Abstract
The linear and nonlinear optical properties of polymer/inorganic nanocomposites have received a great interest because of their potential application such as optical limiting devices. A flexible foil like polymer/ZnO nanocomposites and polymer/ZnO/CuO nanocomposites have been prepared via casting method. ZnO and CuO nanoparticles were used as filler, while four different types of polymer were used as polymer matrix. The purity and composition of the nanocomposites were confirmed via EDX analysis and EDS mapping. Surface morphology of samples was tested by FESEM that were shown the dispersion of ZnO and CuO nanoparticles successfully. To study the influence of adding CuO nanoparticles on polymer/ZnO nanocomposites; the liner transmittance was measured and linear absorption coefficient was calculated. The results show a decrease in linear transmittance and increase in linear absorption coefficient when CuO nanoparticles was added. Then, the absorption coefficient and refractive index of the as-prepared sample were analysed using an open and closed aperture single beam Z-scan technique via Q-switched Nd-YAG pulse laser at 532 nm. The nonlinear refractive index was in the order of 10−12 cm2/W with a negative sign whereas the nonlinear absorption coefficient was in the order of 10−7 cm/W. The real part, imaginary part and the absolute value of the third order nonlinear optical susceptibility χ(3) were calculated. The χ(3) was in the order of 10−6 esu. The effect of adding CuO nanoparticles to nanocomposites was enhanced their nonlinear optical properties. Consequently, a good optical limiting was obtained. The optical limiting threshold of the samples was measured. The results showed that the prepared nanocomposites can be considered as an excellent candidate for optical limiting devices, which clearly affected by the adding CuO nanoparticles and the type of polymer matrix. Nanocomposites PMMA/ZnO/CuO and PS/ZnO/CuO showed the low optical limiting threshold, which were equal to 60 and 50 Mw/cm2, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, polymer inorganic nanocomposites have focused by significant scientific and technological interest. Those nanocomposites are a sort of composite materials comprising of inorganic nanoparticles, which are dispersed in a polymer matrix uniformly. Both properties of inorganic nanoparticles and polymer will be changed when inorganic nanoparticles are added in a polymer matrix. Thus, it will be enhanced, and advanced new functions to the nanocomposite (Li et al. 2010). A single material with novel properties can be obtained by successfully joining materials of different characteristics to prepare nanocomposites. Consequently, it can be used to provide high-performance novel materials and can be applied in many scientific fields. The optical properties of nanocomposites have received much attention because they are different from individual polymers. In addition, they offer unique properties which greatly differ from that of conventional materials (Indolia and Gaur 2013).
Zinc oxide (ZnO) is one of the groups of II–VI semiconductors. It is the most attractive semiconductor due to the unique combination of electrical and optical properties, and important features like biocompatibility, long-term environmental stability, non-toxicity and low cost (Jeeju et al. 2012). The future where ZnO devices become part of our daily lives is already approaching (Irimpan 2008). ZnO has been added as fillers in many polymers, such as PMMA, PS, PVDF, PVA, PVC and PC (Indolia and Gaur 2013; Jeeju et al. 2012; Chen et al. 2003; Sangawar and Golchha 2013; El-Kader et al. 2013; Al-Taa et al. 2014; Al Jaafari and Ayesh 2011).
Copper oxide (CuO) can be defined as a p-type semiconductor oxide with a narrow band gap of about 1.2–1.4 eV at room temperature. CuO has a monoclinic crystal structure. The copper atom is surrounded by four oxygen atoms in an approximately square planar design (Al-Gaashani 2012). It has been attracting attention due to its multipurpose applications in optoelectronic devices which are effective at ultraviolet and blue regions. Its excitation binding energy is very large (60 meV) at room temperature. It has two absorption peaks at 391 and 485 nm (Jundale et al. 2013). CuO nanoparticles showed different peak absorption at 370–391 nm depending on the particle size; i.e., they exhibit blue shift at the absorption peaks with decreasing of nanoparticle size (Dagher et al. 2014).
The literature revealed that doping another nanoparticles material modified the ZnO nanoparticles (Li et al. 2010).
Ando et al. (1995), Chen et al. (2009), and Shahmiri et al. (2013) stated that CuO nanoparticles have been showing high optical nonlinearity, χ(3).
Chen et al. (2013) and Anand et al. (2014) mentioned that CuO nanoparticles film was found to be a good optical limiter under high repetition rate laser with ultrafast pulse duration.
Optical limiter is one of the most important types of devices used to control the amplitude of high light intensity. For an ideal optical limiting material, high transmission is observed at normal light, whereas low transmission is detected at intense light. So, it is used as a protection for human eyes and optical detectors. The self-defocusing in conjunction with the nonlinear absorption process in semiconductors will enhance the optical limiting performance (Aleali and Mansour 2010).
In spite of the many studies that had been conducted, there is a need to enhance the understanding of all photonics technologies and contribute to the knowledge pertaining to the suitability of using nonlinear optical materials as optical limiter, especially the common usage of optical detectors, and sensors for different scientific purposes (Jeeju et al. 2012). The optical limiting devices are needed to protect the photosensitive components from high intensity laser radiation.
The importance of studying linear and nonlinear optical properties is consider as an opportunity to determine and choose the nanoparticles of materials and nanocomposites in which the linear and nonlinear optical properties are known so it can be used in optoelectronics applications. This work is considered as a contribution to fill the lack of nonlinear optical studies on polymer–ZnO and polymer–ZnO–CuO nanocomposites.
In this work, flexible foil like polymer/ZnO and polymer/ZnO/CuO nanocomposites with different polymer matrix; PMMA, PVDF, PVA, and PS are prepared. For as-prepared samples, the linear transmittance is measured and the linear absorption coefficient is calculated. Besides, the nonlinear optical properties such as nonlinear refractive index and nonlinear absorption coefficient are determined using Z-scan technique, then the third order nonlinearity is calculated. As well as the optical limiting property is explored using transmittance technique. To the best of the author’s knowledge, the preparation of polymer/ZnO/CuO nanocomposites and study their linear and nonlinear optical properties are considered to be the first work.
2 Method and materials
Four different types of polymers were used as polymer matrix; poly (methyl methacrylate) (PMMA), poly (vinylidene fluoride) (PVDF) polyvinyl alcohol (PVA), and polystyrene (PS). All of them were supplied by Sigma-Aldrich.
