Abstract
The cerebellum facilitates and modulates cognitive functions using forward and inverse internal models to predict and control behavior, respectively. Despite neuroimaging evidence that regions of the cerebellum are active during executive function (EF) tasks in general, little is known about the cerebellum’s role in specific EFs and their underlying neural networks. Inhibitory control specifically may be facilitated by cerebellar internal models predicting responses during proactive control (withholding), and controlling responses during reactive control (inhibiting). The stop signal task (SST) is an inhibitory control task often used in neuroimaging studies to measure neural responses to both proactive and reactive control. Thus, in this review, we examine evidence for the cerebellum’s role in inhibitory control by reviewing studies of healthy adults that utilized the SST in event-related functional magnetic resonance imaging (fMRI) experiments. Twenty-one studies that demonstrated cerebellar results were eligible for review, including 749 participants, 28 contrasts, and 38 cerebellar clusters. We also performed activation likelihood estimation (ALE) meta-analysis of contrasts derived from reviewed studies. This review illustrates evidence for the cerebellum participating in inhibitory control independent of motor control. Most significant cerebellar clusters were located in the left posterior cerebellum, suggesting that it communicates with the established cortical right-lateralized inhibitory control network. Cerebellar activity was most consistently observed for contrasts that measured proactive control, and ALE analysis confirmed that left Crus I is most likely to be activated in studies of proactive control measuring monitoring and anticipation. Results suggest that the left posterior cerebellum may communicate with right frontal and parietal cortices, using forward models to predict appropriate responses. Reactive control contrasts indicated a possible role for cerebellar regions in enhancing inhibition efficiency through inverse models, but ALE meta-analysis did not confirm this hypothesis. Limitations in the current literature, clinical implications, and directions for future research are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum, long known to be an important motor modulator, is now also known to be a modulator of thought (Keren-Happuch, Chen, Ho, & Desmond, 2014; Stoodley & Schmahmann, 2009). Koziol, Budding, and Chidekel (2011) proposed that the cerebellum participates in executive functions (EFs) in particular, due to its unique structure and function. Cognitive and behavioral deficits were observed after cerebellar lesions as early as the 1800s (Schmahmann & Sherman, 1998), and more recently, functional magnetic resonance imaging (fMRI) studies have demonstrated cerebellar activity during EF tasks broadly (Keren-Happuch et al., 2014; Niendam et al., 2012). Inhibitory control may be one EF influenced by the cerebellum specifically, as disorders of poor inhibitory control (e.g. attention deficit/hyperactivity disorder [ADHD] and substance abuse) also often show cerebellar dysfunction (Lipszyc & Schachar, 2010; Nowrangi, Lyketsos, Rao, & Munro, 2014; Wilcox, Dekonenko, Mayer, Bogenschutz, & Turner, 2014). However, neuroimaging studies tend to have a cortico-centric bias, often failing to report or discuss cerebellar findings. There are also few studies of cerebellar activity during specific EFs, which contain both shared and unique variance (Miyake & Friedman, 2012). Therefore, the cerebellum’s role in inhibitory control in healthy individuals and its potential clinical significance remain understudied.
The cerebellum has a modular functional architecture based on cortical input (Caligiore et al., 2016; Ramnani, 2006; Stoodley & Schmahmann, 2009). Tracing studies have illustrated closed-loop, multi-synaptic anatomical connections between the cerebellum and contralateral cortical areas that generate motor or cognitive commands (Bostan, Dum, & Strick, 2013; Hoshi, Tremblay, Féger, Carras, & Strick, 2005). The anterior and inferior cerebellum are active during motor tasks, whereas the posterior cerebellum is active during cognitive tasks (Stoodley & Schmahmann, 2010). See Fig. 1 for an illustration of cerebellar lobules. The cerebellum’s densely interconnected neurons and diverse connections with cortical and subcortical areas indicate it is capable of modulating numerous cognitive processes, and importantly, most of the cerebellar cortex appears not to be associated with motor function (Ramnani, 2006).
Cerebellar topography, according to Diedrichsen’s (2006) Spatially Unbiased Infra-Tentorial Template (SUIT) atlas. Left, coronal view; right, sagittal view. Left side of the figure represents left side of the cerebellum
Functionally, control theory posits that the cerebellum utilizes forward and inverse internal models to predict and control behavior, respectively (Caligiore et al., 2016; Ito, 2008; Ramnani, 2006; Wolpert & Kawato, 1998). A forward model creates an efference copy of a controlled object, such as a plan or a movement of a limb, compares the outcome to the prediction, and sends feedback to the input system to adjust future commands. Inverse models create signals that act on the controlled object after receiving feedback from the control system (Ramnani, 2014). Koziol et al. (2011) argued that that cerebellar internal models facilitate and automate efficient planning and coordination of EFs because executive functioning (i.e., working memory, cognitive flexibility, and inhibitory control (Diamond, 2013)) essentially refers to predicting and controlling behavior.
Previous neuroimaging meta-analyses demonstrated posterior cerebellar activity during EF tasks in lobules VI and VIIb and Crus I and II (Keren-Happuch et al., 2014; Stoodley & Schmahmann, 2009). However, these meta-analyses limited searches to studies that included the term “cerebellum” or variants, which likely resulted in overlooking some studies in which cerebellar findings were not discussed in the text. Additionally, these meta-analyses only included one study of inhibitory control, which is correlated with other executive functions and is also highly correlated with a “common EF” latent variable, indicating that it may be superordinate to other EFs (Miyake & Friedman, 2012; Miyake et al., 2000).
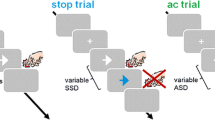
Inhibitory control is the ability to suppress inappropriate or irrelevant actions (Verbruggen & Logan, 2009), and there are multiple cognitive processes that result in a cumulative effect of successful response inhibition (Aron, 2011; Bari & Robbins, 2013; Braver, 2012). The stop signal task (SST) is a model inhibitory control task that measures these processes; it includes a period in which participants monitor for a stop signal and suppress responses proactively (proactive control), a period after stop signal presentation in which the response is inhibited reactively (reactive control), and an error processing period when participants respond incorrectly (Chevrier, Noseworthy, & Schachar, 2007). During the SST, individuals perform a reaction time (RT) task, responding to “go” stimuli as quickly as possible (Matzke, Verbruggen, & Logan, 2018; Verbruggen & Logan, 2008b). On a minority of trials, a “stop” signal is presented at varying delays after the go stimulus, which signals the participant should not respond (see Fig. 2 for a detailed description of the SST and independent race model of inhibition).
Typical SST design and independent race model. A) A fixation symbol is usually presented for a period of 1–5 s, followed by a “Go” signal. On a minority of trials (25–33%), a “Stop” signal is presented after the Go signal. The critical stop signal delay (SSD) is usually varied using a staircase procedure, whereby the SSD is increased by a fraction of a second following a successful stop and decreased following an unsuccessful stop. The critical SSD is the SSD at which the participant achieves 50% correct on Stop trials. Stop signal reaction time (SSRT) is computed by subtracting the critical SSD from the mean or median go reaction time (RT) and is an indirect measure of inhibitory control ability. A trial is completed when the participant responds or after the stop signal is presented for approximately one second. B) The independent race model assumes that going and stopping are independent processes that are initiated upon presentation of the go and stop signal respectively. Response inhibition occurs when the stop process finishes before the go process. Proactive control is considered to occur prior to stop signal presentation, whereas reactive control is initiated after stop signal presentation
The primary advantage of using the SST over other inhibitory control tasks (i.e., Go/No Go) is that it indirectly measures latency of response inhibition (stop signal reaction time; SSRT; Bari & Robbins, 2013). Shorter SSRT reflects a relatively faster “stop” process and represents better inhibitory control ability. Thus, inhibitory control ability of different populations and effects of different experimental manipulations can be compared (Matzke et al., 2018). While different populations may have different speeds of responding and the distribution of go RT can affect the probability of responding or inhibiting, the SST is designed so that all participants achieve 50% successful stops, regardless of go RT. This strategy enables computing the SSRT, and thus inhibition efficiency, from the stop signal delay needed to achieve 50% accuracy.
