Abstract
The change of firing rates depends on how synaptic features interact with intrinsic properties of cells in neural system. Considering the chemical synaptic features, we design a controllable memristive synapse with magnetic coupling that are voltage-controlled, nonlinear, and unidirectional. To explore the effect of firing rates on interactions between synapse and neuron, the memristive synaptic current involving excitation and inhibition is then mapped into a generalized neuronal model. We observe and characterize the appearance of counterintuitive behavior that increased excitatory memristive synaptic current leads to the decrease in firing rates, and increased inhibitory memristive synaptic current leads to the increase in firing rates in the neural circuit. For the counterintuitive phenomenon, we utilize a geometric dynamics method to provide an underlying dynamics mechanism how the excitatory or inhibitory current impacts the decrease or increase in firing rates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The excitatory and inhibitory inputs have an important effect on the electrical activities of neural system [1,2,3]. There are many neurons in neural system, and each has much synaptic connections with other neurons. Neuron is thought to be a fundamental component. Due to the existence of ion channels, neuron can generate a pulse named action potential when the external forcing current is injected. The action potential or spike is responsible for signaling transmission along the axon with a certain velocity [4]. The generation of spike was confirmed via the biophysical experiments [5]. Meanwhile, neural excitability has been categorized as three classes by observing the interplay between firing rates and the external forcing current [6]. According to the qualitative dynamics analysis, Class 1, 2, and 3 excitabilities occur through, respectively, a saddle node on invariant circle bifurcation, a Hopf bifurcation, and a quasi-separatrix crossing when the appropriate parameters and the forcing currents are selected [7].
Further, some input currents from synaptic component may also regulate neural electrical activities. In typical pacemaker neurons, increased inhibitory input rates cause the increase in firing rates, and increased excitatory input rates cause the decrease in firing rates [8]. This view is a counterintuitive behavior than one traditional view that the excitatory synapses always tend to enhance firing rates, and the inhibitory synapses tend to reduce firing rates [9,10,11]. Indeed, both excitatory inputs and inhibitory inputs are simultaneously mapped into the pyramidal neurons so that firing rates become increased [12]. The balance between excitation and inhibition is of fundamental importance to regulate the electrical activities of neural systems [13,14,15,16]. The brief time interval between inhibition and excitation is able to control the generation of spikes in auditory circuits based on experimental observations and theoretical analysis [17]. The interplay between excitation and inhibition plays a key role in facilitating or suppressing spikes in auditory system [18,19,20,21]. Based on the morphological evidence, the synapse that connects with the same neuron’s dendrites, called autapse, has inhibitory effects on repetitive firing and increased the current threshold for evoking spikes, which provides a novel and powerful form of feedback inhibitory synaptic transmission in one class of cortical interneurons [22]. In summary, the synaptic current may affect firing rates and firing patterns in neural systems.
Thus, the mathematic physical model and the analog electronic system have been extensively used to characterize and explore dynamics properties in the neural circuits involving electrical or chemical synapse. Employing Ohm’s law, the current through a resistor was added into the integrate-and-fire neuronal model to regulate electric activities [23]. The mathematic model with conductance and time-delayed effect is intuitively used to describe autaptic coupling current in a stochastic Hodgkin–Huxley model [24]. Control of the autaptic strength and the time delay can exhibit the complex distribution of firing rates. And the autaptic model with time-delayed feedback has an effect on the firing rates in Hodgkin–Huxley model under sinusoidal stimulus [25]. The effects of electrical and chemical autaptic model on firing regularity have been investigated in neural systems [26]. It is found that autaptic time delay and appropriately tuned conductance can induce multiple coherence resonance. Autapse is able to enhance transmission for weak signal across the scale-free neural networks and improve the transmission of pacemaker rhythm [27, 28]. The chemical synaptic coupling function plays a critical role in the emergence of electric activities and chimera states in bursting neural system [29]. The complex firing regularities may arise from the neural system with the electrical and chemical coupling or from the uncoupled neurons with the multilayer structure [30,31,32]. The time-varying conductance model is employed to characterize excitatory and inhibitory coupling current so that strong inhibitory inputs guarantee effectively inhibition of action potentials [33, 34]. And the nonlinear conductance with time-varying feature is often use to characterize excitatory or inhibitory synaptic current associated with the short/long-term plasticity [35,36,37,38,39,40,41,42,43]. The conductance involving exponential function is able to describe chemical synaptic current participated with the various type of receptors [44,45,46,47,48,49].