Zinc Oxide (ZnO) was purchased from Sigma-Aldrich. It was used as a filler in polymer/ZnO and polymer/ZnO/CuO nanocomposites. The size of the nanoparticles was 50 < size < 100 nm. This particle size was chosen because nonlinear optical properties are enhanced as the particle size of nanoparticles that are used as fillers in nanocomposite increases (Jeeju et al. 2012; Sreeja et al. 2010; Haripadmam et al. 2012).
Copper oxide (CuO) was purchased from Sigma-Aldrich; the size of the nanoparticles was 25 < size < 50 nm. It was added to polymer/ZnO nanocomposites to enhance the linear and nonlinear optical properties of the samples. The ZnO nanoparticles were also modified to avoid aggregation and agglomeration that may happen during their dispersion in the polymer matrix.
To prepare polymer/ZnO nanocomposites one of the four polymers (PMMA, PVDF, PVA, and PS) was used as the polymer matrix and ZnO nanoparticles were used as fillers. Then CuO nanoparticles was added to each one of four polymer/ZnO nanocomposites to prepare polymer/ZnO/CuO nanocomposites.
3 Preparation of polymer/ZnO nanocomposites
Nanocomposites PMMA/ZnO were prepared in two steps; firstly, the PMMA solution was prepared by adding chloroform (CHCl3) to the (PMMA). Secondly, Zinc oxide (ZnO) with concentrations of 10 wt% was added to the mixture of PMMA/chloroform. The procedures of preparation was maintained in our previous literature (Shanshool et al. 2016).
PVDF/ZnO nanocomposites were prepared using the same procedures applied for the preparation of PMMA/ZnO nanocomposites. PVDF was dissolved in DMF as solvent using a magnetic stirrer (angular velocity of 400 rpm and time duration of 2 h at 60 °C). Zinc oxide (ZnO) with concentrations 8 wt% were added to the mixture of PVDF/DMF. Then a sonicator was used for 15 min to disperse the nanoparticles in the solution. After that, the solution was stirred at room temperature for 2 h by a magnetic stirrer (angular velocity 400 rpm) at room temperature to get a homogeneous solution.
PVA/ZnO nanocomposites was prepared by the same procedures used to prepare PMMA/ZnO nanocomposites. Firstly, PVA solution was prepared by adding distilled water (H2O) to the PVA. A magnetic stirrer (angular velocity of 400 rpm and duration time 2 h at 70 °C) was used to help it dissolve. Secondly, zinc oxide (ZnO) with concentrations of 10 wt% were added to the mixture of PVA/water.
PS/ZnO nanocomposite was prepared by applying the same procedures used to prepare PMMA/ZnO nanocomposites. Firstly, PS was dissolved in toluene to prepare PS solution. Then it was stirred using a magnetic stirrer (angular velocity of 400 rpm and time duration of 2 h at 60 °C). Secondly, zinc oxide (ZnO) with concentrations of 10 wt% was added to the mixture of PS/toluene. The same steps which used after adding ZnO nanoparticles to mixture of PMMA/Chloroform was followed when adding ZnO nanoparticles to PVA/water and PS/toluene.
Casting technique was used to prepare foils like PMMA/ZnO, PVDF/ZnO, PVA/ZnO, and PS/ZnO. PMMA/ZnO solution was casted uniformly on a glass petri dish and each one of three other solutions were casted uniformly on a ceramic petri dish at room temperature. After few hours the film was pulled out easily as flexible foil. Next, the foil was kept at room temperature for one day for PMMA/ZnO and at 60 °C for one day for PVDF/ZnO, PVA/ZnO, and PS/ZnO to solidify. Later, the foils like polymer/ZnO of four different types of polymer matrix were collected.
4 Preparation of polymer/ZnO/CuO nanocomposites
Copper oxide (CuO) nanoparticles were added to the polymer/ZnO nanocomposites. The PMMA-ZnO, PS/ZnO and PVA/ZnO nanocomposites with 10% of ZnO, and PVDF/ZnO with 8% of ZnO were chosen to be added 1% CuO. These nanocomposites with ZnO and CuO concentrations were prepared as the requirements of the study because they have suitable transmittance that can be used in the Z-scan technique with a laser source at a wavelength of 532 nm. After preparing polymer–ZnO nanocomposites as mentioned above, CuO nanoparticles (1 wt%) were added to these nanocomposites. A sonicator was used for 15 min to disperse the CuO nanoparticles in the solution. After that, the solution (polymer/ZnO/CuO) was stirred at room temperature for 2 h using a magnetic stirrer (angular velocity of 400 rpm) to get a homogeneous solution. By using casting technique, each one of the solutions (PVDF/ZnO/CuO, PVA/ZnO/CuO and PS/ZnO/CuO) was casted uniformly on a ceramic dish at room temperature, whereas PMMA/ZnO/CuO was casted on a glass dish using the same procedure. Then, the first three nanocomposites were kept in the oven at 60 °C for 1 day, while PMMA/ZnO/CuO samples were kept at room temperature for one day. After that, the film was pulled out easily from the substrate as a flexible foil.
5 Characterization of the samples
UV–Vis spectrophotometer (PerkinElmer instruments-Lambda 900 UV/VIS Spectrometer) was used to measure the linear transmittance and reflectance spectra of the as-prepared samples. The reflectance was measured in a separate parameter using UV–Vis spectroscopy itself which has the features to measure the R values.
The surface morphologies of the as-prepared nanocomposites were characterized using field emission scanning electron microscope (FESEM, Carl Zeiss, Merlin Compact, Oxford Instrument, 55VP) with accelerating voltage of 3.0 kV. Prior to the analysis, the samples were coated with a fine layer of platinum in order to reduce the charging effect.
Energy dispersive X-ray (EDX) was used to analyze the compositional and purity of the as-prepared nanocomposites materials. An EDS mapping is an image used to show the spatial distribution of elements in as-prepared samples. EDX analysis and EDS mapping were obtained from the plug in the analysis of FESEM.
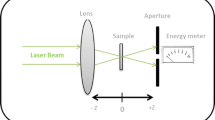
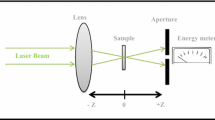
The setup of single beam Z-scan technique is shown in Fig. 1. A Q-switched Nd: YAG pulse laser, (Beijing Mini laser Technology Co., Ltd.) which gives second harmonic at 532 nm (7 ns, 5 Hz) was used as the light source. A 10 cm lens was used to focus the laser beam. The transmitting light energy in the far field, which passed through the aperture, was recorded by energy meter (OPHIR Photonics, A Newport Company). The procedures of Z-scan measurements were explained in our previous studies (Shanshool et al. 2015, 2016).