Functional neuroimaging studies use the SST, contrasting neural activity during different phases of the task, to measure brain activation for both proactive and reactive control, as well as attention, error, and motor processing. For example, contrasting the blood oxygen level dependent (BOLD) hemodynamic response on successful stop trials with go trials or unsuccessful stop trials presumably reveals brain regions underlying to inhibitory control (Verbruggen & Logan, 2008b). Likewise, contrasting go trials with successful stop trials reveals motor activity. Investigators also measure parametric effects of different phases of the task, such as anticipation of the stop signal, by including parametric regressors in their models (S. Hu, Ide, Zhang, & Li, 2015).
A widespread brain network is consistently activated by the SST. The right inferior frontal cortex (IFC) is most often associated with reactive inhibitory control, but studies also demonstrate bilateral IFC activity during the SST (Bari & Robbins, 2013; Swick, Ashley, & Turken, 2011). Other important regions include the basal ganglia, pre-supplementary motor area (pre-SMA), thalamus, and parietal cortex (Aron, 2011; Botvinick, Braver, Barch, Carter, & Cohen, 2001; Braver, 2012; S. Hu, Ide, Zhang, Sinha, & Li, 2015). Further, reactive inhibitory networks may be primed before stop signal presentation (Aron, 2011), and the medial and dorsolateral prefrontal cortex (PFC) may be specific to proactive control (Braver, 2012; Verbruggen & Logan, 2008b).
Cerebellar activity has also been observed during the SST, but investigations tend to have had a cortical bias, thus potentially overlooking the cerebellum’s significance. The cerebellum may communicate with the IFC during the SST, as meta-analytic connectivity modeling demonstrated that the cerebellum is often active during the same tasks as the IFC (Sebastian et al., 2016). Additionally, the posterolateral cerebellum is structurally and functionally connected to much of the prefrontal and parietal cortices associated with EF (Bostan et al., 2013; Buckner, Krienen, Castellanos, Diaz, & Yeo, 2011). A few recent SST functional connectivity studies included the cerebellum in their analysis, suggesting that the posterior cerebellum may be functionally connected to the fronto-parietal network during successful reactive inhibition, and the anterior cerebellum may influence motor control during errors (Zhang & Li, 2012; Zhang et al., 2015). However, previous functional neuroimaging meta-analyses of inhibitory control either did not include the cerebellum in analyses, only investigated reactive control (Swick et al., 2011), or did not investigate the SST (Keren-Happuch et al., 2014; Niendam et al., 2012; Stoodley & Schmahmann, 2009), and thus were unable to comment on the cerebellum’s role in proactive versus reactive control or inhibition efficiency.
Brain circuits involved in inhibitory control are important transdiagnostic factors in mental illness (Insel et al., 2010), and when investigating these circuits, it is important to include all possible regions, both cortical and subcortical. The Research Domain Criteria (RDoc) framework developed by the National Institutes of Mental Health (NIMH) designated cognitive control and specifically response inhibition as meaningful constructs of analysis in the etiology of mental disorders (Insel et al., 2010; Patrick & Hajcak, 2016). Indeed, structural and functional cerebellar deficits are observed in disorders marked by inhibitory control challenges such as attention deficit/hyperactivity disorder (ADHD), substance dependence, obsessive-compulsive disorder (OCD), and schizophrenia (Arnsten & Rubia, 2012; Bari & Robbins, 2013; Lipszyc & Schachar, 2010; Nowrangi et al., 2014; Schmahmann, 2004; Stoodley, 2015; Wilcox et al., 2014), suggesting clinical importance for understanding cerebellar function specifically regarding inhibitory control. In addition, cerebellar noninvasive stimulation can modulate both cognition and emotions, which indicates it could be a treatment target for psychiatric and neurological conditions (Grimaldi et al., 2016, 2014).
In general, there is evidence for cerebellar involvement in inhibitory control processing and disorders marked by inhibitory control deficits; however, specific cerebellar regions that contribute to successful inhibitory control in healthy individuals have yet to be comprehensively explored. Therefore, we aimed to review functional magnetic resonance imaging (fMRI) investigations of the SST in healthy control participants to specify which cerebellar subregions are active during the SST and hypothesize how the cerebellum may influence both proactive and reactive inhibitory control. We also aimed to quantify these review findings with activation likelihood estimation (ALE) meta-analysis (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012).
Methods
Search and Inclusion/Exclusion
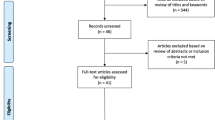
Searches were performed using the PubMed, PsycInfo, and MEDLINE databases, including the terms “stop signal AND fMRI.” The term “cerebellum” was not included because studies often find cerebellar activations but do not report them in the text. See Fig. 3 for search strategy and results.
Search strategy and exclusion. Search results were filtered by age (over 18), species (humans), and empirical articles in peer-reviewed journals. Studies were included if they reported cerebellar activity during the SST. Specifically, articles were included if they employed event-related fMRI utilizing the SST, reported whole-brain activation results for healthy adults (ages 18–65), were group studies, included the cerebellum in both imaging and analysis, and reported cerebellar activity in standard coordinates
Search results were filtered by age (over 18), species (humans), and empirical articles in peer-reviewed journals. In keeping with previous work (e.g., Keren-Happuch et al., 2014; Bernard & Mittal, 2015), the primary inclusion criterion was reporting cerebellar activity during the SST, as we were specifically interested in cerebellar regions active during proactive and reactive control. Articles were eligible for inclusion if they employed event-related fMRI utilizing the SST, reported whole-brain activation results for healthy adults (ages 18–65), were group studies, included the cerebellum in both imaging and analysis, and reported cerebellar activity in standard coordinates. Event-related designs were utilized specifically because the vast majority of SST studies use event-related designs and it is an effective way of establishing the prepotency of the “go” response across the entirety of the task (Garavan, Ross, & Stein, 1999). Event-related designs also allow for the direct comparison of specific trials and exclusion of other trials (such as errors), in contrast to block designs (Garavan et al., 1999). Titles and abstracts of 247 articles were screened for appropriate methods and population. Full-texts and supplementary material were then examined for cerebellar activations. Imaging parameters of studies that were eligible for inclusion were assessed for a minimum 120 mm field of view in the z-direction, which likely covers the entire cerebellum in at least 10% of participants (Mennes et al., 2014). Using a more stringent cutoff would have excluded nearly all studies (but see discussion for limitations).
Following full-text examination, 16 articles were determined to have null cerebellar findings, either due to observation of cerebellar activity in a non-control group or group contrast, or contacting authors to verify cerebellum inclusion in statistical analyses. Although observing cerebellar activity in clinical groups is important, the aim of this review was to investigate cerebellar activity in healthy adults, so future work with clinical populations may have a baseline for comparison. Four of these studies did not report coordinates of their contrasts within the main text or supplementary materials. In addition, 14 articles did not report any cerebellar activity but it is still unclear if the cerebellum was part of their analyses. Presumably, of the 14 remaining studies that were unclear about cerebellar inclusion, some had null cerebellar findings and others had significant findings that were not reported. Further, two studies showed cerebellar activity in a figure, but the authors were unable to provide coordinates. A total of 21 articles that reported cerebellar activity were included in this review.
Within articles, results were examined for contrast measure and location, size, and T or Z statistic of significant activation clusters. MRIcron (Rorden, 2007) was utilized to determine lobular locations, using both the Spatially Unbiased Infra-tentorial Template (Diedrichsen, Balsters, Flavell, Cussans, & Ramnani, 2009) and Automated Anatomic Labeling (Tzourio-Mazoyer et al., 2002) atlases. The SUIT probabilistic atlas was utilized to determine location and probability of coordinate location based on normalization methods (i.e., normalized to the MNI template using SPM or FSL programs). When coordinates were reported in Talairach space, the MNI-2-Tal online conversion tool was used to convert coordinates to Montreal Neurological Institute (MNI) space for localization using the AAL atlas (Lacadie, Fulbright, Constable, & Papademetris, 2008). Additionally, study quality was assessed using eight criteria, established based on recommendations for reporting in fMRI studies (Poldrack et al., 2008) and recommendations for imaging the cerebellum (Diedrichsen, 2006), as well as criteria specific to the goals of this review (Table 1).
