Abstract
In our study we investigate the characteristic differences, with diagnostic meaning, in the synchronous fluorescence spectra (SFS) of cancerous and healthy colorectal tissues, ex vivo. The main observed fluorophores which fluorescence has a diagnostic meaning are tyrosine, tryptophan, NADH, FAD, collagen, elastin, and porphyrines. In the SFS of three colorectal tumours and healthy tissue originated from one patient we observed the formation of alterations in the fluorescence, which can be addressed to specific dysplastic changes in the tissues and could be used for tumour stage evaluation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is a major health problem worldwide, and the second most common cause of death following cardiovascular diseases in many world regions. Since elderly people are most amenable to cancer and the average age of the population is increasing the spread and incidence of cancer are bound to grow. Colorectal cancer is recognized as the third most common cancer among the cancer patients (Ferlay et al. 2012). The mortality among the colorectal cancer patients is only lower than mortality among the lung, liver and stomach cancer patients (Bernard and Christopher 2015). The probability for 5-year survival correlates with the cancer stage at diagnosis, varying between 89% for stage I and 9% for advanced stage cancer (Drouillard et al. 2015). The accurate stage evaluation could be critical for choosing appropriate treatment for gastrointestinal cancer. Treatments like surgery, chemotherapy and/or radiotherapy could lead to positive outcome, but also lead to decreased quality of life. Although those are crucial treatments for late stage cancer, early stage cancer could be effectively treated with low-invasive, endoscopic and more patient friendly therapies.
Therefore an early diagnostic of colorectal cancer is essential for a positive treatment outcome (Drouillard et al. 2015). The standard clinical practice for colorectal cancer diagnostic at the moment is the white light endoscopy. This method relays entirely on the physicians experience, subsequently the diagnostic accuracy is of the high definition white light endoscopy is between 68 and 84% (Rastogi et al. 2011) and varies as much as 30% between trainees and expert gastroenterologists (Kuiper 2013).
Different optical techniques are under development for their possible implementation in the standard endoscopy, as an add-on technique for differentiation between healthy and cancerous tissues (Song and Ang 2014; Fujiya and Kohgo 2013; Johansson et al. 2008; Hasan and Wallace 2009). Techniques as dye-based chromoendoscopy, narrow-band imaging and autofluorescence spectroscopy are available for image enhanced endoscopies (Fujiya and Kohgo 2013).
Chromoendoscopy allows detection of mucosal lesions by revealing distinguishing pit pattern of the mucosa tissue, however for now its application in the daily practice is time-consuming and cumbersome (Fujiya and Kohgo 2013; Kiesslich et al. 2001). Narrow-band imaging is an endoscopic technique that uses narrow bandwidth filters in the red– green– blue sequential illumination system, which is used for enhanced imaging of the vascular system; irregular vascular pattern is an essential cancer diagnostic criterion. Recently the main disadvantages of the narrow-band imaging endoscopy, related to poor light intensity and dark image, has been overcome and its feasibility into the clinical practice is yet to be clearly justified (Subramanian and Ragunath 2014). Autofluorescence spectroscopy is one of the most intensively investigated add-on techniques for endoscopic gastrointestinal diagnostics, it is a powerful technique for noninvasive analyze of tissues (Hasan and Wallace 2009; Song and Ang 2014; Fujiya and Kohgo 2013; Johansson et al. 2008; Broek et al. 2007). Autofluorescence imaging (AFI) is effective as a “red-flag” technique in improving the diagnostic accuracy of less experienced endoscopists (Tada et al. 2011), which could reduce unnecessary biopsy sampleing. Some of the major drawbacks of this technique are high false positive rates, as a result of low selectivity and poor image resolution (Subramanian and Ragunath 2014; Song and Ang 2014; Aihara et al. 2009). The optimization of the available systems is mainly directed to noise reduction, color contrast and development of different parameters and excitation and/ or detection wavelength algorithms (Aihara et al. 2009; Song et al. 2011).