6 Results and discussion
6.1 Field emission scanning electron microscope (FESEM)
Figure 2 presents the top-view FESEM images of the surface morphology of foil like polymer/ZnO/CuO nanocomposites for four types of polymer where two different magnifications; were used (10 and 50 K) each type of nanocomposites. Figure 2a, b represent PMMA/ZnO/CuO nanocomposites, (c) and (d) represent PVDF/ZnO/CuO nanocomposites, (e) and (f) represent PVA/ZnO/CuO nanocomposites and (g) and (h) represent PS/ZnO/CuO nanocomposites. A homogeneous dispersion of ZnO and CuO nanoparticles can be observed in the polymeric matrix, however that dispersion in polymer/ZnO/CuO was better than the desperation obtained in polymer/ZnO which indicate that ZnO nanoparticles were modified when CuO nanoparticles were added to the nanocomposites. Also, the effective size of the ZnO nanoparticles was calculated via imageJ software. Interestingly, it is noted that irregular-shaped ZnO nanoparticles and CuO nanoparticles were formed. For PMMA/ZnO/CuO, the size ranged from 74 to 289 nm, For PVDF/ZnO/CuO, it ranged from 75 to 102 nm, For PVA ZnO/CuO, it ranged from 37 to 62 nm and for PS/ZnO/CuO it ranged from 78 to 107 nm.
6.2 Energy dispersive X-ray (EDX) and EDS mapping
To check the composition and distribution of the element existed in the as-prepared foil like polymer/ZnO/CuO nanocomposites, EDX survey scan and EDS mapping analysis were performed on these samples. Figure 3a represents the EDX of PMMA/ZnO/CuO nanocomposites which showed the four elements detected in the sample which were carbon (C) that arose from the PMMA structure, oxygen (O) that also arose from the PMMA structure, zinc (Zn) and copper (Cu) from the dopant ZnO and CuO. Figure 3b represents the EDX of PVDF/ZnO/CuO nanocomposites which showed the five elements detected in the sample which were carbon (C) and fluorine (F) that arose from the PVDF structure and oxygen(O), zinc(Zn), and copper(Cu) that arose from dopant ZnO and CuO. Figure 3e represents the EDX spectrum of PVA/ZnO/CuO nanocomposite which showed the four elements detected which were carbon (C) that arose from the PVA structure, oxygen(O) that arose from the PVA structure and dopant ZnO and CuO, and zinc (Zn) and (Cu) elements from the compounds (ZnO) and (CuO). Figure 3f represents the EDX spectrum of PS/ZnO/CuO nanocomposite that showed four elements detected in the sample which were carbon (C) that arose from the PS structure, and oxygen (O), zinc (Zn) and copper (Cu) which arose from the dopant ZnO and CuO. This result suggests that the as-prepared samples were in high-purity condition. Figure 3c, d, g, h show the EDS mapping of PMMA/ZnO/CuO, PVDF/ZnO/CuO, PVA/ZnO/CuO, and PS/ZnO/CuO nanocomposites, respectively. The good spatial distribution was shown obviously from the same elements in the EDX spectra of every nanocomposite.
6.3 Transmittance
Figure 4 represent the transmittance spectra; (a) pure PMMA, PMMA/ZnO and PMMA/ZnO/CuO, (b) pure PVDF, PVDF/ZnO, and PVDF/ZnO/CuO, (c) pure PVA, PVA/ZnO and PVA/ZnO/CuO, (d) pure PS, PS/ZnO and PS/ZnO/CuO. Pure PMMA showed high transparency in the visible region (approximately 90%) but relatively lower in the UV region (around 80%). Meanwhile pure PVA showed transparency around 85% in the visible region and lower in the UV region. Pure PS showed transparency approximately 80% in the UV and visible regions. However pure PVDF showed the lowest transparency in the UV and visible regions for all nanocomposites, it was around 15%. The effect of adding ZnO nanoparticles to all polymers was clear, polymer/ZnO nanoparticles showed lower transmittance than pure polymer in all four nanocomposites. These results are in accordance with the studies by Anžlovar et al. (2012) and Khan et al. (2014) for PMMA/ZnO, Indolia and Gaur (2013) and Bhunia et al. (2014) for PVDF/ZnO, Kumar et al. (2014) and Mallika et al. (2015) for PVA/ZnO, and Jeeju et al. (2012) for PS/ZnO.
The effect of adding CuO nanoparticles in nanocomposites was observed clearly. The transmittance spectrum of polymer/ZnO/CuO nanocomposite showed lower value than nanocomposite that contained only ZnO nanoparticles for four different nanocomposites. The highest effect was noticed in PS/ZnO/CuO nanocomposites, while the lowest effect was noticed in the PVA/ZnO/CuO nanocomposite.
6.4 Linear absorption coefficient (α)
The linear absorption coefficient (α) for foil samples was calculated according to Hamdalla et al. (2015):
where d is the thickness of the sample, T is the transmittance, and R is the reflectance that was obtained from the data of UV–visible spectroscopy.
The thickness of the prepared samples like the flexible foil was determined using a digital micrometre at different places in each film and an average was determined; the thickness was around 75 μm for PMMA/ZnO/CuO, 35 µm for PVDF/ZnO/CuO, 90 µm for PVA/ZnO/CuO, and 120 µm for PS/ZnO/CuO.
Figure 5 represent the linear absorption coefficient spectra; (a) pure PMMA, PMMA/ZnO and PMMA/ZnO/CuO, (b) pure PVDF, PVDF/ZnO, and PVDF/ZnO/CuO (c) pure PVA, PVA/ZnO and PVA/ZnO/CuO, (d) pure PS, PS/ZnO and PS/ZnO/CuO. In all nanocomposites, the results showed that α increased with the increase of ZnO nanoparticles contained, especially in short wavelengths. This means that there is a high probability of the transition to be directly allowed to take place (Nwanya et al. 2016). The presence of absorption edges was observed, which is an indication of a good degree of crystallinity of nanocomposite samples (Al-Hussam and Jassim 2012). In addition, α depends on the wavelength of light that is actually being absorbed. The effect of CuO nanoparticles is observed clearly in which the addition of the nanoparticles increased the linear absorption coefficient of the nanocomposites. The highest effect is noticed in the PS/ZnO/CuO nanocomposite.
6.5 Nonlinear optical properties
Z-scan technique (Opened and Closed Aperture) was used to determine nonlinear absorption coefficient and nonlinear refractive index, besides third-order nonlinear optical susceptibility χ(3) of as-prepared samples was calculated.
