (total density of states) although ions contribution of different band structures is analyzed by PDS (partial density of states). The TDOS and PDOS are used to explicate the decrement in the band gap. Figure 3a–c shows pressure is further increased, its value tends to decrease and reaches the lowest value of 8.96 at 100 GPa. This characteristic peak shifts towards the right through the pressure is increased toward 100 GPa. The P state is prevailing above and below the Fermi level in PDOS. Moreover, in p states, the number of states increases as the pressure increases. As a result, p states show more prominent contributions than s and d states as shown in Fig. 4. By increasing the external static pressure s-p states hybridization increases, and as a result decrement in a band gap. These results indicate that MoS2 is an appropriate material for photocatalytic applications.
When external static pressure is implied the VB moves toward a lower energy level while the CB shifts towards the higher energy, which is the fundamental reason for the contraction of the band gap. With the help of all observations, we can conclude that the partial density of states shifts towards smaller and lower energies as a result of variation of pressures, leading to a decrement in the band gap, which also implied the optical properties described below.
2.3 Optical properties
The optical properties of MoS2 are intriguing and have significant applications in photocatalytic activity. The complex \(\varepsilon \left(\omega \right)\) dielectric parameters describe the response of 2D layered structured MoS2 to an electric field. It is divided into two components, real dielectric function (RDF) and imaginary dielectric function (IDF), and is dependent on the crystal's optical band structure.
The optical characteristics of 2D layered structured MOS2 may be explained with the help of electronic structure moreover relative permittivity, reflectivity, refractive index, coefficient of absorption as well as energy loss function. These Optical characteristics are calculated through GGA (Generalized Gradient Approximation) and PBE (Perdew Burke-Ernzerhof), furthermore these are very helpful in suggesting the viability and suitability of 2D layered structured MOS2 material in photocatalytic applications. All of the optical characteristics are the consequence of the interaction of 2D layered structured MOS2 material and waves (electromagnetic waves). All these characteristics are interlinked with one another, and the complex dielectric function is utilized which is given by Ali et al. (2016); Hoseinzadeh et al. 2018).
To study the optical response to the effect of external static pressure effect of RDF \(\left( {\varepsilon_{1} \omega } \right)\) and IDF \(\varepsilon_{2} \left( \omega \right)\) of the dielectric function \(\varepsilon \left( \omega \right)\) can be calculated through the following Eqs. (2) and (3) (Williamson et al.xxxxLi et al. 2014; Jameel et al. 2021a).
The dielectric constants RDF \(\left( {\varepsilon_{1} \omega } \right)\) and IDF \(\varepsilon_{2} \left( \omega \right)\) presents the relative permittivity of the 2D layered structured MOS2 materials. If we take the term dielectric literally, it shows how much an electric field is permitted to go through 2D-MoS2 material at different external static pressure. This mainly shows how much polarization 2D-MoS2 material can be tolerated at different external static pressure. As a consequence, an ideal electrical conductor should have zero value because no field can be present within conductor boundaries. The RDF \(\left({\varepsilon }_{1}\omega \right)\) gives information regarding the polarization intensity of MoS2 material at different external static pressure 0–100 GPa. The main peak of dielectric function at 0 GPa appears at 4.28 eV and 100 GPa at 27.5 eV. It seems to decrease towards a lower value of 7.5 eV when external static was maintained at 0, 50, 75, and 100 GPa respectively. The optical properties are related to energy dissipation and are influenced by IDF \({\varepsilon }_{2}\left(\omega \right)\) function. The imaginary dielectric function (IDF) can be shown in Fig. 5b. As the pressure is gradually increased, imaginary dielectric function (IDF) peaks shift slightly to the lower value, and energy also shifts towards higher values. All peaks slightly move toward higher energy when pressure increases. These dielectric characteristics results indicate that 2-D MoS2 is an appropriate material for photocatalytic applications.
2.4 Refractive index and extinction
By carefully examining the extinction co-efficient k(w) and refractive index n(w) of 2D-MoS2 transition material the absorbance of electromagnetic radiations and the prediction of optical transparency, can be shown in Fig. 6a–b. The behavior of refractive index n(w) and extinction coefficient k(w) in the region of 0–60 eV with an external static pressure of 2D layered transition MoS2 material.