Colorectal tissue is a multilayered type of tissue, consisted of three main layers mucosa, submucosa and muscularis propria. The fluorescence of the structural proteins collagen and elastin arises mainly from the submucosa layer; NADH and FAD are mostly present in gland cells (located in the mucosa layer), mitochondria and lysosomal granules (digestive cells), and porphyrin in the mitochondria of red blood cells and gland cells (Aihara et al. 2012). The main endogenous fluorophores responsible for the fluorescence signal in biological tissues are the structural proteins, enzymes and coenzymes, and amino acids. They are listed in Table 1, with their excitation and emission maxima. We expect to observe and to be able to address their fluorescence in the fluorescence signal of our samples.
Newly developed approach for fluorescence investigation is the synchronous fluorescence spectroscopy (SFS). The SFS application and basic theory are presented by Lloyd (1971) and implemented in biomedical research by Vo-Dinh (2000). The SFS method is based on simultaneous scanning of both the excitation and emission wavelength, while a constant wavelength (offset) is kept between them. For a chosen wavelength interval one or a few relevant spectral features of the investigated sample will be much more pronounced and resolvable, as a result spectral overlapping is reduced. SFS’s high sensitivity, non-invasive character and relatively fast performing are the main factors for the rapid widespread exploitation of the method for qualitative and quantitative analysis of food and beverages.(Pis et al. 2011; Dankowska et al. 2013) The SFS find its application in biomedical researches as a promising modality for investigation of blood and urine samples for cancer diagnostic and for differentiation between normal and pathologically altered cells and tissues (Al-Thunayan 2006; Dramićanin et al. 2012). The diagnostic potential of SFS for identification and localization of dysplastic tissues has been investigated for breast, cervical and thyroid gland cancers. Conclusions of those investigations support the superiority of SFS in sensitivity and specificity for differentiating cancerous and healthy tissue on the basis of their fluorescence spectra (Masilamani et al. 2012; Giubileo et al. 2005; Jeyasingh et al. 2010). The method enables greater sensitivity and selectivity, which is one of the main drawbacks of the current AFI modality (Zhang et al. 2015).
We have previously presented our work on detailed investigations of the fluorescence of tumour and healthy tissues originating from skin (Borisova et al. 2016; Zhelyazkova et al. 2015; Pavlova et al. 2010; Borisova et al. 2014) and gastrointestinal tract (Borisova et al. 2015) and their potential for diagnostic applications. In this article we will present an unique for our research case of three tumours, with the same histopathological diagnosis, originating from one patient, which allowed us to observe the fluorescence spectra of tumours in different stages with eliminated patient-to-patient spectral differences and to evaluate SFS feasibility to assess the stage of the investigated tumour.
2 Methods and materials
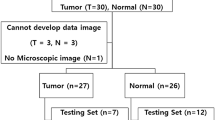
The investigated ex vivo tissue samples are obtained during standard surgical procedure for colorectal neoplasia lesions removal, performed in University Hospital “Tsaritsa Yoanna-ISUL”, Sofia. All patients received and signed written informed consent and this research is approved by the Ethics Committee of the University Hospital “Tsaritsa Yoanna-ISUL”, Sofia. Immediately after the excision the tissue samples are placed in safe-keeping solution and transported in isothermal conditions from the hospital to the spectral laboratory. The fluorescence of the safe-keeping solution has been investigated and its intensity is statistically negligible in comparison with the intensity of the investigated tissue autofluorescence.
The measurements are performed with spectrofluorimeter FluoroLog 3 (HORIBA Jobin Yvon, France). This systems’ light source is a Xenon lamp with power 300 W, performance range of 200–750 nm and PMT detector with performance range of 220–850 nm for fluorescence detection. Since our samples vary in shape and dimensions, their fluorescence was investigated with additional fiberoptical module F - 3000 of Fluorolog 3, which allows investigation of samples outside of the sample chamber.
SFS measurements were performed with excitation wavelength in the spectral range of 280–720 nm and wavelength interval in the range of 10–280 nm with increment of 10 nm.