The radius of the laser beam at the focus wo was calculated to be 26.6 μm. The irradiance of the laser beam at the focus Io was 0.9 GW/cm2. The Rayleigh length, Zo was calculated to be 4.17 mm. The procedures have been described by Sheik-Bahae et al. (1989), Bahae et al. (1990) and Van Stryland and Sheik-bahae (1998) were used to analyse the obtained data. The high nonlinear effect observed in the present work belonged to the ZnO and CuO nanoparticles, whereas the pure PMMA, PVDF, PVA, and PS had a negligible nonlinear optical response at 532 nm when measured using the same technique. These results are in accordance with Dorranian et al. (2012) for PMMA, Husaini et al. (2012) for PVA, Haripadmam et al. (2014) for PS, and Shanshool et al. (2015) for PVDF, all of them were prepared as thin film. In this work, to the best of the author’s knowledge, study on the nonlinear optical properties of polymer/ZnO/CuO nanocomposites as flexible foils is considered to be the first work. Therefore, the results could not be compared with previous literature.
6.6 Nonlinear refraction index (closed aperture)
Figure 6 shows the measurements of the normalized transmittance versus the sample position in the closed-aperture Z-scan, (a), (c), (e) and (g) for polymer/ZnO and (b), (d), (f) and (h) for polymer/ZnO/CuO. For polymer/ZnO nanocomposites, it can be observed that the value of ΔTP–V for PMMA/ZnO nanocomposite showed the highest value while PVDF/ZnO showed the lowest value. For polymer/ZnO/CuO nanocomposites, the effect of adding CuO nanoparticles for all nanocomposites is clear whereby, the value of ΔTP-V increased. The highest increment is shown in the PS/ZnO/CuO nanocomposite, whereas the lowest increment is shown in the PVDF/ZnO/CuO nanocomposite.
A peak followed by a valley is the hallmark of a negative nonlinear refractive index in all types of polymer/ZnO and polymer/ZnO/CuO nanocomposites, which is due to self-defocusing (Irimpan 2008). Therefore, the figures demonstrated that the samples have exhibited self-defocusing effect, i.e., they have negative nonlinearity. This means that, these samples will diverge the high intense beam which is incident on them (Nagaraja et al. 2013).
There are electronic and thermal effects to the nonlinear refractive index. However, the separation between them is not easy (Jacinto et al. 2006; Freitas et al. 2008).
From Fig. 6a–h the distance between peak and valley (ΔZP-V) was found to be approximately 7 mm in nanocomposites of PMMA/ZnO and PMMA/ZnO/CuO, PVDF/ZnO and PVDF/ZnO/CuO, and PS/ZnO and PS/ZnO/CuO;when compared to ΔZP-V = 1.7 Zo which satisfied the condition of third-order nonlinearity (Bahae et al. 1990; Aranda et al. 1995; Battaglin et al. 2001; Yang et al. 2002; Kumari et al. 2012). Thus confirming the presence of pure electronic third-order nonlinearity. However, for ΔZP-V > 1.7 Zo for PVA/ZnO and PVA/ZnO/CuO nanocomposites, Fig. 6e, f the occurrence of thermal nonlinearity was clearly shown and the nonlinear effect observed represented the third-order process (Mathews et al. 2007; Tamgadge et al. 2014). Usually, the thermal effect has a dominant contribution to the nonlinear optical mechanism when continuous wave laser is used. On the other hand, electronic contribution dominates in nonlinear optical mechanism when pulsed laser is used, as in the current work, except in some limited cases in which thermal contribution will dominate. This limited case took place when it satisfied the condition that states that the thermal response time of the sample will be in the range of pulse duration of pulsed laser (Boyd 2007). Even when using pulsed laser of short pulses, the cumulative thermo-optical effects will arise in the sample in which its refractive index is temperature dependent (i.e. dn/dT ≠ 0) (De Nalda et al. 2002) hence thermal lens is formed. Consequently, it contributes to the result of closed aperture Z-scan. The expression of a nonlinear refractive index n2 of thermal effect is (Boyd 2007; Tamgadge et al. 2015):
where \(\left( { \frac{dn}{dT}} \right)\) is the thermo-optical coefficient which is defined as the variation of the refractive index with temperature (Ghosh 1998), α is the linear absorption coefficient. \(\omega_{o}\) is the radius of the laser beam at the focus, and \(k\) is the thermal conductivity \(\left( {k = \left[ {\rho C_{p} } \right]D} \right)\), where ρ is the density, \(C_{p}\) is the specific heat at constant pressure, \(\left( {\rho C_{p} } \right)\) represents the heat capacity, and D is the thermal diffusivity. These parameters affected the determination of thermal response time. As ΔZP-V was more than 1.7 Zo, the thermal response time of PVA/ZnO nanocomposite was considered in the range of pulse duration; hence thermal contribution was dominant (Boyd 2007).
By using the variable transmittance values of the closed-aperture Z-scan, the nonlinear phase shift \(\Delta \Phi_{0}\) and the nonlinear refractive index n2 were determined.
\(\Delta \Phi_{0}\) can be calculated using (Bahae et al. 1990; Dorranian et al. 2012):
ΔTpv is the change in transmittance between the peak and valley in a closed aperture Z-scan, and is defined as:
where Tp and Tv are the normalized peak and valley transmittances as seen in Fig. 5.
The ratio of the light passing through the aperture to the light in front of the aperture was defined as S. The details of calculation S was mentioned in previous study (Shanshool et al. 2015, 2016).
Thus the values of \(\Delta \Phi_{0}\) can be calculated and was used to calculate the nonlinear refractive index n2 using the equation (Irimpan 2008):
where λ is the wavelength of the laser source, Io is the irradiance of the laser beam at the focus, and \(L_{eff }\) is the effective length of the sample, which is calculated as (Haripadmam et al. 2012; Bahae et al. 1990):
where \(\alpha\) is the linear absorption coefficient and d is the thickness of the sample.
The values of \(\Delta \Phi_{0} , L_{eff }\) and n2 of all polymer/ZnO and polymer/ZnO/CuO nanocomposites are listed in the Tables 1, 2, 3, and 4. The obtained values of n2 for as-prepared samples are in order 10−12 cm/W that are larger than values in previous studies which was in order 10−14 cm2/W for PS/ZnO nanocomposite prepared as thin film (Jeeju et al. 2012), 10−13 cm2/W for PMMA/ZnO nanocomposite prepared as thin film (Jeeju 2012), and 10−14 cm2/W for PVA/ZnO nanocomposite prepared as thin film (Jeeju et al. 2014).