On the other hand, in order to characterize the function of bio-synapse, the electronic and manufactured components have been attracted considerable attention in recent some years. For the neuromorphic circuits, there are many excellent cases to emulate synapse based on the properties of memristor [50,51,52]. For example, the hybrid system composed of complementary metal–oxide semiconductor neurons and memristor synapses is able to realize spike timing-dependent plasticity [52]. The electronic system composed of the memristor emulator exhibits various firing patterns, including three classes excitabilities, regular spiking, intrinsic bursting, chattering, and fast spiking [53, 54]. The devices of three-terminal memristor are capable of emulating short-term and long-term plasticity concomitantly [50]. And a review enumerated the many excellent cases about artificial synapses and neurons based on memristive devices [55]. Over recently, two Colpitts oscillators connected by the magnetic coupling have complex dynamical behaviors [56]. The multimode oscillations and clustered patterns appear in the electronic system connected by both magnetic and resistive coupling [57]. Therefore, whether the synapse emulated by electronic components may be used to regulate firing patterns and firing rates. Considering memristor concept and magnetic coupling as well as synaptic feature, we propose a memristive synapse involving magnetic coupling to regulate firing rates in the neural circuits.
The rest of the present paper is organized as follows. In Sect. 2, we design a memristive electronic system with magnetic coupling. The formula of memristive synapse is deduced and described. In Sect. 3, we analyze and discuss the dynamical behaviors in a neuronal model with excitatory and inhibitory memristive synaptic current. The underlying mechanism is also investigated by the fast/slow decomposition. The conclusion is given in Sect. 4.
2 Model description
Considering the memristor proposed by Chua [58], the most basic mathematical definition of a voltage-controlled memristor reads
where w is the state variable of the electronic circuit, and G is the nonlinear conductance dependent on the internal state. In 1976, the memristor concept was further generalized by Chua and Kang to satisfy a much broader class of nonlinear electronic circuits called memristive systems [59]. And its form is described by the differential equations
Inspired by the memristive system and the magnetic coupling in Refs. [49, 50], we design a controllable memristive system composed of the voltage follow, magnetic coupling, and two multipliers. The schematic diagram is shown in Fig. 1.
The analog circuit composed of two voltage follows (U1, U2), one transformer (T2) to characterize magnetic coupling, one integrator (U3), and two multipliers (A1, A2). The two red oblique 8-type curves are plotted via analog circuit and numerical stimulation, respectively. Note that the red arrow denotes input voltage and the black arrow denotes output current. (Color figure online)
Note that our designed memristive system is a voltage-controlled and time-varying device with the unidirectional output current \(i_{M}\). This system also exhibits current–voltage hysteresis in the inset of Fig. 1. Applying the Kirchhoff’s law into the magnetic coupling part in circuit mentioned above, the coupling equations are obtained by
where \(i_{1}\), \(V_{1}\), and \(i_{2}\), \(V_{2}\) represent the current and voltage in primary coil and secondary coil, respectively. The voltage follow U1 can guarantee the unidirectional current input, and \(V_{1}\) is equal to \(V_{\mathrm{pre}}\). R, L, and M are the resistance, self-inductance, and mutual inductance. Due to the existence of a voltage follow U2, the current in secondary circuit is zero as \(i_{2} = 0\). Thus, Eq. (3) is rewritten as
where \(R_{1}\) is a controllable resistance to mediate the oscillatory frequency so that there is a different relationship between current and voltage in the whole circuit. Through an integrator U3, we get
Through the first analog multiplier A1, we get
The eventual output current through the second analog multiplier A2 reads
Further, the potential scale transformation is considered as following equations
Substituting Eq. (8) into Eq. (7), the memristive synaptic current is given by
where \(i_{M}\) denotes the output current through a memristive system involving magnetic coupling, w is a dimensionless internal state, and x is also a dimensionless voltage. \(k_{M}\) is a memristive coefficient roughly proportional to quadratic mutual inductance M. To realize the red oblique 8-type curve of memristor, \(k_{M}\), \(x_{\mathrm{rev}}\), and \(k_{1}\) can be selected as 1, 0, and 0.2.
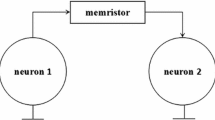
Form the standpoint of neuroscience, synapse in a neural system makes neuron produce the firing patterns such as resting, spiking, and bursting, even to chaotic state. The synapse is roughly divided into the chemical connection and gap junction or electrical connection. Chemical synapse is probabilistic, time-delayed, nonlinear, and unidirectional. Electrical synapse is resistive and bidirectional. The interaction between two kinds of synaptic currents is of fundamental important to regular electric activities in neuronal system. So we will try to use the memristive synapse to emulate bio-synaptic connection in terms of the regulating firing rates. In order to simplify and analyze in circuit, we further assume the postsynaptic potential \(V_{\mathrm{post}}\) and presynaptic potential \(V_{\mathrm{pre}}\) are equal and set as x. The schematic diagram is shown in Fig. 2.