At 0 eV 2D layered structured MoS2 has a refractive index (n) of 2.1, 2.9, 4.2, and 4.9 with 0 -100 GPa external static pressure correspondingly. The refractive move toward decrement significantly with energy eV up to 5 eV. The refractive index (n) fluctuates from 0 to 5 in the range of energy 0 to 40 eV outstanding to the diverse frequencies of the inner-transition band. The lower refractive index shows smaller polarization in the eminent energy range. The following equations are used to calculate the additional optical factors such as reflectivity, absorption, refractive index, and energy loss (Jameel et al. 2021b; Li et al. 2012).
2.5 Absorbance and reflectivity
The absorption of any material is efficiently linked to the ability of absorbance luminescent electromagnetic radiation in disparity to photons with appropriate energy (E = \(\hbar \omega\)), moreover, the energy loss function L(\(\omega\)) describes the energy dissipation when the material comes into range with incident photons. The absorbance of 2D layered structured MoS2 is increasing by increasing external static pressure (0–100 GPa) as shown in Fig. 7a. The photon energy increase through increasing the external static pressure, and the absorption coefficient of MoS2 rises, achieving 4 × 105 \({\mathrm{cm}}^{-1}\) at 12 eV with 100 GPa. The results indicate that 2D layered MoS2 has high optical conductivity as well as absorbance and is a more consistent and appropriate material for photocatalytic applications.
2.6 Optical conductivity and reflectivity
Optical conductivity explains the conductance of photo-generated electrons by the photoelectric phenomenon. Electromagnetic radiation EM is used to break the bonding of particles. Figure 8a–d the optical conductance of 2D layered structured MoS2 at 0 −100GPa in the range of 0–60 eV. The real peaks of optical conductance \(\sigma 1(w)\) originate from the origin point and touch its maximum conductivity values of 1.5 cm−1, 2.8 cm−1, 6 cm−1, and 7 cm−1 at the energy range 15–35 eV with 0,50, 75, and 100GPa external pressure. The real optical conductivity of 2D layered structured MoS2 initially decreases from the starting zero point and at 5 eV suddenly increasing till 20 eV.
On the other hand, imaginary optical conductivity \(\sigma 2(w)\) shows the largest value at 5 eV 2.8 cm−1, 4.1 cm−1, 9 cm−1, 14 cm−1 for external static pressure 0, 50,75, 100GPa. The optical conductivity is increasing with increased external static pressure. These results of optical conductivity indicate that 2D layered structured MoS2 is appropriate material for photocatalytic applications.
The reflectivity of any material can be used to examine its surface behavior. The reflectivity surface behavior of 2D layered structured MoS2 material at different pressure at 0 -100 GPa is shown in Fig. 7. At 0 eV the value of reflectivity for MoS2 material 0.1 at 0 GPa, 0.22 at 50 GPa, 0.9 at 70 GPa, and 0.7 at 100 GPa respectively. These values show that the reflectivity peak slightly shifts toward the higher values of frequency as the pressure is increasing. The reflectance falls rapidly to 0.9 at 22 eV as the frequency increase. These results of reflectivity indicate that 2D layered structured MoS2 is appropriate material for photocatalytic applications.
2.7 Photocatalytic mechanism
A schematic diagram shows that the sunlight conciliates photo-degradation application of layered structured MoS2 is shown in Fig. 9. Layered structured MoS2 with a large surface area absorbs the waste material more proficiently through active surface sites as Eq. (14). Under the sunlight, revelation layered structured MoS2 electrons and holes in the conduction (C.B) and valance band (V.B) correspondingly as Eq. (15). Organic particle becomes excited in the exposure of sunlight it moves from HOMO toward LUMO of pollutant level. Furthermore, electrons are moved from the valance to the conduction band of layered structured MoS2 and increase electron density with an increase in the external static pressure0 -100 GPa. The photo-captured electrons are positioned in the conduction level of layered structured MoS2 renovating the oxygen molecule into super-oxide \({\dot{}\mathrm{O}}_{2}^{ -}\) radical as Eq. (16). At a similar moment, \(O\dot{H}\) hydroxyl radical produced the holes in the valance level through water splitting molecules as Eq. (17, 18). Both superoxide and hydroxyl (unsaturated form) changed the pollutant into CO2 and H2O as Eq. (19). Underexposure of sunlight photocatalytic activity of organic waste pollutants by layered structured MoS2 is given below (Whitfield Reaves and Bong 2010; Ali et al. 2021; Saleem 2022; Lahourpour et al. 2019).