6.7 Nonlinear absorption coefficient (open aperture)
By removing the aperture, open aperture Z-scan was used to investigate the nonlinear absorption coefficient. During this process, the transmitted beam was collected by the detector without any limitation.
In order to evaluate the nonlinear absorption coefficient, Fig. 7 shows the measurements of the normalized transmittance versus the sample position in the open-aperture Z-scan for polymer/ZnO and polymer/ZnO/CuO, (a) represent PMMA/ZnO and PMMA/ZnO/CuO, (b) represent PVDF/ZnO and PVDF/ZnO/CuO, (c) represent PVA/ZnO and PVA/ZnO/CuO, and (d) represent PS/ZnO and PS/ZnO/CuO. The transmittance was sensitive to nonlinear absorption. The transmittance showed a minimum value at the focus Z = 0 and then increased steadily on both sides of the focus, that represented a valley. From the resulting data as comparison between four nanocomposites of polymer/ZnO, the valley of PMMA/ZnO nanocomposites is found to be deeper than the other three nanocomposites, whereas the PVDF/ZnO nanocomposites had the lowest deep valley among all the studies of nanocomposites. This indicates that PMMA/ZnO nanocomposite exhibits stronger nonlinear absorption performance than other nanocomposites. The two-photon absorption caused the change in the transmittance in the samples (Mahdi and Altaify 2009). From the data, a symmetric valley indicates a positive nonlinear absorption coefficient β for all types of nanocomposites which represents two-photon absorption.
The effect of adding CuO nanoparticles for all nanocomposites is clear. The valley of the nanocomposite that contains CuO nanoparticles in addition to ZnO nanoparticles is deeper than nanocomposite containing only ZnO nanoparticles that is due to the red shift of energy gap. The energy band gap of polymer/ZnO/CuO nanocomposites is shifted to the lower value as the CuO nanoparticles was added in every one of polymer/ZnO/CuO nanocomposites. Here the shift of the energy gap enhanced two photon absorptions (Sreeja et al. 2010; Vinitha et al. 2010). Thus, the results of the open aperture Z-scan of the polymer/ZnO/CuO nanocomposites show that the nonlinear absorption coefficient values were enhanced with the addition of CuO nanoparticles.
To determine the value of nonlinear absorption coefficient β, the following equation (Vinitha et al. 2010) was used:
where Δt is the one valley value obtained from the data of the open Z-scan curve.
The values of \(\beta\) calculated from Eq. (7) for all types of nanocomposites are listed in Tables 1, 2, 3, and 4. The obtained values of β for as-prepared samples are in order 10−7 cm/W that are larger than values obtained in previous studies for polymer/ZnO nanocomposites which was in order 10−9 cm/W for PS/ZnO nanocomposite prepared as thin film (Jeeju et al. 2012), and 10−9 cm/W for PMMA/ZnO nanocomposite prepared as thin film (Jeeju 2012).
6.8 Third-order nonlinear optical susceptibility χ(3)
χ(3) is the third order susceptibility that explains processes like third harmonic generation. The nonlinear susceptibility should be practically large as the main step to select materials for nonlinear optical applications. Its value varies with different of nonlinear materials and normally, the third order susceptibility of semiconductors is in the range of 10−13–10−10 esu. The real part of the third order susceptibility is dependent on the nonlinear refraction index n2, while the imaginary part of the third order susceptibility is dependent on the nonlinear absorption coefficient β (Nagaraja et al. 2013). The real and imaginary parts of the third-order nonlinear optical susceptibility χ(3) can be calculated by using the refractive index n2 and nonlinear absorption coefficient β, according to the following equations (Mathews et al. 2007):
where \(\varepsilon_{o}\) is the dielectric constant in a vacuum, c is the speed of light in a vacuum, no is the linear refractive index and λ is the laser wavelength.
The absolute value of the third-order nonlinear optical susceptibility χ(3) can be calculated by:
Third order nonlinearity is usually measured in esu, and sometimes in m2/V2. The values of polymer/ZnO/CuO nanocomposites are tabulated in Tables 1, 2, 3, and 4. The obtained values of third optical susceptibility χ(3) for as-prepared samples are in order 10−6 esu that are larger than the values obtained in previous studies which was in order 10−12 esu for PS/ZnO nanocomposite prepared as thin film (Jeeju et al. 2012), 10−13 and 10−11 esu for PMMA/ZnO nanocomposite prepared as thin film (Kulyk et al. 2009; Jeeju 2012) respectively.
6.9 Optical limiting
The suitability of a sample to be used as an optical limiter depends on the sign and the value of the nonlinear refractive index, n2. Besides that, the presence of a strong nonlinear absorption produces good optical limiting (Kumar et al. 2008). The negative sign of n2 indicates the suitability of the sample to be used as an optical limiter for laser radiation due to self-defocusing, which means it will diverge the radiation. According to the results that were obtained in Fig. 6 and Tables 1, 2, 3, and 4, the sign of n2 which were obtained for all as-prepared nanocomposites were negative. Hence, they are considered as promising candidates to be used as optical limiter devices at a wavelength of 532 nm (Ryasnyanskiy et al. 2007). The property of optical limiting is mostly found to be absorptive nonlinearity. Hence, it is related to the imaginary part of the third order susceptibility (Mathew et al. 2012). The optical limiting threshold reduced when the size of the nanoparticle increased and the optical limiting performance was enhanced (Hari et al. 2011).
6.10 Measurements of optical limiting threshold
The setup of the transmittance technique, which was used to determine the optical limiting threshold of the as-prepared samples, is same as the setup of the Z-scan technique, but the sample should be fixed at the focal point (i.e. it will be constant at this point and will not move along the z-axis as in the Z-scan measurements). Figure 8 represents the setup of the transmittance technique. Variable beam splitter (attenuator) was used in the path of the laser to vary the output power of the laser source. The transmittance in the far field was measured using an energy meter and was normalized, and then plotted as a function of input fluence (Mw/cm2). It should be mentioned that the absorption spectra of the as-prepared samples were checked after irradiating them with a laser source and it was found that almost no change happened in the pattern and its intensity, hinting that the as-prepared samples have good photo stability.