ALE Meta-Analysis
We used the data extracted for the systematic review (Tables 2 and 3) to perform a confirmatory activation likelihood estimation (ALE) meta-analysis (Eickhoff et al., 2012; Eickhoff, Laird, Grefkes, Wang, Zilles, & Fox, 2009; Turkeltaub, Eickhoff, Laird, Fox, Wiener, & Fox, 2012). All papers were entered into the BrainMap database and the Sleuth 3.0.1 software was used to compile all contrasts and coordinates reported in Table 3, in MNI space (Fox et al., 2005; Fox & Lancaster, 2002; Laird, Lancaster, & Fox, 2005). Because one study’s results were received through personal communication and the cerebellar cluster was part of a larger cuneus cluster (Hu & Li, 2012), it was not included in the ALE analysis. We separated coordinates into two files: one that corresponded to proactive control activations and one that corresponded to reactive control activations, as reported in Table 3. Sleuth converted Talairach coordinates to MNI coordinates using the Lancaster transform (Laird et al., 2010; Lancaster et al., 2007) and created a text file of activations by experiment that we then entered into the GingerALE 3.0 program for meta-analysis (Eickhoff et al., 2012, 2009; Turkeltaub et al., 2012). Activation likelihood estimation reveals consistencies in studies by estimating the probability that a given voxel is activated for a task of interest (ALE statistic). GingerALE performs a nonparametric permutation test of statistical significance, testing the null that foci are evenly spread throughout the brain. We used a cluster forming threshold of p < .001, a cluster-based familywise error (FWE) corrected threshold of p < .05, and 1000 permutations, as suggested by Eickhoff et al. (2012).
We ran six ALE analyses to test both cerebellar and whole brain activity. We first performed analyses exclusively with cerebellar coordinates to generate patterns of cerebellar activity specifically, similar to previous work (Keren-Happuch et al., 2014; Bernard & Mittal, 2015). The proactive control analysis included 483 subjects, 13 experiments (individual contrasts), and 28 foci. The reactive control analysis included 348 subjects, 12 experiments, and 22 foci. Then, we performed analyses of the same contrasts but included whole brain coordinates to observe if the cerebellar activity was still present in the context of whole brain activation. The proactive control analysis included 483 subjects, 13 experiments, and 206 foci. The reactive control analysis included 394 subjects, 12 experiments, and 211 foci.
Finally, we ran whole-brain ALE analyses including the 12 studies with null cerebellar findings (see supplementary references). Within the 16 articles that were found to have null cerebellar findings, four could not be included in meta-analysis because they did not present coordinates of their whole brain findings in the control group alone. Seven (nine experiments) were included in the whole brain proactive control meta-analysis and five (six experiments) were included in the reactive control meta-analysis. Notably, seven out of nine proactive control contrasts added measured a form of risk-taking or pre- or post-error processing. The proactive control analysis included 739 subjects, 22 experiments, and 292 foci. The reactive control analysis included 441 subjects, 18 experiments, and 279 foci.
Results
Data Extraction
Data extraction for results is presented in Tables 2 and 3. Table 2 lists studies alphabetically; Table 3 lists them according to inhibitory control function measured and includes neuroimaging details. Figures 4 and 5 illustrate locations of cerebellar activations according to function. Studies included 749 participants, 28 contrasts, and 38 cerebellar clusters. Most SSTs (n = 11) utilized a simple RT task, with stop signal presentation on 25–30% of trials. In addition, most of these studies used the same task. Eight studies used a choice RT task, and two utilized complex visual stimuli. All studies used a staircase procedure based on the race model to determine the critical SSD that resulted in 50% success on stop trials. This method was employed by starting with an SSD of approximately 200 ms and increasing the delay by 30–70 ms if the response was inhibited or decreasing the delay if the response was not inhibited. In most studies (n = 16), the go and stop stimuli were visual; five studies used a tone stop signal. One study designated relevant (inhibit) and irrelevant (do not inhibit) stop (or not stop) or go signals of different colors or shapes (Aron, Behrens, Smith, Frank, & Poldrack, 2007). While studies demonstrated much consistency in their stimuli and behavioral analyses, they varied substantially in their neuroimaging analyses, yielding a variety of contrasts that encompass various cognitive processes involved in inhibitory control. The most common contrast for proactive control was the parametric effect of the hemodynamic response at either go or fixation cross onset (8 contrasts). The most common reactive control contrast was contrasting the hemodynamic response on successful stop trials with go trials (8 contrasts). Only four studies contrasted successful stop trials with stop error trials.
Approximate locations of cerebellar clusters, plotted on the Spatially Unbiased Infra-tentorial Template (SUIT) cerebellum (Diedrichsen, 2006). Peak activation of each individual cluster was plotted, according to function measured by the contrast. Activations with a focus outside the cerebellum were not plotted. Left side of the figure is left side of the cerebellum and numbers represent MNI coordinates. Locations are approximate to within 5 mm for visualization purposes
Study quality was determined to be comparable across studies. Using a system in which each study could earn a total of 24 points (8 categories in which they could earn 1, 2, or 3 points for low, medium, and high quality), 17 studies scored above 16. Five studies earned scores of 20 or higher. The four studies that scored 14 or 15 all used 1.5 T scanners. The greatest weaknesses across studies were imaging parameters and preprocessing techniques: most studies likely did not obtain full cerebellar coverage in all participants and most used a relatively large smoothing kernel for investigating cerebellar activity (≥ 8 mm). These weaknesses will be discussed further regarding limitations of this review, but studies were generally comparable in quality.
Cerebellar Correlates of Proactive Control
Anticipation
Five studies investigated proactive control during the fore-period as participants awaited presentation of the go signal. Three studies from the same group measured stop signal anticipation using a Bayesian model in which predicted probability of encountering a stop signal is assumed to affect behavior and neural activity during go trials (J. Hu et al., 2016; S. Hu, Ide, Zhang, & Li, 2015; S. Hu, Ide, Zhang, Sinha, et al., 2015). It is hypothesized that these processes represent conflict anticipation and proactive control. These studies found that greater activity in left Crus I during go trials was associated with greater stop signal anticipation. In addition, greater anticipation was associated with longer go RT, interpreted as the behavioral effect of withholding responses. Internal models are also hypothesized to work via Bayesian prediction, so using a Bayesian framework for SST tasks may tap into cerebellar internal models specifically (Wolpert & Ghahramani, 2000).
Two studies investigated anticipation during the fore-period without Bayesian prediction. Chevrier, Cheyne, Graham, and Schachar (2015) modeled activity of go trials at fixation and go onset together, finding activation during the fore-period in medial left Crus I/vermis that was positively correlated with SSRT (greater activity was related to less efficient inhibition). The authors interpreted this relationship to represent preparation for motor execution rather than preparation to inhibit. Hu and Li (2012) used two linear models to separate activity related to preparation for inhibition and preparation for motor execution. Their study showed activity in a large cluster that encompassed right lobule V and bilateral lobule VI (though the focus of the lobule VI clusters was outside the brain according to the SUIT atlas) before successful inhibition. However, this cerebellar cluster is part of a larger cluster with a peak in the cuneus, which may have arisen partially due to registration and smoothing methods (Schlerf, Wiestler, Verstynen, & Diedrichsen, 2014).
Two studies of the same participants parametrically modeled anticipation at go signal onset, showing activity in right lobule VIIIb and left Crus II (Chevrier et al., 2015, 2007). Right lobule VIII contains a secondary motor representation of the right hand (Buckner et al., 2011), so lobule VIII activity could represent an inverse model for withholding a response after the go signal until a decision is made, whereas left Crus II activity could represent a forward model for predicting the trial outcome or withholding, similar to Hu et al.’s Bayesian model. The authors argued that this activity is measuring a withholding process that is engaged after go signal onset, as opposed to the preparation-related activity that is engaged at fixation onset (the anticipation period).
Most cerebellar activity during anticipation was observed in the left superior posterior cerebellum, which demonstrates modularity for cognitive function (Stoodley & Schmahmann, 2010). Therefore, it is possible that left Crus I is creating internal models of task instructions that are governed by the prefrontal cortex. However, there is disagreement about how to best model this activity, lenient thresholds were used which increases the likelihood of false-positive results, and all studies were from two research groups, so these findings should be considered preliminary.
Monitoring
Conflict and error monitoring studies compared neural effects of monitoring for a salient signal or adjusting behavior based on previous trials. One study measured monitoring using different types of stop trials in which participants were either required to stop or to respond anyway despite a conflicting signal (Aron et al., 2007). This method purportedly measured inhibition preparation and conflict-induced slowing. Aron et al. (2007) observed similarly located activity during both monitoring and inhibition, in multiple clusters located in left Crus I and II and near left lobule VIIb. However, the focus of one large cluster was outside of the brain, reflecting issues in cerebellar imaging and normalization, and an image of these clusters was not available. Despite some weaknesses in imaging methodology and a small sample size, Aron et al.’s (2007) study tentatively suggests activity during monitoring and inhibition may represent coupled forward and inverse models for predicting and controlling responses, respectively (Wolpert & Kawato, 1998).