3 Results
Presented results are obtained after investigation of three different lesions, originating from one patient and with the same histological diagnosis Ca-Colli Asc. colon cancer. The fluorescence of the tissue, part from the safety zone excised around the lesions, is given as a healthy tissue fluorescence.
Main differences observed in the contour maps of SFS for healthy (Fig. 1) and cancerous tissue (Figs. 2, 3, 4) are in the intensity of the fluorescence originating from the amino acids tyrosine and tryptophan (exitation at 300 nm and emission at 360 nm), the enzymes and coenzymes NADPH (excitation at 325 nm and emission at 475 nm), NADH (excitation at 350 nm and emission at 450 nm) and FAD (exitation at 450 nm and emission at 500 nm), from the structural proteins elastin and collagen (exitation at 330 nm and emission at 390 nm) and from pophyrins (exitation at 400 nm and emission at 630 nm). Those differences arise from the different metabolic state and structural characteristic of lesions to healthy tissues (Ramanujam 2000). Higher intensity of the tyrosine and tryptophan amino acids is a result of their overproduction in cancer cells, concerning unrestricted cell proliferation and growth (Hirayama et al. 2009). We observe lower intensity of the fluorescence of NADPH, NADH and structural proteins. Depending on the site of the investigated cancer higher (Muller et al. 2003) or lower (Lim et al. 2014) levels of NADH are observed, since cancer cells have different metabolic paths than the typical for the site cells NADPH’s and NADH’s function and properties changes. Cancer cells use metalloproteinase enzymes to disrupt the surrounding extracellular matrix for tumour’s needs for new vascularisation and this results in degradation of structural proteins, hence we observe lower intensity of their fluorescence in tumour’s spectra (DeClerck 2000). There is also difference in the fluorescence of FAD as a result of alterated metabolic pathways (Skala et al. 2007). The observed by us spectral characteristics of tumour and healthy tissue correlate well with previously reported data (Borisova et al. 2015; Bottiroli et al. 1995; Barr et al. 1998; Liu et al. 2012; Richards-Kortum et al. 1991).
The observed gradually intensification of the fluorescence of porphyrines is typical for tumours. Porphyrines are precursures in the cycle of heme synthesis, the inhibited activity of the enzyme ferrohelatases in tumour’s cells causes bypassing in the heme synthesis cycle, hence excessive accumulation of porphyrines (Kemmner et al. 2008; Moesta et al. 2001).
For wavelength interval corresponding of the Stoke’s shift of a particular fluorophore in the investigated sample, the fluorescence maximum of this fluorophore will appear more pronounced, in comparison with fluorescence maxima of other present fluorophores. Therefore we have chosen the wavelengths intervals matching the Stokes shift of the main observed fluorophores as the measurement parameters for SFS, that result in the most diagnostically valuable fluorescent spectra. The selected SFS spectra are obtained for wavelength intervals of 60, 90, 120, 240 nm. Figure 5 presents the SFS for offcet 60 nm of the three tumour samples and healthy tissue, the main peaks are at 350, 400, 450 nm and corresponds to tyrosine and tryptophan, collagen and NADH fluorescence, respectivley. Figure 6 presents the SFS for offcet 90 nm of the three tumour samples and healthy tissue, the main peaks are at 380 and 450 nm and corresponds to tryptophan and NADH fluorescence, respectivley. Figure 7 presents the SFS for offcet 120 nm of the three tumour samples and healthy tissue , the main peaks are at 450, 630 nm and corresponds to NADH and porphyrines fluorescence, respectivley. Figure 8 presents the SFS for offcet 240 nm of the three tumour samples and healthy tissue, the main peaks are at 530, 630 nm and corresponds to FAD and porphyrines fluorescence, respectivley.
The spectrum from sample tumour 1 is the most similar, among the three tumour samples, to the spectrum obtained from healthy tissue, but it also showcases spectral alterations, which we typically observe in tumour tissue spectra higher intensity of the amino acids fluorescence and lower intensity of the collagen and NADPH/NADH fluorescence. In comparison of the spectrum of sample tumour 1 with the spectra of samples tumour 2 and tumour 3, the fluorescence of the porphyrines, which is typical for more advanced stage tumours, is with much lower intensity. Thus we consider that sample tumour 1 is a tumor at an earlier stage of development, according to tumours in sample 2 and 3. Further gradually the fluorescence hallmarks of cancerous tissue alterations are enhanced from sample tumour 2 to sample tumour 3. Therefore we suggest that among the three investigated tumours tumour 3 is in latest stage of development.