For the black node in Fig. 2, based on the Kirchhoff’s law, the generalized Hindmarsh–Rose neuronal model [60] connected by the memristive synapse is written as
where the variable x, y, or z denotes the membrane potential, recovery current, and slow adaption current, respectively. \(I_{M}= k_{M} w^{2}(x_{\mathrm{rev}}-x)\) and \(I_{R} = k_{R}(x_{\mathrm{rev}}-x)\) represent the memristive synaptic current and resistive coupling current. \(k_{M}\) is a memristive coefficient. \(k_{R}\) is a resistive coefficient (or conductance). \(x_{\mathrm{rev}}\) is a bias voltage. Modulating \(x_{\mathrm{rev}}\) can obtain excitatory or inhibitory current. For example, in the auditory brainstem, the synaptic current \(I_{\mathrm{syn} }=G(t)(E_{\mathrm{syn}}-V)\) is added into a leakage conductance model \({ C\mathrm{d}V/\mathrm{d}t }= -I_{\mathrm{leak}}+I_{\mathrm{syn}}\), where G(t) is the time-varying conductance [27]. And selecting \(E_{\mathrm{syn}}\) such as 0 mV or \(-80\) mV designates the excitatory or inhibitory synaptic current. So modulating \(x_{\mathrm{rev}}\) (greater than the maximum of x, or less than the minimum of x) such as 2.5 or \(-4\) gets the excitation or inhibition. \(I_{\mathrm{ext}}\) is an external forcing current. \(I_{\mathrm{AMCC}}\) or \(I_{\mathrm{ARCC}}\) denotes an average memristive or resistive coupling current during a period T as
The parameter values are selected as \(a=1.0\), \(b=3.0\), \(c=1.0\), \(d=5.0\), \(r=0.001\), \(s=4.0\), \(k_{1}=0.2\). The bifurcation diagram is calculated via the software XPPAUT. In the numerical studies, the fourth Runge–Kutta algorithm is used to obtain solutions of nonlinear system based on the ODE45 in MATLAB 2017.
3 Results
One traditional view is that excitatory synapse always trends to promote electrical activity or increase firing rate. Inhibitory synapse, on the other hand, is thought to depress electrical activity or decrease firing rate. However, simple examples demonstrate that it may not always be the case. For example, there exists the significant result that increased excitatory inputs can decrease firing rates, and increased inhibitory inputs can enhance firing rates [8]. To explore counterintuitive phenomenon, a goal of this section is to consider three-variable neuronal models connected with different types of emulated synapses. And the synaptic current, by modulating different bias voltage \(x_{\mathrm{rev}}\), can be roughly divided into the excitatory and inhibitory current. To begin, we use the excitatory memristive and resistive current to regulate firing rates. Next, the inhibitory memristive current increases firing rates. Eventually, we try also to provide a potential geometric dynamics mechanism for this counterintuitive behavior based on the fast–slow decomposition method.
3.1 Excitatory current
In order to emulate the counterintuitive phenomenon for excitatory memristive synapse, we start from the period-11 bursting as initial firing mode when the external forcing current is fixed at \(I_{\mathrm{ext}}=2.1\), as shown in Fig. 3a. After increasing the memristive coefficient \(k_{M}\) and with only the memristive synapse, it is found that there appears a transition from initial state, to period-6 bursting, to period-4 bursting, and to period-1 firing, as depicted in the black lines of Fig. 3b–d, respectively. Based on the classification of bursting oscillation in Refs. [61, 62], this bursting belongs to a square-wave bursting. From Fig. 4a, the average memristive coupling current becomes more positive, indicating that excitatory degree becomes strong. Furthermore, we calculate the interspike interval (ISI) and mean firing frequency f. According to the ISI shown in Fig. 4b, the electrical activities of a neuronal model with the memristive synapse belongs to an inverse period-adding bifurcation. And it is confirmed that the increase in excitatory memristive current lowers firing frequencies as illustrated in the red curve of Fig. 4b.
Temporal evolution of the membrane potential (black) and memristive synaptic current (red) in HR model at different memristive synaptic coefficient as a\(k_{M}=0.0\); b\(k_{M}=1.0\); c\(k_{M}=2.0\); d\(k_{M}=4.0\). The resistive coupling current is zero (\(k_{R}=0\)), and modulating the reversal potential as \(x_{\mathrm{rev}}=2.5\) gets excitatory current. (Color figure online)
Similarly, we also analyze dynamical behavior of neuronal model with only the excitatory resistive current. Under same condition, with increase in the resistive coupling gain \(k_{R}\), it is found that the neural circuit exhibits a transition between square-wave bursting and plateau-like bursting by observing trajectory of the temporal evolution of membrane potential, as shown in Fig. 5a–c. The average value of resistive coupling current is excitatory, as shown in Fig. 6a. Meanwhile, the ISI and mean firing rates are calculated in Fig. 6b. The neuronal model connected with an excitatory resistive current exhibits a complex evolution. In the red rectangle of regions, this excitatory resistive current plays a natural role in enhancing the electrical activities. In other words, its firing frequency should be increasing, which is analogous to our results by observed in the same red rectangle of Fig. 6b. After through a critical point, the mean firing rate will, however, lower if when we continuously increase resistive coupling gain. This potential mechanism is that the stronger input current destroys the normal function of system, resulting the whole system appears a resting state.