Another conflict monitoring study demonstrated anterior cerebellum activity for motor activity when stop signals were common (and go signals were more salient), left posterior cerebellar activation for inhibition activity when stop signals were rare (salient), and left posterior cerebellum and vermis activation for the conjunction of inhibition success when stop signals were salient (Manza et al., 2016). They did not report coordinates for the first two contrasts, so it is unclear where the foci of these clusters were, though their figures clearly depict cerebellar activity. These findings suggest that the cerebellum possibly predicts or controls the more salient response based on previous trials. The cerebellum may therefore be part of a conflict-monitoring network, helping to predict and slow responses until conflict is resolved by conditional rule retrieval (Aron et al., 2011).
Two studies measured error monitoring, but in different ways. S. Hu, Ide, Zhang, and Li, (2015) observed cerebellar activation in left Crus I following Bayesian prediction error (thought to represent surprise when a stop signal was expected but did not occur), which may represent updating internal models (den Ouden, Kok, & de Lange, 2012). Spunt, Lieberman, Cohen, and Eisenberger (2012) used a mixed design including SST blocks and go-only blocks, finding right lobule V activation for stop errors during SST blocks. This activity is likely motor-related because lobule V is known to contain motor modules and stop error includes a motor response.
Regarding post-trial behavioral adjustment, Hendrick, Ide, Luo, and Li (2010) contrasted activity during post-conflict cognitive control (post-stop stop success – post-go stop success), finding activations in left Crus II and right Crus I. Chevrier and Schachar (2010) measured post-error slowing by combining parametric effects of BOLD responses to error responding, error detection, and slowing on the trial subsequent to the error. They observed activity in right lobule VIIb. Interestingly, the cerebellar cluster was more strongly positively associated with post-error slowing than other clusters, and activity in dopamine-related areas was negatively associated with post-error slowing, suggesting a potential role for the posterior cerebellum in mediating reward signaling in response to errors. In contrast, Li, Chao, and Lee (2009) found right Crus II activity during post-go speeding (“risk-taking”). Notably, both post-error adjustment studies demonstrated right hemisphere activity and suggest a potential reward circuit process.
Generally, monitoring studies have been more variable in their approaches than anticipation studies, but cerebellar activity was fairly consistent. Most studies seem to suggest that the left posterior cerebellum predicts the appropriate response, in agreement with anticipation studies. Conflict monitoring and post-trial behavioral adjustment in posterior cerebellar lobules may represent fine-tuning and automation of task performance (Ramnani, 2006) or response to reward. Few studies observed cerebellar responses to error processing, so its primary role in proactive control may be predicting.
Cerebellar Correlates of Reactive Control
Inhibition
Eight studies observed cerebellar effects during reactive control, including studies that compared relevant and irrelevant “stop” signals, studies that compared successful stop trials and go trials, and studies that measured inhibition efficiency. Two utilized multiple types of trials in which participants were instructed to either stop or ignore the stop signal. Cai and Leung (2011) found activation in the left dentate nucleus (the output nucleus of the cerebellum) for successful stopping during a mixed event-related and block design incorporating a SST and “Not Stop Task”. Dentate activity suggests that the cerebellum is sending information to the cortex during the SST. However, it is unclear how accurate this result is because this study likely did not image the inferior cerebellum in many participants. Aron et al. (2007) found activity in left posterior (Crus I and II) and midline inferior (left lobule VIIb) cerebellar regions during successful stopping in tasks that included uncertain and certain go trials. Both studies required participants to keep rules in mind about which stimuli were associated with stopping, but Cai and Leung (2011) indicated rules at the beginning of trial blocks, presumably reducing cognitive load. Therefore, participants in Aron’s study may have been more engaged in conflict monitoring and rule retrieval on single trials, engaging the superior posterior lobules that are also associated with proactive control and other EFs.
Regarding studies that compared stop trials with go trials, Rubia et al. (2007) observed left lobule V and right lobule VI activity during successful inhibition, opposite to what would be expected when participants were using their right hand. Wilbertz et al. (2014) found activity for all stop trials (successful and failed) in left Crus I and a main effect of success in right lobule VI. Their task used two different stop stimuli that indicated whether the participant would get a reward or not for successful inhibition; therefore, participants may have been more motivated to perform well on some trials than others.
Four studies investigated neural relationships with inhibition efficiency, two that included SSRT as a predictor in the neuroimaging model and two that contrasted long and short SSRTs. The two studies that included SSRT as a predictor in their neuroimaging model found that in general, greater cerebellar activity was associated with better inhibitory control performance. Ghahremani et al. (2012) found that shorter SSRT was associated with bilateral posterior cerebellum activity, with a focus in right Crus II. The second study to include SSRT in their neuroimaging model investigated the relationship between successful stopping and SSRT across time (Jimura et al., 2014). Right lobule V activity was negatively correlated with SSRT over the entirety of the task, possibly representing motor modules influencing inhibition efficiency. Further, whereas left Crus II activation was associated with shorter SSRT during the first half of the task, left lobule VIIIa activation was associated with shorter SSRT during the second half of the task. The lobule VIIIa cluster was the largest and strongest result in their study. The relationship between SSRT and left lobule VIIIa became significantly more negative during the task, due to stronger activation in good performers and lower activation in poor performers; therefore, lobule VIIIa may have helped to optimize performance in good performers (Ramnani, 2014).
Two studies of efficiency that utilized the same task investigated differences between short and long SSRTs. A within-subjects study observed that stopping success during shorter SSRT blocks was associated with greater activation in left lobule I-IV (Chao, Luo, Chang, & Li, 2009). Differences in left lobule I-IV when participants were using their right hand is curious, but they used a larger smoothing kernel than other studies (10 mm), which may blur cerebellar activations (Schlerf et al., 2014). Nevertheless, this study suggests that motor-related modules may influence inhibition efficiency. In contrast, a between-subjects study found activations in right lobule I-IV, vermis VI, and a small cluster with a maximum outside the brain but near left lobule VIIIa during successful stopping in individuals with longer SSRT (Li, Yan, Sinha, & Lee, 2008). As a longer SSRT decreases the probability of inhibiting a response (Matzke et al., 2018), this ipsilateral motor lobule activity may represent a compensatory process in contrast to contralateral motor lobule activity observed by Chao et al. (2009).
In general, studies of efficiency demonstrated activity in lobules with motor representations (lobules I-V and VIII). Studies that investigated inhibition efficiency across the entirety of the task observed activity in the anterior (motor) lobules; however, Jimura et al. (2014) suggested that neural activity may change to secondary motor lobules over the course of the task. These studies of inhibition efficiency demonstrated stronger cerebellar than cortical activity, whereas studies that did not include efficiency in their models generally observed stronger cortical activity. Thus, motor-related cerebellar lobules (I-V and VIII) may be the brain regions most strongly associated with performance optimization as a person becomes more experienced with the task, consistent with control theory (Ramnani, 2014).
Locations of reactive control-related activity are more variable than those of proactive control, though some patterns emerged. There was activity in similar regions to proactive control contrasts, including left Crus I and II, as well as distinct activity in the left inferior posterior lobules. Anterior lobules appear to be implicated in studies of inhibition efficiency in particular, as well. Therefore, different cerebellar internal models may be facilitating proactive and reactive control, and both may be active during different phases of the SST. Additionally, it appears that studies with more complex stimuli or rules demonstrated activity in prediction-related regions, possibly related to greater cognitive processing demands. Notably, inhibition-related findings may include proactive control effects because they are not parsed apart when comparing successful stopping and going (Chevrier et al., 2007).
Motor Activity
Two studies observed cerebellar activation during go trials. Berkman, Kahn, and Merchant (2014)Footnote 1 observed activity in bilateral lobule VI when comparing go and successful stop trials, with left lobule VI activity stronger than right. Because this cluster was located in the anterior part of lobule VI, part of this cluster may encompass motor-related lobule V, and part of the hand representation in the cerebellum is located in contralateral lobule VI, as well (Buckner et al., 2011). Jahfari, Waldorp, Ridderinkhof, and Scholte (2015) reported cerebellar activity in a large cluster that likely includes much of the left hemisphere; however, the maximal focal location appears to be outside the brain and no image was available for inspection.