The computed results show that the visible, visible active sites of the 2-D Heterojunction layer structure Molybdenum disulfide (MoS2) may be proficient to make excellent photocatalytic activity.
3 Conclusion
A First-principles study was consummate to examine the various characteristics of 2-D Heterojunction layer structure Molybdenum disulfide (MoS2) materials such as structural, electronic, and optical properties through variation of external static pressure 0–100 GPa with a 4-steps escalation such as 0, 50, 75 100 GPa. Scheming bandgap tuning by the external static pressure engineering of 2-D layered MoS2 is a relatively new approach for tailoring different properties. Owing to the increasing interest in photocatalytic activity MoS2 is a remarkable and prominent 2D layered structure material with unique characteristics. The bandgap of MoS2 is found to be decreasing from 1.65 to 0.0 eV with increased external static pressure from 0 to 100 GPa. By increasing the external static pressure in MoS2 extra gamma states are produced into an energy band gap (Eg). The bandgap nature originated indirect moreover bandgap represented that material is an eminent semiconductor. MoS2 is a proficient photo-catalysis have a large surface area for light absorption and decreases the electron–hole pairs recombination rate and increases the charge transport. A significant decrement is found in the reflectivity due to the decrement in the bandgap. It is also examined that increment in energy adsorption peaks at 0 to 35 eV furthermore optical conductivity peaks shifts at larger energy band through increment the static pressure engineering. The optical properties significantly increased by the decrement of Eg. MoS2 has high energy absorption, optical conductivity, and refractive index and is an appropriate material for photocatalytic applications.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Agarwal, M.K., Talele, L.T.: Growth conditions and structural characterization of molybdenum sulphoselenide single crystals:(MoSxSe2− x, 0≦ x≦ 2). Mater. Res. Bull. 20(3), 329–336 (1985)
Ali, A., et al.: The effect of Ca dopant on the electrical and dielectric properties of BaTi4O9 sintered ceramics. Materials (basel) 14(18), 1–11 (2021). https://doi.org/10.3390/ma14185375
Ali, F., Saqib, M., Khan, I., Ahmad Sheikh, N.: Application of Caputo-Fabrizio derivatives to MHD free convection flow of generalized Walters’-B fluid model. Eur. Phys. J. Plus 131, 377 (2016). https://doi.org/10.1140/epjp/i2016-16377-x
El Beqqali, O., et al.: Electrical properties of molybdenum disulfide MoS2. Experimental study and density functional calculation results. Synth. Met. 90(3), 165–172 (1997). https://doi.org/10.1016/s0379-6779(98)80002-7
Guan, Z., Lian, C.S., Hu, S., Ni, S., Li, J., Duan, W.: Tunable structural, electronic, and optical properties of layered two-dimensional c2n and mos2 van der waals heterostructure as photovoltaic material. J. Phys. Chem. C 121(6), 3654–3660 (2017). https://doi.org/10.1021/acs.jpcc.6b12681
Hoseinzadeh, T., et al.: Microstructure, fractal geometry and dye-sensitized solar cells performance of CdS/TiO2 nanostructures. J. Electroanal. Chem. 830–831, 80–87 (2018). https://doi.org/10.1016/j.jelechem.2018.10.037
Jameel, M.H., et al.: First principal calculations of electronic, optical and magnetic properties of cubic K1-xYxNbO3(Y = Fe, Ni)”. Phys. Scr. 96, 125839 (2021). https://doi.org/10.1088/1402-4896/ac198d
Jameel, M.H., et al.: First principal calculations to investigate structural, electronic, optical, and magnetic properties of Fe3O4 and Cd-doped Fe2O4”. Comput. Condens. Matter 30, e00629 (2022). https://doi.org/10.1016/j.cocom.2021.e00629