Temporal evolution of the membrane potential (black) and resistive coupling current (red) in HR model at different resistive coupling coefficient as a\(k_{R}=0.0\); b\(k_{R}=0.1\); c\(k_{R}=0.5\); d\(k_{R}=1.0\). The memristive current is zero (\(k_{M}=0\)), and setting the reversal potential as \(x_{\mathrm{rev}}=2.5\) gets excitatory current. (Color figure online)
Considering the coexistence of chemical and electric synapse in neural system, the interaction between two forms of couplings might be helpful to communicate information conveyed from one neuron to other neuron, rapidly [63]. Hence, together with the memristive electronic system and resistor this allows the better examination of the role that the memristive synapse plays on the regularizing dynamical behavior of neuronal model. In order to emphasize the effect of memristive synapse, the resistive coupling gain is small as \(k_{R}=0.1\). The transition, from period-17 square-wave bursting to period-13 bursting, and then from about period-16 plateau-like bursting to around period-9 bursting, appears in neural system as shown in the black trace of Fig. 7. According to Fig. 8(a), the sum of both memristive and resistive coupling current still exhibits the excitatory current. Further, we characterize in detail the evolution of process via the ISI and the mean firing rate. There are two kinds of mode transitions, such as square-wave bursting in red rectangle A1 and plateau-like bursting in red rectangle A2. The mean firing frequency in each process increases in the two red rectangles of Fig. 8b. This point differs from the only existence of resistive coupling current. Under the coexistence of excitatory memristive and resistive coupling, the firing rates become low no matter how firing transitions change. In summary, our results show that the excitatory memristive synapse can reduce rather than enhance firing rates of neuronal model, which is different from the common viewpoint that excitatory current is able to improve firing activities.
Temporal evolution of the membrane potential (black) and excitatory memristive and resistive current (red) in HR model at different memristive coefficient as a\(k_{M}=0.0\); b\(k_{M}=1.0\); c\(k_{M}=3.0\); d\(k_{M}=5.0\). The resistive coupling current is fixed at \(k_{R}=0.1\), and setting the reversal potential as \(x_{\mathrm{rev}}=2.5\) gets excitatory current. (Color figure online)
a Average memristive and resistive coupling current \(I_{\mathrm{AMCC}}+I_{\mathrm{ARCC}}\); b the ISI (black) and the mean firing frequency (red) in HR model with increasing \(k_{M}\) at \(x_{\mathrm{rev}}=2.5\), \(k_{R}=0.1\). In red rectangle A1 and A2, firing modes are the square-wave bursting and plateau-like bursting, respectively. (Color figure online)
3.2 Inhibitory current
In order to explore the effect of inhibitory memristive synapse, we employ the following parameters as \(I_{\mathrm{ext}} = 1.1\), \(x_{\mathrm{rev}}= -4\). We start from the period-3 bursting as initial firing mode, as shown in Fig. 9a. After increasing the memristive efficient \(k_{M}\) and without considering the resistive coupling current, there exists a transition between period-5 bursting, period-6 bursting, and period-7 firing, as depicted in Fig. 9b–d, respectively. In Fig. 10a, the average memristive coupling current becomes more negative, suggesting that the occurrence of inhibitory current is more obvious. Further, we utilize the interspike interval (ISI) and mean firing frequency f to characterize the transition procedure. As illustrated in the ISI of Fig. 10b, dynamical behavior of a neuronal model with only an inhibitory memristive synapse belongs to a period-adding bifurcation. And it is confirmed that increased inhibitory memristive current can enhance firing frequencies as show in the red curve of Fig. 10b. We try also to calculate electrical activities of the neuronal model associated with only an inhibitory resistive coupling. The results show that firing rates fail to increase while reaching a resting state. In sum, the excitatory and inhibitory memristive synapse from our design, mapped into a general neuronal model, can realize above-mentioned results (see Figs 4, 10), qualitatively consistent with the findings that increased inhibitory inputs can enhance firing rates, and increased excitatory inputs can reduce firing frequencies [8].