Concurrent Activity outside the Cerebellum
Cerebellar activity was present with cortical and subcortical regions previously associated with the SST (Bari & Robbins, 2013; Swick et al., 2011). The thalamus, which is a relay station between the cerebellum and cortex (Buckner, 2013), was active during most studies, providing evidence that the cerebellum is sending information to the cortex. The inferior parietal lobule (IPL) and insula, parts of attention/executive and salience networks (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2006), were active most often during proactive control studies. Thus, anticipation may be more related to attention, monitoring, and maintaining task sets than directly inhibiting, which echoes the predictive role of forward models. The Hu group studies all demonstrated pre-SMA and Crus I activation during anticipation, but studies of reactive control did not observe pre-SMA activity and less frequently reported Crus I activity, suggesting Crus I may communicate with the pre-SMA to predict appropriate motor responses.
The right orbitofrontal cortex (OFC) was also frequently active during proactive control, most often with left Crus I and II. The OFC is associated with numerous functions, including inhibitory control, reward processing, prediction error, and cognitive mapping of task demands (Fettes, Schulze, & Downar, 2017; Kringelbach & Rolls, 2004; Stalnaker, Cooch, & Schoenbaum, 2015). The OFC and cerebellum have both been associated with prediction, and thus may communicate to predict the appropriate response during proactive control. Stalnaker et al. (2015) hypothesized that the OFC abstractly represents task requirements which suggests it could provide the posterior cerebellum with rules for forming internal models. The OFC has been strongly associated with processing of abstract reward and emotion, as well (Kringelbach & Rolls, 2004), which may be related to motivation to perform well on the SST or reaction to errors (punishment). If the OFC is communicating with the cerebellum, error signals from the cerebellum may influence performance through reward or rule processing in the OFC.
The IFC was active for nearly all reactive control contrasts and a few monitoring studies; it was not active during anticipation. Thus, it may be the center responsible for generating stopping commands that inform cerebellar internal models of inhibiting, consistent with previous work (Swick et al., 2011). Motor-related lobules (I-V and VIII) were only active during reactive control, so they may contain inverse models for controlling motor inhibition from commands originating in the IFC (Ramnani, 2006). However, this idea is speculative because two contrasts with results in lobule VIIIa were from the same study (Jimura et al., 2014) and results from reactive control studies were inconsistent.
Potential Moderators
A few potential moderators of cerebellar activity during SST performance were observed in this review. For example, complex stimuli may engage more perceptual or attentional processing than simple tasks. Some simple SST studies demonstrated inferior results, whereas choice SSTs more often had superior posterior results, and potentially reflect more monitoring-related activity (e.g., Aron et al., 2007; Cai & Leung, 2011; Wilbertz et al., 2014). However, the study with the most complex stimuli only observed motor activity (Jahfari, Waldorp, Richard Ridderinkhof, & Steven Scholte, 2015), which appears to be counterintuitive.
Jimura et al.’ (2014) observation of changes in brain-behavior relationships over time indicates that imaging results may be affected by practice or a large number of trials, consistent with the cerebellum’s role in associative learning and optimization (Ramnani, 2014). Other studies finding activity in inferior cerebellar regions employed either long practice runs (Cai & Leung, 2011) or over 400 trials (Cai & Leung, 2011; Jimura et al., 2014; Manza et al., 2016). Thus, superior cerebellar regions may be active early in the task when more conscious processing is required, and activity may shift to inferior regions as internal models are updated and processing becomes more automatic. Alternatively, internal model function may shift from predicting to controlling appropriate responses.
Emotional or motivational factors may also impact SST performance and neural activity (Braver, 2012; Leotti & Wager, 2010), as studies that measured these processes (i.e., risk-taking, reward, frustration) found right cerebellar hemisphere activity in lobules V and VI (Li et al., 2008; Wilbertz et al., 2014; Spunt et al., 2012). Keren-Happuch et al. (2014) and Stoodley and Schmahmann (2009) both observed unique emotion-related activity in their meta-analyses in right lobule VI, suggesting that emotion may influence cerebellar activity, as well.
ALE Meta-Analysis
Cerebellar ALE analyses revealed two significant posterior cerebellum clusters during proactive control contrasts. The first cluster was centered on left Crus I, extending to left lobule VI and left dentate nucleus. The second cluster was also located in left Crus I, medial to the first cluster. During reactive control, one significant cluster was present in left lobule VIIIa and left Crus II. Figures 6 and 7 and Table 4 display these significant results.
Cerebellar results of activation likelihood estimation (ALE) meta-analyses for proactive and reactive control contrasts. Analyses were performed in the cerebellum only and the whole brain. Further whole-brain analysis included studies with null cerebellar results, but no significant cerebellar activations were observed. Analyses utilized 1000 permutations, a cluster-forming threshold of uncorrected p < .001, and cluster-level family-wise error corrected threshold of p < .05. Results were plotted on the SUIT template using MRIcron (nitrc.org/projects/mricron). The left side of the figure represents the left side of the brain
Cortical results of whole-brain activation likelihood estimation (ALE) meta-analyses for proactive and reactive control contrasts. Analyses were performed with papers reviewed and including studies with null cerebellar results. Analyses utilized 1000 permutations, a cluster-forming threshold of uncorrected p < .001, and cluster-level family-wise error–corrected threshold of p < .05. Results were plotted on the Colin27 template using MRIcron (nitrc.org/projects/mricron). The left side of the figure represents the left side of the brain
With regard to proactive control, whole brain ALE analyses replicated cerebellum-specific analyses and also included a wider network of activation. Proactive control contrasts activated the same, but smaller, clusters centered on left Crus I, and also activated right OFC, bilateral IPL, right thalamus, and right red nucleus. When studies of proactive control that had null cerebellar results were included in the meta-analysis, the cerebellar, thalamic, and red nucleus clusters were no longer significant. Whole brain reactive control ALE analysis demonstrated significant clusters in the left IFG and right thalamus, but no significant clusters were present in the cerebellum. Reactive control ALE including null papers showed the same IFG and thalamus clusters, plus further activation in the left insula and left claustrum.
Discussion
This review provides evidence that the cerebellum participates in cognitive inhibitory control processes during the SST. Contrasts measuring proactive control demonstrated cerebellar activity most often in the superior posterior cerebellum, and ALE analyses of these same contrasts confirmed high likelihood of activation spanning left Crus I/II and lobule VI. Studies of reactive control showed more variable activity in both anterior and posterior lobules, with more activations located in the posterior cerebellum. ALE analysis including only cerebellar coordinates showed a high likelihood of activation during reactive control in left lobule VIIIa; however, this result was not replicated with whole-brain ALE analysis, leaving this association tenuous. Results of this review and meta-analysis support previous findings of posterior cerebellar activation during EF processes (Buckner et al., 2011; Keren-Happuch et al., 2014; Stoodley & Schmahmann, 2009), and extend them to proactive and reactive inhibitory control specifically.
Performing both cerebellum-specific and whole brain ALE meta-analyses allowed us to quantitatively show which regions of the cerebellum are most likely active, and whether the cerebellum is consistently among the brain regions active during proactive and reactive control. Consistent with the findings of the review, we demonstrated that given studies of the SST that find cerebellar activity, studies of proactive control are more likely to activate the left Crus I/II and lobule VI; studies of reactive control are more likely to activate lobule VIIIa and Crus II. Second, we showed that these same studies of proactive control activate a fronto-parietal-thalamo-cerebellar circuit, consistent with known cerebello-cortical connectivity (Bostan et al., 2013; Buckner et al., 2011) and previous literature on executive functioning (Keren-Happuch et al., 2014; Niendam et al., 2012). While including proactive control contrasts with null cerebellar findings eliminated the significant cerebellar and thalamic activity, it is important to consider that the studies that did observe cerebellar activations mainly investigated stop signal anticipation and conflict monitoring (10/13 contrasts), whereas the majority of the null studies measured activity related to making errors or risk-taking (7/9 contrasts). Therefore, these two meta-analyses may actually be capturing different cognitive processes. We originally classified any papers that included error monitoring, conflict monitoring, anticipation, or risk-taking as proactive control because these processes are ongoing prior to stop signal presentation and there were very few studies that measured any aspect of proactive control; however, the results of the meta-analysis indicate that multiple cognitive processes are occurring during proactive control. What our findings suggest is that the posterior cerebellum is likely active during conflict monitoring and predicting when a stop signal will occur. It may be less important for error processing and risk-taking/aversion. In addition, while ALE analysis did show activity during reactive control in the left IFG and right thalamus, well-established inhibitory control regions, it also suggests that the cerebellum may not be necessary for reactive control, at least on the SST (Swick, Ashley, & Turken, 2008; Swick et al., 2011).