4 Conclusions
The observed differences in the SFS spectra of cancerous and healthy tissues are related to specific pathologic alterations affecting particular fluorophores; hence they have diagnostic value, specific for cancerous tissues. We have chosen Stokes shift to match those particular fluorophores, fluorescence’s specifications to define spectral parameters, with great diagnostic value and potential for stage evaluation, out of the resultant SFS spectra. After statistical evaluation of the significance of the defined parameters we will choose the most suitable ones for development of a robust diagnostic algorithm for implementation in the clinical practice with the proper equipment.
References
Aihara, H., Tajiri, H., Suzuki, T.: Application of autofluorescence endoscopy for colorectal cancer screening: rationale and an update. Gastroenterol. Res. Pract. 2012, 5 (2012). doi:10.1155/2012/971383
Aihara, H., Sumiyama, K., Saito, S., Tajiri, H., Ikegami, M.: Numerical analysis of the autofluorescence intensity of neoplastic and non-neoplastic colorectal lesions by using a novel videoendoscopy system. Gastrointest. Endosc. 69, 726–733 (2009)
Al-Thunayan, M.: Cancer diagnosis by synchronous fluorescence spectra of blood and urine components. PhD thesis, King Saud University (2006)
Barr, H., Dix, T., Stone, N.: Optical spectroscopy for the early diagnosis of gastrointestinal malignancy. Lasers Med. Sci. 13, 3–13 (1998)
Bernard, S., Christopher, P.: World cancer report 2014. World Health Organization pp. 24–69 (2015) http://www.who.int/mediacentre/factsheets/fs297/en/
Borisova, E., Angelova, L.: Endogenous and exogenous fluorescence skin cancer diagnostics for clinical applications. IEEE J. Selected Topics Quantum Electronics 20(2), 1–12 (2014). doi:10.1109/JSTQE.2013.2280503
Borisova, E., Genova, T., Zhelyazkova, A., Angelova, L., Keremedchiev, M., Penkov, N., Terziev, I., Semyachkina-Glushkovskaya, O., Avramov, L.: Synchronous autofluorescence spectroscopy of gastrointestinal tumours tool for endogenous fluorophores evaluation. Optoelectron Adv Mat 9(9–10), 1234–1238 (2015)
Borisova, E., Zhelyazkova, A., Keremedchiev, M., Penkov, N., Semyachkina-Glushkovskaya, O., Avramov, L.: Endogenous synchronous fluorescence spectroscopy (sfs) of basal cell carcinoma-initial study. Opt. Spectrosc+. 120(1), 38–44 (2016)
Bottiroli, G., Croce, A., Locatelli, D., Marchesini, R., Pignoli, E., Tomatis, S., Cuzzoni, C., Di Palma, S., Dalfante, M., Spinelli, P.: Natural fluorescence of normal and neoplastic human colon: a comprehensive “ex vivo” study. Lasers Surg. Med. 16, 48–60 (1995). doi:10.1002/lsm.1900160107
Broek, F., Fockens, P., Dekker, E.: Review article: new developments in colonic imaging. Aliment Pharmacol. Ther. 26, 91–99 (2007)
Dankowska, A., Małecka, M., Kowalewski, W.: Discrimination of edible olive oils by means of synchronous fluorescence spectroscopy with multivariate data analysis. Grasas y aceites 64(4), 425–431 (2013)
DeClerck, Y.: Interactions between tumour cells and stromal cells and proteolytic modifcation of the extracellular matrix by metalloproteinases in cancer. Eur. J. Cancer 36, 1258–1268 (2000)
Dramićanin, T., Lenhardt, L., Zeković, I., Miroslav, D.: Support vector machine on fluorescence landscapes for breast cancer diagnostics. J. Fluoresc. 22, 1281–1289 (2012)