Temporal evolution of the membrane potential (black) and inhibitory memristive synapse (blue) in HR model at different memristive coupling coefficient as a\(k_{M} = 0.0\); b\(k_{M}= 0.3\); c\(k_{M}= 0.6\); d\(k_{M} = 1.0\). The resistive coupling current is zero (\(k_{R}=0.0\)), and setting the reversal potential as \(x_{\mathrm{rev}} = -4\) gets inhibitory current. (Color figure online)
Bifurcation structure of the fast subsystem and phase trajectory of the whole system. Left: the excitatory memristive current in Fig. 4. Right: the inhibitory memristive current in Fig. 10. The red point is a supercritical Hopf, the black point is a saddle node, and the green point is a saddle homoclinic. (Color figure online)
3.3 Dynamics analysis
Based on above-mentioned results, we further try to explore a possible mechanism for the decrease and the increase in firing rates under the excitatory and inhibitory memristive synapse. Thus, the fast–slow decomposition method is employed in this section [64,65,66]. Due to the existence of a small singular parameter \(r<<1\), the neural system for bursting involves multiple timescales. The derivative of remaining variables (x, y, w) in Eqs. (6) and (7) constructs a fast subsystem responsible for spikes generation. The derivative of variable z in Eq. (7) is a slow subsystem responsible for the slow modulation of silent and active phases. The silent phase of a bursting trajectory passes near a manifold of fixed points of the fast subsystem, whereas the active phase or the repetitive spikes of a bursting trajectory passes near a manifold of periodic solutions of the fast subsystem. The slow variable modulates fast dynamics between these two phases. As illustrated in the inset of Fig. 11, there exists a Z-type curve composed of the stable (solid line) and unstable (dashed line) equilibrium in fast subsystem. And the maximal and minimal values of periodic oscillation arise from a supercritical Hopf bifurcation (red point), when the slow variable z in fast subsystem is viewed a bifurcation parameter. For the generation of a black square-wave burster in Fig. 11, the trajectory of active phase originates at a saddle-node point (fold bifurcation) and terminates at a homoclinic orbit to the middle branch of Z-type curve. That is, the square-wave burster is denoted as fold/homoclinic.
Furthermore, we elucidate the geometric dynamics features of counterintuitive phenomenon in the neuronal model with excitatory and inhibitory memristive synapse. For the excitatory memristive synapse in the left-hand side of Fig. 11, the black saddle-node (SN) point and the green saddle-homoclinic (SH) point occur a rightward shift in large and small scale along with the red arrow, respectively. The result is that there appears a shrinking distance between SN (beginning point) and SH (terminal point) of a burster, indicating that the number of spikes per burster will decrease under excitatory current. For the inhibitory memristive synapse in the right-hand side of Fig. 11, the SN point and the SH point have a leftward shift in large and small scale along with the blue arrow, respectively. While there is an extending distance between SN and SH of a burster, suggesting that the number of spikes per burster will increase under inhibitory current. These results may be helpful to understand the evidence that increased inhibitory input rates can enhance firing rates, while increased excitatory input rates can reduce firing rates.
Given the above analysis and investigation, the topic of memristive synapse is currently attracting considerable interest as the physically underlying nonlinear coupling arising in the dynamics system. The quadratic magneto-electric coupling can occur the nonlinear vibrations having phonon frequencies dependent upon the electrical polarization and magnetization [67]. And we have also in detail discussed the function of quadratic term in chaotic system dependent upon the initial values [68, 69]. Thus, the nonlinear coupling involving the quadratic term is raising hope for exploring the control of electrical activities in neuromorphic system composed of neuron and synapse.
4 Conclusions
Complex firing patterns are believed to play a critical role in many brain functions. The synaptic properties of connections between neurons have a dramatic influence on the dynamical behavior of neural systems. And the intrinsic property of cell is a very important factor. Complex rhythms and firing transitions arise from the interactions between neuron and synapse. Thus, the memristive synapse is used for emulating bio-synapse in this paper. Memristive synapse involving magnetic coupling has some features like chemical synapse, such as voltage-controlled, nonlinear, and unidirectional. The dynamical behavior may depend not only on whether the synapses are excitatory or inhibitory, but on the intrinsic properties of neuron in neural system. Thus, memristive synapse with excitation or inhibition is mapped into the general neuronal model. We focus on exploring the regulating firing rates in neuronal circuit by activating memristive synapse. According to the interspike interval and firing rate of analysis, it is found that excitatory memristive synaptic current causes the decrease in firing rates. And inhibitory memristive synaptic current is able to increase firing rates. Based on the geometric dynamics properties, excitatory memristive synapse makes the distance per burster shrink so that the number of spikes per burster become sparse, suggesting that excitation decreases firing rates. While inhibitory memristive synapse makes the distance per burster extend so that the number of spikes per burster become dense, suggesting that inhibition increases firing rates. These results provide a better insightful description and understanding for underlying dynamics mechanism of the counterintuitive phenomenon that increased excitatory inputs can decrease firing rates, and increased inhibitory inputs can increase firing rates.