Krishnan, U., Kaur, M., Singh, K., Kumar, M., Kumar, A.: A synoptic review of MoS 2: synthesis to applications. Superlattices Microstruct. 128, 274–297 (2019). https://doi.org/10.1016/j.spmi.2019.02.005
Lahourpour, F., Boochani, A., Parhizgar, S.S., Elahi, S.M.: Structural, electronic and optical properties of graphene-like nano-layers MoX2(X:S, Se, Te): DFT study. J. Theor. Appl. Phys. 13(3), 191–201 (2019). https://doi.org/10.1007/s40094-019-0333-4
Li, H., et al.: From bulk to monolayer MoS 2: evolution of Raman scattering. Adv. Funct. Mater. 22(7), 1385–1390 (2012). https://doi.org/10.1002/adfm.201102111
Li, H., Wu, J., Yin, Z., Zhang, H.: Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 47(4), 1067–1075 (2014). https://doi.org/10.1021/ar4002312
Mouhat, F., Coudert, F.X.: Necessary and sufficient elastic stability conditions in various crystal systems. Phys Rev. B - Condens. Matter Mater. Phys. 90, 4–7 (2014). https://doi.org/10.1103/PhysRevB.90.224104
Neupane, H.K., Adhikari, N.P.: Structure, electronic and magnetic properties of 2D Graphene-Molybdenum diSulphide (G-MoS2) Heterostructure (HS) with vacancy defects at Mo sites. Comput. Condens. Matter 24, e00489 (2020). https://doi.org/10.1016/j.cocom.2020.e00489
Pan, F., Chen, C., Wang, Z.S., Yang, Y.C., Yang, J., Zeng, F.: Nonvolatile resistive switching memories-characteristics, mechanisms and challenges. Prog. Nat. Sci. Mater. Int. 20(1), 1–15 (2010). https://doi.org/10.1016/s1002-0071(12)60001-x
Saleem, S., et al.: Modification in structural, optical, morphological, and electrical properties of zinc oxide (ZnO) nanoparticles (NPs) by metal (Ni, Co) dopants for electronic device applications”. Arab. J. Chem. 15, 103518 (2022). https://doi.org/10.1016/j.arabjc.2021.103518
Sikder, M.A.M., Chakma, U., Kumer, A., Islam, M.J., Habib, A., Alam, M.: The exploration of structural. Electron. Opt. Properties DFT Func First Principle Approach. 1, 103–113 (2021)
Tao, L., Duan, X., Wang, C., Duan, X., Wang, S.: Plasma-engineered MoS2 thin-film as an efficient electrocatalyst for hydrogen evolution reaction. Chem. Commun. 51(35), 7470–7473 (2015). https://doi.org/10.1039/c5cc01981h
Whitfield Reaves, B., Bong, C.: Shoulder pain: the supraspinatous muscle, Part 2. Acupunct. Today 11, 1–20 (2010)
Williamson, I., et al., “Structural , electrical , phonon , and optical properties of Ti- and V-doped two-.”
Zan, W., Geng, W., Liu, H., Yao, X.: Influence of interface structures on the properties of molybdenum disulfide/graphene composites: a density functional theory study. J. Alloys Compd. 649, 961–967 (2015). https://doi.org/10.1016/j.jallcom.2015.05.149
Zhao, W., et al.: Metastable MoS2: crystal structure, electronic band structure, synthetic approach and intriguing physical properties. Chem. - A Eur. J. 24(60), 15942–15954 (2018). https://doi.org/10.1002/chem.201801018
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number (L.R.G.P2/74/44). This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R1), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Large Groups Project under grant number (L.R.G.P2/74/44). This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R1), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
MHJ designed the computational model framework and analyzed the data. MHJ performed the all calculations and wrote the first draft manuscript. MABA suggested a computational model framework, and MSbR, TA, AB commented on the manuscript and review it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jameel, M.H., Roslan, M.S.b., Agam, M.A.B. et al. Effect of external static pressure on structural, electronic, and optical properties of 2-D hetero-junction MoS2 for a photocatalytic applications: A DFT study. Opt Quant Electron 55, 619 (2023). https://doi.org/10.1007/s11082-023-04853-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-04853-2