Drouillard, A., Bouvier, A., Rollot, F., Faivre, J., Jooste, V., Lepage, C.: Conditional net survival: relevant prognostic information for colorectal cancer survivors. A french population-based study. Dig. Liver Dis. 47(7), 597–601 (2015)
Ferlay, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D., Forman, D., Bray, F.: Worldwide data. France: International Agency for Research on Cancer p E359–E386 (2012) http://www.wcrf.org/int/cancer-facts-figures/worldwide-data
Fujiya, M., Kohgo, Y.: Image-enhanced endoscopy for the diagnosis of colon neoplasms. Gastrointest. Endosc. 3, 545–555 (2013)
Giubileo, G., Colaoa, F., Puiub, A., Panzironi, G., Brizzi, F., Rocchini, P.: Fluorescence spectroscopy of normal and follicular cancer samples from human thyroid. Spectroscopy 19, 79–87 (2005)
Hasan, M., Wallace, M.: Image-enhanced endoscopy. Clin. Update 16, 1–5 (2009)
Hirayama, A., Kami, K., Sugimoto, M., Sugawara, M., Toki, N., Onozuka, H., Kinoshita, T., Saito, N., Ochiai, A., Tomita, M., Esumi, H., Soga, T.: Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 69, 4918–4925 (2009). doi:10.1158/0008-5472.CAN-08-4806
Jeyasingh, E., Prakasarao, A., Singaravelu, G.: Synchronous fluorescence spectroscopy for the detection and characterization of cervical cancers in vitro. Photochem. Photobiol. 86, 77–86 (2010)
Johansson, A., Kromer, K., Sroka, R., Stepp, H.: Clinical optical diagnostics-status and perspectives. Med. Laser Appl. 23 (2008)
Kemmner, W., Wan, K., Rüttinger, S., Ebert, B., Macdonald, R., Klamm, U., Moesta, K.: Silencing of human ferrochelatase causes abundant protoporphyrin-ix accumulation in colon cancer. FASEB 22, 500–509 (2008). doi:10.1096/fj.07-8888com
Kiesslich, R., Mv, Bergh, Hahn, M., Hermann, G., Jung, M.: Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy 33, 1001–1006 (2001)
Kuiper, T.: Advancements in colorectal imaging. PhD thesis, University of Amsterdam, (2013) http://hdl.handle.net/11245/1.385781
Lim, L., Brandon, R., Migden, M., Rajaram, N., Reichenberg, J., Markey, M., Ross, M., Tunnella, J.: Clinical study of noninvasive in vivo melanoma and nonmelanoma skin cancers using multimodal spectral diagnosis. J. Biomed. Opt. 19(11), 117,003 (2014)
Liu, L., Nie, Y., Lin, L., Li, W., Huang, Z., Xiea, S., Li, B.: Pattern recognition of multiple excitation autofluorescence spectra for colon tissue classification. Photodiagn. Photodyn. 10(2), 111–119 (2012)
Lloyd, J.: Synchronized excitation of fluorescence emission spectra. Nat. Phys. Sci. 231, 64–65 (1971)
Masilamani, V., AlSalhi, M., Vijmasi, T., Govindarajan, K., Rathan Rai, R., Atif, M., Prasad, S.: Fluorescence spectra of blood and urine for cervical cancer detection. J. Biomed. Opt. 17(9), 0980011–0980016 (2012)
Moesta, K., Ebert, B., Handke, T., Nolte, D., Nowak, C., Haensch, W., Pandey, R., Dougherty, T., Rinneberg, H., Schlag, P., AS, A.: Protoporphyrin ix occurs naturally in colorectal cancers and their metastases. Cancer Res. 61, 991–999 (2001)
Muller, M., Valdez, T., Georgakoudi, I., Backman, V., Fuentes, C., Kabani, S., Laver, N., Wang, Z., Boone, C., Dasari, R., Shapshay, S., Feld, M.: Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer 97, 1681–92 (2003)
Pavlova, P., Borisova, E., Avramov, L., Petkova, E., Troyanova, P.: Investigation of relations between skin cancer lesions images and their fluorescent spectra. Laser Phys. 20, 596–603 (2010)