Regarding location, two broad results suggest the cerebellum facilitates successful performance in the SST through influencing executive control. First, posterior cerebellar activity during contrasts that controlled for motor activity indicates that the posterior cerebellum participates in cognitive processing, as previous work demonstrated cerebellar segregation for cognitive activity in posterior lobules (Stoodley & Schmahmann, 2010). Second, left cerebellar hemisphere activity suggests that the cerebellum works with the right-lateralized cortical inhibition network, because the cerebellum is mainly contralaterally connected to the cortex (Bostan et al., 2013). Activation of a fronto-parietal-thalamo-cerebellar circuit during proactive control was confirmed via whole-brain meta-analysis, indicating that left Crus I/II and lobule VI assist the OFC and IPL to predict and coordinate appropriate behavior.
Altogether, the cerebellum is likely more involved in proactive control (i.e., anticipation and conflict monitoring) than reactive control, supporting the hypothesis that the cerebellum largely acts as a short-term predictor via internal models (Caligiore et al., 2016; Ito, 2008; Ramnani, 2006, 2014). It may utilize forward models in left Crus I and II to predict the appropriate response guided by cortical networks and therefore facilitate withholding inappropriate responses. Speculatively, left crus I and II may receive information regarding rules, goals, and intentions from the PFC, and then create internal models to predict and monitor task performance that are updated as the task progresses via sensory information received from the parietal lobes (Fettes et al., 2017; Munakata et al., 2011). See Fig. 8 for hypothesized fronto-parietal-thalamo-cerebellar circuits for proactive control. Behaviorally, the studies reviewed suggest that resulting behavior may include preemptive RT slowing and improved conflict monitoring (Aron et al., 2007; Chevrier et al., 2015, 2007; S. Hu, Ide, Zhang, & Li, 2015).
Hypothesized cerebello-cortical circuits involved in proactive (dark gray) and reactive (light gray) control. Proactive control is hypothesized to utilize internal models in the superior posterior cerebellum to predict appropriate behavior via rules encoded in the orbitofrontal cortex and sensory information synthesized by the parietal cortex. Reactive control may function via forward and/or inverse models to control motor responses when inhibition is initiated by the inferior frontal gyrus, and may function to optimize performance. Dashed lines indicate that there was less empirical support for the reactive control pathway as it was not supported by whole-brain meta-analysis
However, because fMRI cannot determine which specific neural processes are occurring, future work should aim to determine whether and how the cerebellum creates internal models during inhibitory control (Huettel, 2012; Logothetis, 2008). It is possible that concurrent activation patterns observed are simply coincidental, but the literature supports functional and anatomical connections between these cerebellar and cortical regions (Bostan et al., 2013; Buckner et al., 2011). Future investigations should characterize these connections utilizing effective functional connectivity with whole-brain and region of interest methods, including the cerebellum (Friston, 2011). These methods can elucidate directional information flow to demonstrate if the cortex initiates inhibitory control commands and then the cerebellum creates internal models, sending updated information back to the cortex. Bayesian prediction models of proactive control may measure forward models specifically, so future studies using this framework may elucidate the nature of internal models during inhibitory control (Wolpert & Ghahramani, 2000). Methods with better temporal resolution such as electroencephalography (EEG) and combined fMRI/EEG could also possibly help characterize interactions among brain regions at millisecond resolution to investigate internal model formation during inhibitory control (Laufs, 2008).
Although there was less consistent evidence for a cerebellar role in cognitive aspects of reactive control, it is possible that multiple paired forward and inverse models are operating to predict and control responses, which may be demonstrated by the greater variability of activation locations in anterior and posterior cerebellum (Wolpert & Kawato, 1998). In addition, the cerebellum may be primarily involved in automating and optimizing SST performance, supported by stronger cerebellar than cortical activity within the few studies of inhibition efficiency (Koziol et al., 2011; Ramnani, 2014). Specifically, the three studies that observed cerebellum – SSRT correlations found negative relationships and stronger cerebellar than cortical effects (Chao et al., 2009; Ghahremani et al., 2012; Jimura et al., 2014). This idea is also potentially supported by Jimura et al.’s (2014) finding that cerebellum – SSRT relationships became more strongly negative over time and may have enhanced performance. Automatic and unconscious processing have both been demonstrated during the SST, so the cerebellum may facilitate these processes, eventually reducing processing load on the PFC (van Gaal, Ridderinkhof, van den Wildenberg, & Lamme, 2009; Verbruggen & Logan, 2008a). These changes in activity over time could not be investigated via meta-analysis, but suggest that future studies should investigate dynamic changes in neural activity during the SST and other inhibitory control tasks, and they should specifically include the cerebellum.
Findings of this review may have clinical implications that future studies should elaborate. In accordance with the dysmetria of thought hypothesis (Schmahmann, 2004), if the cerebellum is more of a facilitator than generator of thoughts or actions, cerebellar dysfunction will not completely disrupt inhibitory control, but reduce efficiency or accuracy. Because many disorders are associated with both inhibitory control and cerebellar deficits (Arnsten & Rubia, 2012; Lipszyc & Schachar, 2010; Nowrangi et al., 2014), cerebellar function, or internal models specifically, could be investigated as possible endophenotypes. For example, Bernard and Mittal (2015) argued that malfunctioning internal models in the cerebellum may result in uncoordinated or inefficient EF abilities in schizophrenia.
Regarding potential interventions, studies of cerebellar stimulation have shown promise for regulating prefrontal activity and working memory, so this work may be extended into other domains (Caligiore et al., 2016; Grimaldi et al., 2016, 2014; Pope & Miall, 2012). This review only focused on studies of healthy individuals, but future work can build from this review’s findings and investigate relationships between cerebellar activity and inhibitory control across disorders.
While patterns emerged that generally support previous literature and theories of cerebellar function, limitations must be considered. There is still disagreement among researchers about the most effective way to measure inhibitory control with neuroimaging contrasts due to myriad processes involved in the SST (Li et al., 2008); therefore, there was substantial heterogeneity among contrasts. This was evidenced by the proactive control contrasts particularly, as when we added the null studies of risk-taking and error processing into our meta-analysis, our cerebellar cluster became nonsignificant. The SST also relies on many assumptions, but investigators attest to its utility as a model paradigm (Bari & Robbins, 2013). Logan et al. (2014) and Matzke et al. (2018) noted that the independent race model continues to be the most useful model for go and stop processes, but that assumptions may be violated. For instance, proactive control may violate the independence assumption if processing during the fore-period affects go and stop processes. All studies included in this review referenced the independent race model, but future work may consider exploring other models that may better account for interactions between go and stop processes during different task phases (Logan et al., 2014; Matzke et al., 2018). These considerations may be especially important for studies of proactive control. Importantly, we did not investigate multiple EFs and thus cannot directly assess the similarities and differences between inhibitory control and other subcomponents of executive functioning; however, this study adds to the broader literature on the cerebellum’s role in EF by analyzing proactive and reactive control in a task that has not been included in previous reviews or meta-analyses. Further, our review and meta-analysis suggest that the posterior cerebellum is likely working with the PFC to predict responses, which is a process common to other EFs (Miyake et al., 2000). The SST neuroimaging literature is still quite limited compared to other EF tasks, but with more studies published each year, hopefully an updated EF meta-analysis can be completed in the future.