References
Denève, S., Machens, C.K.: Efficient codes and balanced networks. Nat. Neurosci. 19, 375–382 (2016)
Pecka, M., Han, Y., Sader, E., Mrsic-Flogel, T.D.: Experience-dependent specialization of receptive field surround for selective coding of natural scenes. Neuron. 84, 457–469 (2014)
Sun, Y.J., Wu, G.K., Liu, B.H., Li, P., Zhou, M., Xiao, Z., Tao, H.W., Zhang, L.I.: Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature 465, 927–931 (2010)
Hodgkin, A.L., Huxley, A.F.: Action potentials recorded a Mauthner from inside. Nature 144, 710–711 (1939)
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
Hodgkin, A.L.: The local electric changes associated with repetitive action in a non-medullated axon. J. Physiol. 107, 165–181 (1948)
Prescott, S.A., De Koninck, Y., Sejnowski, T.J.: Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput. Biol. 4, e1000198 (2008)
Perkel, D.H., Schulman, J.H., Bullock, T.H., Moore, G.P., Segundo, J.P.: Pacemaker neurons: effects of regularly spaced synaptic input. Science 145, 61–63 (1964)
Kuffler, S.W., Eyzaguirre, C.: Synaptic inhibition in an isolated nerve cell. J. Gen. Physiol. 39, 155–184 (1955)
Park, T.J., Grothe, B., Pollak, G.D., Schuller, G., Koch, U.: Neural delays shape selectivity to interaural intensity differences in the lateral superior olive. J. Neurosci. 16, 6554–6566 (1996)
Sanes, D.: An in vitro analysis of sound localization mechanisms in the gerbil lateral superior olive. J. Neurosci. 10, 3494–3506 (1990)
Chance, F.S., Abbott, L.F., Reyes, A.D.: Gain modulation from background synaptic input. Neuron. 35, 773–782 (2002)
Zador, A.M., Stevens, C.F.: Input synchrony and the irregular firing of cortical neurons. Nat. Neurosci. 1, 210–217 (1998)
Mitchell, S.J., Silver, R.A.: Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 38, 433–445 (2003)
Carvalho, T.P., Buonomano, D.V.: Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input–output functions. Neuron 61, 774–785 (2009)
Harsch, A., Robinson, H.P.C.: Postsynaptic variability of firing in rat cortical neurons: the roles of input synchronization and synaptic NMDA receptor conductance. J. Neurosci. 20, 6181–6192 (2000)
Dodla, R., Svirskis, G., Rinzel, J.: Well-timed, brief inhibition can promote spiking: postinhibitory facilitation. J. Neurophysiol. 95, 2664–2677 (2006)
Beiderbeck, B., Myoga, M.H., Müller, N.I.C., Callan, A.R., Friauf, E., Grothe, B., Pecka, M.: Precisely timed inhibition facilitates action potential firing for spatial coding in the auditory brainstem. Nat. Commun. 9, 1771 (2018)
Kopp-Scheinpflug, C., Sinclair, J.L., Linden, J.F.: When sound stops: offset responses in the auditory system. Trends Neurosci. 41, 712–728 (2018)
Myoga, M.H., Lehnert, S., Leibold, C., Felmy, F., Grothe, B.: Glycinergic inhibition tunes coincidence detection in the auditory brainstem. Nat. Commun. 5, 1–13 (2014)
Kotak, V.C., Korada, S., Schwartz, I.R., Sanes, D.H.: A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J. Neurosci. 18, 4646–4655 (1998)
Bacci, A., Huguenard, J.R., Prince, D.A.: Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J. Neurosci. 23, 859–866 (2003)
Perkel, D.H., Mulloney, B., Budelli, R.W.: Quantitative methods for predicting neuronal behavior. Neuroscience 6, 823–837 (1981)
Li, Y., Schmid, G., Hänggi, P., Schimansky-Geier, L.: Spontaneous spiking in an autaptic Hodgkin–Huxley setup. Phys. Rev. E 82, 061907 (2010)
Wang, H., Chen, Y.: Influence of autapse on mode-locking structure of a Hodgkin–Huxley neuron under sinusoidal stimulus. J. Theor. Biol. 358, 25–30 (2014)
Yilmaz, E., Ozer, M., Baysal, V., Perc, M.: Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci. Rep. 6, 30914 (2016)
Baysal, V., Yilmaz, E., Özer, M.: Blocking of weak signal propagation via autaptic transmission in scale-free networks. Istanb. Univ. J. Electr. Electron. Eng. 17, 3081–3085 (2017)
Yilmaz, E., Baysal, V., Ozer, M., Perc, M.: Autaptic pacemaker mediated propagation of weak rhythmic activity across small-world neuronal networks. Phys. A. 444, 538–546 (2016)
Bera, B.K., Ghosh, D., Lakshmanan, M.: Chimera states in bursting neurons. Phys. Rev. E. 93, 012205 (2016)
Majhi, S., Ghosh, D.: Alternating chimeras in networks of ephaptically coupled bursting neurons. Chaos 28, 083113 (2018)
Bera, B.K., Rakshit, S., Ghosh, D., Kurths, J.: Spike chimera states and firing regularities in neuronal hypernetworks. Chaos 29, 053115 (2019)