Figure 9 represents the normalized transmittance as a function of input fluence for four types of polymer/ZnO and polymer/ZnO/CuO nanocomposites that are (a) PMMA/ZnO and PMMA/ZnO/CuO, (b) PVDF/ZnO and PVDF/ZnO/CuO, (c) PVA/ZnO and PVA/ZnO/CuO, and (d) PS/ZnO and PS/ZnO/CuO. Initially, the output power increased as the input power increased, meanings the output power varies linearly with the input power, and the linear transmittance obeyed Beer’s law:
where I is the incident energy, \(I_{o }\) is the output energy, α is the linear absorption coefficient and d is the thickness of the sample, until it reaches a certain threshold value when the sample starts to defocus the laser beam, thus causing the cut off of a greater part of the beam cross-section by the aperture (Manshad and Hassan 2012).
The arrow in the figure indicates the approximate fluence in which the normalized transmission begins to deviate from linearity which is defined as the limiting threshold (Hassan et al. 2013).
From Fig. 9a, it is clear that the addition of CuO nanoparticles reduced the optical limiting threshold from 140 Mw/cm2 for PMMA/ZnO to 60 Mw/cm2 for PMMA/ZnO/CuO. Figure 9b shows the reduction of the optical limiting threshold of PVDF/ZnO, which is equal to 350–300 Mw/cm2 for PVDF/ZnO/CuO. The value of optical limiting threshold of PVA/ZnO is equal to 130 Mw/cm2, while it is reduced to 80 Mw/cm2 for PVA/ZnO/CuO as shown in Fig. 9c. The PS/ZnO/CuO nanocomposite shows the lowest optical limiting threshold which is 50 Mw/cm2, while it is 250 Mw/cm2 for the PS/ZnO nanocomposite, which is clearly shown in Fig. 9d. The PS/ZnO/CuO nanocomposite has the lowest value of optical limiting threshold, so it is considered the best optical limiter, representing the lowest energy band gap of four types of polymer/ZnO/CuO nanocomposites. Besides, it had a deeper valley in the trace of open aperture Z-scan (nonlinear absorption coefficient) (Fig. 7) compared to the other three nanocomposites. Also, it had the highest imaginary part of third order susceptibility (Table 4). The highest optical limiting threshold of 300 Mw/cm2 shown by the PVDF/ZnO/CuO nanocomposite which had the highest energy band gap, and the lower imaginary part of the third order susceptibility (Table 2) was of the other three nanocomposites.
7 Conclusion
A flexible foil-like pure polymer, polymer/ZnO and polymer/ZnO/CuO nanocomposite of four different polymer matrix, which are PMMA, PVDF, PVA and PS have been prepared successfully with ZnO and CuO nanoparticles which were used as filler. For polymer/ZnO nanocomposites, the low linear transmittance was observed in the UV region due to the high absorption of ZnO nanoparticles, whereas, the lower transmittance was noticed for polymer/ZnO/CuO. The linear absorption coefficient of polymer/ZnO/CuO nanocomposites show increment than its value of polymer/ZnO nanocomposites. The EDX analysis and EDS mapping confirmed the purity of the as-prepared samples. The homogeneous dispersion of ZnO and CuO nanoparticles in the polymer matrix was observed in nanocomposites samples through the FESEM test. The nonlinear refractive index had a negative sign for all as-prepared nanocomposites, while they showed strong nonlinear absorption which is increased by adding CuO nanoparticles. The absolute value of the third order nonlinear optical susceptibility χ(3) of as-prepared samples was in the order of 10−6 esu. These obtained values, for n2, β and χ(3), are larger than the values stated in previous studies. The effect of adding CuO nanoparticles enhanced the optical limiting property of nanocomposites; the four different polymer/ZnO/CuO nanocomposites showed lower optical limiting threshold than their values with only ZnO nanoparticles. The lowest optical limiting threshold was obtained by PS/ZnO/CuO which was equal to 50 Mw/cm2, therefore it was considered the best optical limiter than other as-prepared nanocomposites.
References
Al Jaafari, A.A., Ayesh, A.S.: Effect of ZnO nano-particles on the dielectric relaxation behavior and thermal stability of polycarbonate host. J. Thermoplast. Compos. Mater. 24(6), 837–852 (2011)
Aleali, H., Mansour, N.: Nonlinear responses and optical limiting behavior of Ag nanoparticle suspension. J. Sci. Islam. Repub. Iran 21(3), 273–278 (2010)
Al-Gaashani, R.A.H.: Synthesis of metal oxide nanopowders by microwave assisted process and thermal decomposition method. Doctoral disseration, UKM (2012)
Al-Hussam, A.M.A., Jassim, S.A.-J.: Synthesis, structure, and optical properties of CdS thin films nanoparticles prepared by chemical bath technique. J. Assoc. Arab. Univ. Basic Appl. Sci. 11(1), 27–31 (2012)
Al-Taa, W., Nabi, M.A., Yusop, R.M., Yousif, E., Abdullah, M., Salimon, J., Salih, N., Zubairi, S.I.: Effect of nano ZnO on the optical properties of poly (vinyl chloride) films. Int. J. Polym. Sci. 2014, 1–6 (2014)
Anand, B., Kaniyoor, A., Swain, D., Baby, T.T., Venugopal Rao, S., Sankara Sai, S.S., Ramaprabhu, S., Philip, R.: Enhanced optical limiting and carrier dynamics in metal oxide-hydrogen exfoliated graphene hybrids. J. Mater. Chem. C 2(47), 10116–10123 (2014)
Ando, M., Kadono, K., Haruta, M., Sakaguchi, T.: Large third-order optical nonlinearities in transition-metal oxides. Nature 374, 625627 (1995)
Anžlovar, A., Crnjak Orel, Z., Kogej, K., Žigon, M.: Polyol-mediated synthesis of zinc oxide nanorods and nanocomposites with poly(methyl methacrylate). J. Nanomater. 2012, 1–9 (2012)