Imaging parameters of studies in this review are a major concern, because they are not optimized to investigate cerebellar activity (Diedrichsen et al., 2009; Schlerf et al., 2014). Researchers often sacrifice cerebellar coverage in favor of frontal cortex coverage and shorter scan times. While this review excluded studies that reported partial cerebellar coverage, many studies probably only covered the inferior cerebellum in a minority of participants, potentially biasing results by excluding regions such as lobule VIII (Mennes et al., 2014). Notably, Jimura et al. (2014) ensured complete cerebellar coverage and they found robust lobule VIII activity, so there may be a “file drawer” issue concerning this lobule. In addition, Diedrichsen (2006) pointed out that traditional normalization methods distort the cerebellum, and large smoothing kernels and artifacts from vasculature can blur cerebellar activations. Due to these issues, some activations in this review appeared to be outside the brain, and others are probably inaccurate or even potentially artifacts. Further, because most studies did not present images of cerebellar findings and some did not report cluster size, it is unclear which areas significant clusters spanned. Using the SUIT probabilistic atlas (Diedrichsen et al., 2009) is the most accurate way to determine cluster locations within the cerebellum, but locations should still be considered approximate. Future studies that truly want “whole brain” coverage or are interested in the cerebellum must increase their field of view and optimize registration and spatial smoothing methods (Schlerf et al., 2014).
Finally, this study specifically aimed to investigate cerebellar activity during the SST and therefore may be inherently somewhat biased due to excluding studies without significant cerebellar results from the review. However, by not including “cerebellum” or variants in the search terms, this review included studies that did not discuss significant cerebellar findings and would have been overlooked by a more limited search. No SST studies were specifically interested in cerebellar function, so it is possible that many further investigations observed but did not report cerebellar activation. Most importantly, we performed whole-brain ALE analysis including studies of null cerebellar findings, which ameliorated possible bias and resulted in partial support of our review hypotheses. Based on results of the present literature review and meta-analysis, it is somewhat likely that the posterior cerebellum participates in proactive control in a predictive role, but these findings should still be interpreted cautiously due to the heterogeneity and sparsity of the current literature. It is not the intent of this review to overstate the cerebellum’s role in inhibitory control, but patterns observed do support previous cerebellar research and raise questions for future areas of inquiry. See Fig. 9 for potential research questions generated by this review and possible methods to answer them.
In sum, this review shows that the left posterior cerebellum is likely to be active during the SST in a predictive role, along with the right OFC and IPL, for conflict monitoring and predicting responses. Inferior posterior cerebellar activity may influence efficiency of reactive control, but these findings were less consistent. Future investigators must report cerebellar activations when they are observed and investigate the cerebellum specifically so that a potentially important component of inhibitory control and general EF ability is not overlooked.
Notes
These results were not published in the article, but the analyses were performed as part of the published study and received through personal communication.
References
Arnsten, A. F. T., & Rubia, K. (2012). Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: Disruptions in neurodevelopmental psychiatric disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 51(4), 356–367. https://doi.org/10.1016/j.jaac.2012.01.008
Aron, A. R. (2011). From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry, 69(12), e55–e68. https://doi.org/10.1016/j.biopsych.2010.07.024
Aron, A. R., Behrens, T. E., Smith, S., Frank, M. J., & Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience, 27(14), 3743–3752. https://doi.org/10.1523/JNEUROSCI.0519-07.2007
Bari, A., & Robbins, T. W. (2013). Inhibition and impulsivity: Behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79. https://doi.org/10.1016/j.pneurobio.2013.06.005
Berkman, E. T., Kahn, L. E., & Merchant, J. S. (2014). Training-induced changes in inhibitory control network activity. The Journal of Neuroscience, 34(1), 149–157. https://doi.org/10.1523/JNEUROSCI.3564-13.2014
Bernard, J., & Mittal, V. (2015). Dysfunctional activation of the cerebellum in schizophrenia: A functional neuroimaging meta-analysis. Clinical Psychological Science, 3(4), 545–566. https://doi.org/10.1177/2167702614542463
Bostan, A. C., Dum, R. P., & Strick, P. L. (2013). Cerebellar networks with the cerebral cortex and basal ganglia. Trends in Cognitive Sciences, 17(5), 241–254. https://doi.org/10.1016/j.tics.2013.03.003
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., & Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–652. https://doi.org/10.1037/0033-295X.108.3.624
Braver, T. S. (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. https://doi.org/10.1016/j.tics.2011.12.010
Buckner, R. L. (2013). The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron, 80(3), 807–815. https://doi.org/10.1016/j.neuron.2013.10.044
Buckner, R. L., Krienen, F. M., Castellanos, A., Diaz, J. C., & Yeo, B. T. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(5), 2322–2345. https://doi.org/10.1152/jn.00339.2011
Cai, W., & Leung, H.-C. (2011). Rule-guided executive control of response inhibition: Functional topography of the inferior frontal cortex. PLoS One, 6(6), e20840–e20840. https://doi.org/10.1371/journal.pone.0020840
Caligiore, D., Pezzulo, G., Baldassarre, G., Bostan, A. C., Strick, P. L., Doya, K., … Herreros, I. (2016). Consensus paper: Towards a systems-level view of cerebellar function: The interplay between cerebellum, basal ganglia, and cortex. The Cerebellum, 1–27. https://doi.org/10.1007/s12311-016-0763-3
Chao, H. H., Luo, X., Chang, J. L., & Li, C. R. (2009). Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time – An intra-subject analysis. BMC Neuroscience, 10, 75. https://doi.org/10.1186/1471-2202-10-75
Chevrier, A. D., Cheyne, D., Graham, S., & Schachar, R. (2015). Dissociating two stages of preparation in the stop signal task using fMRI. PLoS One, 10(6), e0130992. https://doi.org/10.1371/journal.pone.0130992
Chevrier, A. D., Noseworthy, M. D., & Schachar, R. (2007). Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Human Brain Mapping, 28(12), 1347–1358. https://doi.org/10.1002/hbm.20355
Chevrier, A. D., & Schachar, R. J. (2010). Error detection in the stop signal task. NeuroImage, 53(2), 664–673. https://doi.org/10.1016/j.neuroimage.2010.06.056
den Ouden, H. E. M., Kok, P., & de Lange, F. P. (2012). How prediction errors shape perception, attention, and motivation. Frontiers in Psychology, 3. https://doi.org/10.3389/fpsyg.2012.00548
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Diedrichsen, J. (2006). A spatially unbiased atlas template of the human cerebellum. NeuroImage, 33(1), 127–138. https://doi.org/10.1016/j.neuroimage.2006.05.056
Diedrichsen, J., Balsters, J. H., Flavell, J., Cussans, E., & Ramnani, N. (2009). A probabilistic MR atlas of the human cerebellum. NeuroImage, 46(1), 39–46. https://doi.org/10.1016/j.neuroimage.2009.01.045
Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L., & Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. https://doi.org/10.1016/j.tics.2008.01.001
Dosenbach, N. U. F., Visscher, K. M., Palmer, E. D., Miezin, F. M., Wenger, K. K., Kang, H. C., & Petersen, S. E. (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812. https://doi.org/10.1016/j.neuron.2006.04.031
Eickhoff, S. B., Bzdok, D., Laird, A. R., Kurth, F., & Fox, P. T. (2012). Activation likelihood estimation meta-analysis revisited. NeuroImage, 59(3), 2349–2361. https://doi.org/10.1016/j.neuroimage.2011.09.017
Eickhoff, S. B., Laird, A. R., Grefkes, C., Wang, L. E., Zilles, K., & Fox, P. T. (2009). Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926