Pis, L., Majek, P., Sádecká, J.: Synchronous fluorescence spectroscopy for differentiating between brandies and wine distillates. Acta Chimica Slovaca 4(1), 47–58 (2011)
Ramanujam, N.: Fluorescence spectroscopy in vivo. In: Meyers, R. (ed.) Encyclopedia of Analytical Chemistry, pp. 20–56. John Wiley & Sons Ltd, Chichester (2000). doi:10.1002/9780470027318.a0102
Rastogi, A., Early, D., Gupta, N., Bansal, A., Singh, V., Ansstas, M., Jonnalagadda, S., Hovisc, C., Gaddam, S., Wani, S., Edmundowicz, S., Sharma, P.: Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest. Endosc. 74(3), 593–602 (2011)
Richards-Kortum, R., Rava, R., Petras, R., Fitzmaurice, M., Sivak, M., Feld, M.: Spectroscopic diagnosis of colonic dysplasia. Photochem. Photobiol. 53, 777–786 (1991)
Skala, M., Riching, K., Gendron-Fitzpatrick, A., Eickhoff, J., Eliceiri, K., White, J., Ramanujam, N.: In vivo multiphoton microscopy of nadh and fad redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. PNAS 104(49), 19,494 –9 (2007)
Song, L., Banerjee, S., Desilets, D., Diehl, D., Farraye, F., Kaul, V., Kethu, S., Kwon, R., Mamula, P., Pedrosa, M., Rodriguez, S., Tierney, W.: Autofluorescence imaging. Gastrointest. Endosc. 73, 647–650 (2011)
Song, M., Ang, T.: Early detection of early gastric cancer using image-enhanced endoscopy: current trends. Gastrointest. Interv. 3, 1–7 (2014)
Subramanian, V., Ragunath, K.: Advanced endoscopic imaging: a review of commercially available technologies. Clin. Gastroenterol. H 12(368–76), e1 (2014)
Tada, K., Oda, I., Yokoi, C., Taniguchi, T., Sakamoto, T., Suzuki, H., Nonaka, S., Yoshinaga, S., Saito, Y., Gotoda, T.: Pilot study on clinical effectiveness of autofluorescence imaging for early gastric cancer diagnosis by less experienced endoscopists. Diagn. Ther. Endoscopy 2011, 7 (2011)
Vo-Dinh, T.: Principle of synchronous luminescence (sl) technique for biomedical diagnostics. Proc. SPIE 3911, 42–49 (2000)
Zhang, X., Fales, A., Vo-Dinh, T.: Time-resolved synchronous fluorescence for biomedical diagnosis. Sensors 15, 21,746–21,759 (2015)
Zhelyazkova, A., Borisova, E., Genova, T., Pavlova, E., Troyanova, P., Terziev, I., Penkov, N., Avramov, L.: Polarization-sensitive autofluorescence spectroscopy of non-melanoma cutaneous tumours. Optoelectron. Adv. Mat. 17(9–10), 1283–1287 (2015)
Acknowledgments
This work is supported in part by the NSF-BMES under Grant #DFNI-B02/9/2014, and in part by the MES-RF under Grant # 17.488.2014/K. E. Borisova and Ts. Genova personally acknowledged BM1205 COST Action, WFS National Scholarship Programme support and Program for support of the young scientists in Bulgarian Academy of Sciences for funding within Grant # DFNP-183-A1.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Advances in the Science of Light.
Guest edited by Jelena Radovanovic, Milutin Stepic, Mikhail Sumetsky, Mauro Pereira and Dragan Indjin.
Rights and permissions
About this article
Cite this article
Genova, T., Borisova, E., Zhelyazkova, A. et al. Colorectal cancer stage evaluation using synchronous fluorescence spectroscopy technique. Opt Quant Electron 48, 378 (2016). https://doi.org/10.1007/s11082-016-0634-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0634-7