Majhi, S., Perc, M., Ghosh, D.: Chimera states in uncoupled neurons induced by a multilayer structure. Sci. Rep. 6, 39033 (2016)
Gjoni, E., Zenke, F., Bouhours, B., Schneggenburger, R.: Specific synaptic input strengths determine the computational properties of excitation-inhibition integration in a sound localization circuit. J. Physiol. 596, 4945–4967 (2018)
Gerstner, W., Kistler, W., Naud, R., Paninski, L.: Neuronal Dynamics From Single Neurons to Networks and Models of Cognition. Cambridge University Press, Cambridge (2014)
Markram, H., Wang, Y., Tsodyks, M.: Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. 95, 5323–5328 (2002)
Brughera, A.R., Stutman, E.R., Carney, L.H., Colburn, H.S.: A model with excitation and inhibition for cells in the medial superior olive. Audit. Neurosci. 2, 219–233 (1996)
Large, E.W., Crawford, J.D.: Auditory temporal computation: Interval selectivity based on post-inhibitory rebound. J. Comput. Neurosci. 13, 125–142 (2002)
Brand, A., Behrend, O., Marquardt, T., McAlpine, D., Grothe, B.: Precise inhibition is essential for microsecond interaural time difference coding. Nature 417, 543–547 (2002)
Li, X., Jia, Y., Wang, Y., Kui, Z.: The collective bursting dynamics in a modular neuronal network with synaptic plasticity. Nonlinear Dyn. 89, 2593–2602 (2017)
Rothman, J.S., Young, E.D., Manis, P.B.: Convergence of auditory nerve fibers onto bushy cells in the ventral cochlear nucleus: implications of a computational model. J. Neurophysiol. 70, 2562–2583 (1993)
Casadiego, J., Maoutsa, D., Timme, M.: Inferring network connectivity from event timing patterns. Phys. Rev. Lett. 121, 054101 (2018)
Roxin, A., Riecke, H., Solla, S.A.: Self-sustained activity in a small-world network of excitable neurons. Phys. Rev. Lett. 92, 198101 (2004)
Yaroslav, F., Kossio, K., Goedeke, S., van den Akker, B.: Growing critical?: self-organized criticality in a developing neural system. Phys. Rev. Lett. 121, 058301 (2018)
Jonas, P., Racca, C., Sakmann, B., Seeburg, P.H., Monyer, H.: Differences in \(Ca^{2+}\) permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron 12, 1281–1289 (1994)
Koh, D.S., Geiger, J.R., Jonas, P., Sakmann, B.: \({\rm Ca}^{2+}\)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J. Physiol. 485, 383–402 (1995)
Jahr, C.E., Stevens, C.F.: Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J. Neurosci. 10, 3178–3182 (1990)
Wang, X.-J.: Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J. Neurosci. 19, 9587–9603 (1999)
Higo, S., Akashi, K., Sakimura, K., Tamamaki, N.: Subtypes of GABAergic neurons project axons in the neocortex. Front. Neuroanat. 3, 25–30 (2009)
Braestrup, C., Nielsen, M.: Strychnine as a potent inhibitor of the brain GABA/benzodiazepine receptor complex. Brain Res. Bull. 5, 681–684 (1980)
Zhang, Z., Li, T., Wu, Y., et al.: Truly concomitant and independently expressed short- and long-term plasticity in a \({\rm Bi}_{2}{\rm O}_{2}\)Se-based three-terminal memristor. Adv. Mater. 31, 1805769 (2019)
Najem, J.S., Taylor, G.J., Weiss, R.J., et al.: Memristive Ion channel-doped biomembranes as synaptic mimics. ACS Nano 12, 4702–4711 (2018)
Jo, S.H., Chang, T., Ebong, I., Bhadviya, B.B., Mazumder, P., Lu, W.: Nanoscale memristor device as synapse in neuromorphic systems. Nano Lett. 10, 1297–1301 (2010)
Flores, E.A., Tsang, K.K., Crowell, J.A., Yi, W., Lam, S.K., Bai, X.: Biological plausibility and stochasticity in scalable \({\rm VO}_{{2}}\) active memristor neurons. Nat. Commun. 9, 4661 (2018)
Babacan, Y., Kaçar, F., Gürkan, K.: A spiking and bursting neuron circuit based on memristor. Neurocomputing 203, 86–91 (2016)
Zhang, T., Yang, K., Xu, X., Cai, Y., Yang, Y., Huang, R.: Memristive devices and networks for brain-inspired computing. Phys. Status Solidi Rapid Res. Lett. 13, 1900029 (2019)
Kana, L.K., Fomethe, A., Fotsin, H.B., Wembe, E.T., Moukengue, A.I.: Complex dynamics and synchronization in a system of magnetically coupled colpitts oscillators. J. Nonlinear Dyn. 2017, 5483965 (2017)
Blaha, K.A., Huang, K., Della Rossa, F., Pecora, L., Hossein-Zadeh, M., Sorrentino, F.: Cluster synchronization in multilayer networks: a fully analog experiment with LC oscillators with physically dissimilar coupling. Phys. Rev. Lett. 122, 014101 (2019)
Chua, L.: Memristor-the missing circuit element. IEEE Trans. Circuit Theory 18, 507–519 (1971)
Chua, L.O., Kang, S.M.: Memristive devices and systems. Proc. IEEE 64, 209–223 (1976)