Aranda, C.L.W.F.J., Lapalli, D., Nrao, V.G.L., Zhov, J.P., Chen, Z., Akkara, J.A., Kaplan, D.L., Roach, F.: Nonlinear optical interactions in bacteriohodospin using Z-scan. Opt. Rev. 2(3), 204–206 (1995)
Bahae, M.S., Said, A.A., Wei, T.H., Hagan, D.J., Van Stryland, E.W.: Sensitive measurements of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 26(4), 760–769 (1990)
Battaglin, F.G., Calvelli, P., Cattaruzza, E., Gonella, R.P.: Z-scan study on the nonlinear refractive index of copper nanocluster composite silica glass. Appl. Phys. Lett. 78(25), 3953–3955 (2001)
Bhunia, R., Ghosh, D., Ghosh, B., Hussain, S., Bhar, R., Pal, A.: Free-standing flexible nanocrystalline-ZnO-impregnated polyvinylidene fluoride composite thin films. J. Compos. Mater. 2014, 1–13 (2014)
Boyd, R.W.: Nonlinear Optics, 3rd edn. (2007)
Chen, Q., Schadler, L.S., Siegel, R.W., Irvin, G.C., Jr., Company, E.K.: ZnO/PMMA thin film nanocomposites for optical coatings. In: Proceedings of SPIE. Nanocrystals, and organic and hybrid nanomaterials, vol. 5222, pp. 158–162. Bellingham (2003)
Chen, A., Yang, G., Long, H., Li, F., Li, Y., Lu, P.: Nonlinear optical properties of laser deposited CuO thin films. Thin Solid Films 517(15), 4277–4280 (2009)
Chen, A., Yang, G., Long, H., Lu, P., Zhang, W., Wang, H.: Optical limiting properties in copper oxide thin films under a high-repetition-rate femtosecond laser. Mater. Lett. 91(September 2015), 319–322 (2013)
Dagher, S., Haik, Y., Ayesh, A.I., Tit, N.: Synthesis and optical properties of colloidal CuO nanoparticles. J. Lumin. 151, 149–154 (2014)
De Nalda, R., Del Coso, R., Requejo-Isidro, J., Olivares, J., Suarez-Garcia, A., Solis, J., Afonso, C.N.: Limits to the determination of the nonlinear refractive index by the Z-scan method. J. Opt. Soc. Am. B 19(2), 289 (2002)
Dorranian, D., Golian, Y., Hojabri, A.: Investigation of nitrogen plasma effect on the nonlinear optical properties of PMMA. J. Theor. Appl. Phys. 6(1), 1–8 (2012)
El-Kader, F.H.A., Hakeem, N.A., Elashmawi, I.S., Ismail, A.M.: Structural, optical and thermal characterization of ZnO nanoparticles doped in PEO/PVA blend films. Aust. J. Basic Appl. Sci. 7(10), 608–619 (2013)
Freitas, L.R., Jacinto, C., Ródenas, A., Jaque, D., Catunda, T.: Time-resolved study electronic and thermal contributions to the nonlinear refractive index of Nd3+:SBN laser crystals. J. Lumin. 128(5–6), 1013–1015 (2008)
Ghosh, G.: Handbook of Thermo-Optic Coefficients of Optical Materials with Applications, vol. 5. Academic Press, Cambridge (1998)
Hamdalla, A., Hanafy, T.A., Bekheet, A.E.: Influence of erbium ions on the optical and structural properties of polyvinyl alcohol. J. Spectrosc. 2015, 1–7 (2015)
Hari, M., Joseph, S.A., Balan, N., Mathew, S., Kumar, R., Mishra, G., Yadhav, R.R., Radhakrishnan, P., Nampoori, V.P.N.: Linear and nonlinear optical properties of gold nanoparticles stabilized with polyvinyl alcohol. J. Nonlinear Opt. Phys. Mater. 20(04), 467–475 (2011)
Haripadmam, P.C., Kavitha, M.K., John, H., Krishnan, B., Gopinath, P.: Optical limiting studies of ZnO nanotops and its polymer nanocomposite films. Appl. Phys. Lett. 101(7), 071103–071103-5 (2012)
Haripadmam, P.C., John, H., Philip, R., Gopinath, P.: Enhanced optical limiting in polystyrene-ZnO nanotop composite films. Opt. Lett. 39(3), 474–477 (2014)
Hassan, Q.M.A., Al-ahmad, A.Y., Al-mudhaffer, M.F., Badran, H.A.: Third-order optical nonlinearities and optical-limiting properties of phloxine b dye doped pmma films investigated by Z-scan technique. Rom. J. Phys. 58(7–8), 962–969 (2013)
Husaini, S., Teng, H., Menon, V.M.: Enhanced nonlinear optical response of metal nanocomposite based photonic crystals. Appl. Phys. Lett. 101(11), 111103 (2012)
Indolia, A.P., Gaur, M.S.: Optical properties of solution grown PVDF-ZnO nanocomposite thin films. J. Polym. Res. 20(1), 1–8 (2013)
Irimpan, L.M.: Spectral and nonlinear optical characterization of ZnO nanocomposites. Doctoral dissertation, Cochin University of Science and Technology (2008)
Jacinto, C., Messias, D.N., Andrade, A.A., Lima, S.M., Baesso, M.L., Catunda, T.: Thermal lens and Z-scan measurements: thermal and optical properties of laser glasses—a review. J. Non. Cryst. Solids 352(32–35), 3582–3597 (2006)
Jeeju, P.P.: Investigations on some technologically important polymer nunocomposite films and semi crystalline Polypyrrole films. Doctoral dissertation, Cochin University of Science and Technology, Cochin, Kerala, India (2012)
Jeeju, P.P., Jayalekshmi, S., Chandrasekharan, K., Sudheesh, P.: Size dependent nonlinear optical properties of spin coated zinc oxide-polystyrene nanocomposite films. Opt. Commun. 285(24), 5433–5439 (2012)
Jeeju, P.P., Jayalekshmi, S., Chandrasekharan, K.: Nonlinear optical properties of ZnO/poly (vinyl alcohol) nanocomposite films. In: OPTOELECTRONICS MATERIALS and Thin Film, AIP Conference Proceedings, vol. 1576, pp. 163–166 (2014)
Jundale, D.M., Navale, S.T., Khuspe, G.: Polyaniline–CuO hybrid nanocomposites: synthesis, structural, morphological, optical and electrical transport studies. J. Mater. Sci.: Mater. Electron. 24, 3526–3536 (2013)
Khan, M., Chen, M., Wei, C., Tao, J., Huang, N., Qi, Z., Li, L.: Synthesis at the nanoscale of ZnO into poly(methyl methacrylate) and its characterization. Appl. Phys. A 117(3), 1085–1093 (2014)