Rose, J.L., Hindmarsh, R.M.R.: A model of neuronal bursting using three coupled first order differential equations. Proc. R. Soc. Lond. B 221, 87–102 (1984)
Rinzel, J.: A Formal Classification of Bursting Mechanisms in Excitable Systems, pp. 267–281. Springer, Berlin (1987)
Barrio, R., Shilnikov, A.: Parameter-sweeping techniques for temporal dynamics of neuronal systems: case study of Hindmarsh–Rose model. J. Math. Neurosci. 1, 6 (2011)
Pereda, A.E.: Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 15, 250–263 (2014)
Rinzel, J.: Bursting Oscillations in an Excitable Membrane Model, pp. 304–316. Springer, Berlin (1985)
Guckenheimer, J., Harris-Warrick, R., Peck, J., Willms, A.: Bifurcation, bursting, and spike frequency adaptation. J. Comput. Neurosci. 4, 257–277 (1997)
Ermentrout, G.B., Terman, D.H.: Mathematical Foundations of Neuroscience, vol. 35. Springer, Berlin (2010)
Sayedaghaee, S.O., Xu, B., Prosandeev, S., Paillard, C., Bellaiche, L.: Novel dynamical magnetoelectric effects in multiferroic \({\rm BiFeO}_{{3}}\). Phys. Rev. Lett. 122, 097601 (2019)
Ma, J., Wu, F., Ren, G., Tang, J.: A class of initials-dependent dynamical systems. Appl. Math. Comput. 298, 65–76 (2017)
Wu, F., Ma, J., Ren, G.: Synchronization stability between initial-dependent oscillators with periodical and chaotic oscillation. J. Zhejiang Univ. A. 19, 889–903 (2018)
Acknowledgements
The authors thank editor Jun Ma and anonymous reviewers for their valuable comments and suggestions that helped to improve the paper. This work is supported by National Natural Science Foundation of China under Grants. 11765011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Model description in phasor domain
Appendix: Model description in phasor domain
Here, we design a controllable memristive system composed of the voltage follow, magnetic coupling, and two multipliers. The schematic diagram is shown in Fig. 12.
The analog circuit composed of two voltage follows (U1, U2), the transformer (T2) to characterize magnetic coupling, and two multipliers (A1, A2). The two red oblique 8-type curves are plotted via analog circuit and numerical stimulation, respectively. Note that the red arrow denotes input voltage and the black arrow denotes output current. (Color figure online)
Note that, our designed memristive system is a voltage-controlled and time-varying devices with only a unidirectional output current \(i_{M}\). This system also exhibits current–voltage hysteresis in the inset of Fig. 12. Applying the Kirchhoff’s law into the magnetic coupling part of circuit mentioned above, the coupling equations are obtained by
where \(i_{1}\), \(V_{1}\), and \(i_{2}\), \(V_{2}\) represent the time-harmonic current and voltage in primary coil and secondary coil, respectively. The voltage follow U1 can guarantee the unidirectional current input, and \(V_{1}\) is equal to \(V_{\mathrm{pre}}\). R, L, and M are the resistance, self-inductance, and mutual inductance. \(j\omega \) denotes the derivative with respect to time variable \(\tau \). \(\omega \) is the angular frequency. Due to the existence of a voltage follow U2, the current in secondary circuit is zero as \(i_{2}= 0\). Thus, Eq. (S1) is rewritten as
where \(R_{1}\) is the controllable resistance to mediate the oscillatory frequency so that there is a different relationship between current and voltage in the whole circuit. Through the analog multiplier A1, we get
The eventual output signal in the phasor-domain representation through the analog multipliers A2 reads
The eventual output current in the time-domain representation reads
Further, the potential scale transformation is considered as following equations
Substituting Eq. (S6) into Eq. (S5), the memristive synaptic current is given by
where \(i_{M}\) denotes the output current through a memristive system involving magnetic coupling and w is a dimensionless internal state. The approach based on the phasor domain is also able to describe the memristive synaptic current of Eq. (S7) consistent with Eq. (9) obtained from the time domain.
Rights and permissions
About this article
Cite this article
Wu, F., Zhang, Y. & Zhang, X. Regulating firing rates in a neural circuit by activating memristive synapse with magnetic coupling. Nonlinear Dyn 98, 971–984 (2019). https://doi.org/10.1007/s11071-019-05239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-019-05239-4