Kulyk, B., Sahraoui, B., Krupka, O., Kapustianyk, V., Rudyk, V., Berdowska, E., Tkaczyk, S., Kityk, I.: Linear and nonlinear optical properties of ZnO/PMMA nanocomposite films. J. Appl. Phys. 106(9), 093102–093102-6 (2009)
Kumar, R., Venugopal Rao, S., Giribabu, L., Narayana Rao, D.: Nonlinear optical properties of alkyl phthalocyanines in the femtosecond, nanosecond, and cw excitation regimes. Proc. SPIE 6875, 68751D (2008)
Kumar, N.B.R., Crasta, V., Praveen, B.M.: Advancement in microstructural, optical, and mechanical properties of PVA (Mowiol 10-98) doped by ZnO nanoparticles. Phys. Res. Int. 2014, 1–9 (2014)
Kumari, V., Kumar, V., Mohan, D., Purnima, Malik, B.P., Mehra, R.M.: Effect of surface roughness on laser induced nonlinear optical properties of annealed ZnO thin films. J. Mater. Sci. Technol. 28(6), 506–511 (2012)
Li, S., Lin, M.M., Toprak, M.S., Kim, D.K., Muhammed, M.: Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 1, 1–19 (2010)
Mahdi, Z.F., Altaify, D.: Nonlinear optical properties of nanoparticles CdS thin film using Z-scan technique. In: 5th Saudi Technical Conference and Exhibition, Riyadh, 11–14 Jan 2009, pp. 1–6 (2009)
Mallika, A.N., Reddy, A.R., Reddy, K.V.: Annealing effects on the structural and optical properties of ZnO nanoparticles with PVA and CA as chelating agents. J. Adv. Ceram. 4(2), 123–129 (2015)
Manshad, R.K.H., Hassan, Q.M.A.: Optical limiting properties of magenta doped PMMA under CW laser illumination. Adv. Appl. Sci. Res. 3(6), 3696–3702 (2012)
Mathews, S.J., Chaitanya Kumar, S., Giribabu, L., Venugopal Rao, S.: Large third-order optical nonlinearity and optical limiting in symmetric and unsymmetrical phthalocyanines studied using Z-scan. Opt. Commun. 280(1), 206–212 (2007)
Mathew, S., Saran, A.D., Joseph, S.A., Bhardwaj, B.S., Punj, D., Radhakrishnan, P., Nampoori, V.P.N., Vallabhan, C.P.G., Bellare, J.R.: Nonlinear optical characterization and measurement of optical limiting threshold of CdSe quantum dots prepared by a microemulsion technique. J. Mater. Sci.: Mater. Electron. 23(3), 739–745 (2012)
Nagaraja, K.K., Pramodini, S., Poornesh, P., Nagaraja, H.S.: Effect of annealing on the structural and nonlinear optical properties of ZnO thin films under cw regime. J. Phys. D Appl. Phys. 46(5), 1–12 (2013)
Nwanya, A.C., Chigbo, C., Ezugwu, S.C., Osuji, R.U., Malik, M., Ezema, F.I.: Transformation of cadmium hydroxide to cadmium oxide thin films synthesized by SILAR deposition process: role of varying deposition cycles. J. Assoc. Arab. Univ. Basic Appl. Sci. 20, 49–54 (2016)
Ryasnyanskiy, A.I., Palpant, B., Debrus, S., Pal, U., Stepanov, A.L.: Optical nonlinearities of Au nanoparticles embedded in a zinc oxide matrix. Opt. Commun. 273(2), 538–543 (2007)
Sangawar, V.S., Golchha, M.C.: Evolution of the optical properties of Polystyrene thin films filled with Zinc Oxide nanoparticles. Int. J. Sci. Eng. Res. 4(6), 2700–2705 (2013)
Shahmiri, M., Ibrahim, N.A., Faraji, N., Yunus, W.M.M., Asim, N., Zainuddin, N.: Third-order nonlinear optical properties of chemically synthesized copper oxide nanosheets. Phys. E: Low-dimens. Syst. Nanostruct. 54, 109–114 (2013)
Shanshool, H.M., Yahaya, M., Yunus, W.M.M., Abdullah, I.Y.: Measurements of nonlinear optical properties of PVDF/ZnO using Z-scan technique. Braz. J. Phys. 45(5), 538–544 (2015)
Shanshool, H.M., Yahaya, M., Yunus, W.M.M., Abdullah, I.Y.: Third order nonlinearity of PMMA/ZnO nanocomposites as foils. Opt. Quantum Electron. 48(1), 1–14 (2016)
Sheik-Bahae, M., Said, A.A., Van Stryland, E.W.: High-sensitivity, single-beam n(2) measurements. Opt. Lett. 14(17), 955–957 (1989)
Sreeja, R., John, J., Aneesh, P.M., Jayaraj, M.K.: Linear and nonlinear optical properties of luminescent ZnO nanoparticles embedded in PMMA matrix. Opt. Commun. 283(14), 2908–2913 (2010)
Tamgadge, Y.S., Sunatkari, A.L., Talwatkar, S.S., Pahurkar, V.G., Muley, G.G.: Linear and nonlinear optical properties of nanostructured Zn(1 − x)SrxO–PVA composite thin films. Opt. Mater. (Amst). 37, 42–50 (2014)
Tamgadge, Y.S., Pahurkar, V.G., Talwatkar, S.S., Sunatkari, A.L., Muley, G.G.: Thermally stimulated third-order optical nonlinearity in Cd-doped CuO–PVA thin films under cw laser illumination. Appl. Phys. B 120(2), 373–381 (2015)
Van Stryland, E.W., Sheik-bahae, M.: Z-scan measurements of optical nonlinearities. In: Characterization Techniques and Tabulations for Organic Nonlinear Materials, pp. 655–692 (1998)
Vinitha, G., Manirahulan, K., Ramalingam, A.: Optical limiting characteristics of core-shell nanoparticles. J. Nonlinear Opt. Phys. Mater. 19(04), 621–628 (2010)
Yang, H.G., Wang, W., Yan, L., Lu, Z.C., Yang, G.: Z-scan determination of the large third-order optical nonlinearity of Rh:BaTiO3 thin films deposited on MgO substrates. Opt. Commun. 209, 445–449 (2002)
Acknowledgements
The authors would like to acknowledge the contribution and the financial support by the Malaysian Ministry of Higher Education and Universiti Kebangsaan Malaysia under research Grant (FRGS/1/2013/SG02/UKM/01/1). The authors would like to thank Dr. Tan Sin Tee for her contribution EDX characterization and conceptual advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shanshool, H.M., Yahaya, M., Yunus, W.M.M. et al. Influence of CuO nanoparticles on third order nonlinearity and optical limiting threshold of polymer/ZnO nanocomposites. Opt Quant Electron 49, 18 (2017). https://doi.org/10.1007/s11082-016-0830-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0830-5