Fettes, P., Schulze, L., & Downar, J. (2017). Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: Promising therapeutic targets in psychiatric illness. Frontiers in Systems Neuroscience, 11. https://doi.org/10.3389/fnsys.2017.00025
Fox, P. T., Laird, A. R., Fox, S. P., Fox, P. M., Uecker, A. M., & Crank, M. (2005). Brainmap taxonomy of experimental design: Description and evaluation. Human Brain Mapping, 25(1), 185–198. https://doi.org/10.1002/hbm.20141
Fox, P. T., & Lancaster, J. L. (2002). Mapping context and content: The BrainMap model. Nature Reviews Neuroscience, 3(4), 319. https://doi.org/10.1038/nrn789
Friston, K. J. (2011). Functional and effective connectivity: A review. Brain Connectivity; New Rochelle, 1(1), 13–36 https://doi.org.ezproxy.gsu.edu/10.1089/brain.2011.0008
Garavan, H., Ross, T. J., & Stein, E. A. (1999). Right hemispheric dominance of inhibitory control: An event-related functional MRI study. Proceedings of the National Academy of Sciences, 96(14), 8301–8306. https://doi.org/10.1073/pnas.96.14.8301
Ghahremani, D. G., Lee, B., Robertson, C. L., Tabibnia, G., Morgan, A. T., & de Shetler, N. (2012). Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural cicuitry in humans. The Journal of Neuroscience, 32(21), 7316–7324. https://doi.org/10.1523/JNEUROSCI.4284-11.2012
Grimaldi, G., Argyropoulos, G. P., Bastian, A., Cortes, M., Davis, N. J., & Edwards, D. J. (2016). Cerebellar transcranial direct current stimulation (ctDCS): A novel approach to understanding cerebellar function in health and disease. The Neuroscientist, 22(1), 83–97. https://doi.org/10.1177/1073858414559409
Grimaldi, G., Argyropoulos, G. P., Boehringer, A., Celnik, P., Edwards, M. J., Ferrucci, R., … Ziemann, U. (2014). Non-invasive cerebellar stimulation: A consensus paper. The Cerebellum; New York, 13(1), 121–138 https://doi.org/10.1007/s12311-013-0514-7
Hendrick, O. M., Ide, J. S., Luo, X., & Li, C. R. (2010). Dissociable processes of cognitive control during error and non-error conflicts: A study of the stop signal task. PLoS One, 5(10), e13155. https://doi.org/10.1371/journal.pone.0013155
Hoshi, E., Tremblay, L., Féger, J., Carras, P. L., & Strick, P. L. (2005). The cerebellum communicates with the basal ganglia. Nature Neuroscience; New York, 8(11), 1491–1493 https://doi.org/10.1038/nn1544
Hu, J., Hu, S., Maisano, J. R., Chao, H. H., Zhang, S., & Li, C.-S. R. (2016). Novelty seeking, harm avoidance, and cerebral responses to conflict anticipation: An exploratory study. Frontiers in Human Neuroscience, 10 Retrieved from http://ezproxy.gsu.edu/login?url=http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2016-54671-001&site=ehost-live&scope=site
Hu, S., Ide, J. S., Zhang, S., & Li, C. R. (2015). Anticipating conflict: Neural correlates of a bayesian belief and its motor consequence. NeuroImage, 119, 286–295. https://doi.org/10.1016/j.neuroimage.2015.06.032
Hu, S., Ide, J. S., Zhang, S., Sinha, R., & Li, C. R. (2015). Conflict anticipation in alcohol dependence — A model-based fMRI study of stop signal task. NeuroImage: Clinical, 8, 39–50. https://doi.org/10.1016/j.nicl.2015.03.008
Hu, S., & Li, C.-S. R. (2012). Neural processes of preparatory control for stop signal inhibition. Human Brain Mapping, 33(12), 2785–2796. https://doi.org/10.1002/hbm.21399
Huettel, S. A. (2012). Event-related fMRI in cognition. Neuroimage, 62(2), 1152–1156. https://doi.org/10.1016/j.neuroimage.2011.08.113
Insel, T., Cuthbert, B., Garvey, M., Heinssen, R., Pine, D. S., & Quinn, K. (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167(7), 748–751. https://doi.org/10.1176/appi.ajp.2010.09091379
Ito, M. (2008). Control of mental activities by internal models in the cerebellum. Nature Reviews. Neuroscience, 9(4), 304–313. https://doi.org/10.1038/nrn2332
Jahfari, S., Waldorp, L., Richard Ridderinkhof, K., & Steven Scholte, H. (2015). Visual information shapes the dynamics of corticobasal ganglia pathways during response selection and inhibition. Journal of Cognitive Neuroscience, 27(7), 1344–1359. Retrieved from psyh. (2015-25200-007)
Jimura, K., Hirose, S., Kunimatsu, A., Ohtomo, K., Koike, Y., & Konishi, S. (2014). Late enhancement of brain-behavior correlations during response inhibition. Neuroscience, 274, 383–392. https://doi.org/10.1016/j.neuroscience.2014.05.058
Keren-Happuch, E., Chen, S.-H. A., Ho, M.-H. R., & Desmond, J. E. (2014). A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Human Brain Mapping, 35(2), 593–615. https://doi.org/10.1002/hbm.22194
Koziol, L. F., Budding, D. E., & Chidekel, D. (2011). From movement to thought: Executive function, embodied cognition, and the cerebellum. The Cerebellum, 11(2), 505–525. https://doi.org/10.1007/s12311-011-0321-y
Kringelbach, M. L., & Rolls, E. T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72(5), 341–372. https://doi.org/10.1016/j.pneurobio.2004.03.006
Lacadie, C. M., Fulbright, R. K., Constable, R. T., & Papademetris, X. (2008). More accurate Talairach coordinates for neuroImaging using nonlinear registration. NeuroImage, 42(2), 717–725. https://doi.org/10.1016/j.neuroimage.2008.04.240
Laird, A. R., Lancaster, J. L., & Fox, P. T. (2005). BrainMap: The social evolution of a human brain mapping database. Neuroinformatics, 3(1), 65–78.
Laird, A. R., Robinson, J. L., McMillan, K. M., Tordesillas-Gutiérrez, D., Moran, S. T., Gonzales, S. M., … Lancaster, J. L. (2010). Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: Validation of the Lancaster transform. NeuroImage, 51(2), 677–683. https://doi.org/10.1016/j.neuroimage.2010.02.048
Lancaster, J. L., Tordesillas-Gutiérrez, D., Martinez, M., Salinas, F., Evans, A., Zilles, K., … Fox, P. T. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28(11), 1194–1205. https://doi.org/10.1002/hbm.20345
Laufs, H. (2008). Endogenous brain oscillations and related networks detected by surface EEG-combined fMRI. Human Brain Mapping, 29(7), 762–769. https://doi.org/10.1002/hbm.20600
Leotti, L. A., & Wager, T. D. (2010). Motivational influences on response inhibition measures. Journal of Experimental Psychology. Human Perception and Performance, 36(2), 430–447. https://doi.org/10.1037/a0016802
Li, C. R., Chao, H. H.-A., & Lee, T.-W. (2009). Neural correlates of speeded as compared with delayed responses in a stop signal task: An indirect analog of risk taking and association with an anxiety trait. Cerebral Cortex, 19(4), 839–848. https://doi.org/10.1093/cercor/bhn132
Li, C.-S. R., Yan, P., Sinha, R., & Lee, T.-W. (2008). Subcortical processes of motor response inhibition during a stop signal task. Neuroimage, 41(4), 1352–1363. https://doi.org/10.1016/j.neuroimage.2008.04.023
Lipszyc, J., & Schachar, R. (2010). Inhibitory control and psychopathology: A meta-analysis of studies using the stop signal task. Journal of the International Neuropsychological Society, 16(6), 1064–1076. https://doi.org/10.1017/S1355617710000895
Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453(7197), 869–878. https://doi.org/10.1038/nature06976
Manza, P., Hu, S., Chao, H. H., Zhang, S., Leung, H.-C., & Li, C. R. (2016). A dual but asymmetric role of the dorsal anterior cingulate cortex in response inhibition and switching from a non-salient to salient action. NeuroImage, 134, 466–474. https://doi.org/10.1016/j.neuroimage.2016.04.055
Matzke, D., Verbruggen, F., & Logan, G. D. (2018). The stop-signal paradigm. In In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience, Methodology. Hoboken, NJ, US: John Wiley & Sons.
Mennes, M., Jenkinson, M., Valabregue, R., Buitelaar, J. K., Beckmann, C., & Smith, S. (2014). Optimizing full-brain coverage in human brain MRI through population distributions of brain size. NeuroImage, 98, 513–520. https://doi.org/10.1016/j.neuroimage.2014.04.030
Miyake, A., & Friedman, N. P. (2012). The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science, 21(1), 8–14. https://doi.org/10.1177/0963721411429458
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734
Munakata, Y., Herd, S. A., Chatham, C. H., Depue, B. E., Banich, M. T., & O’Reilly, R. C. (2011). A unified framework for inhibitory control. Trends in Cognitive Sciences, 15(10), 453–459. https://doi.org/10.1016/j.tics.2011.07.011
Niendam, T., Laird, A. R., Ray, K. L., Dean, Y. M., Glahn, D. C., & Carter, C. S. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. https://doi.org/10.3758/s13415-011-0083-5
Nowrangi, M. A., Lyketsos, C., Rao, V., & Munro, C. A. (2014). Systematic review of neuroimaging correlates of executive functioning: Converging evidence from different clinical populations. The Journal of Neuropsychiatry and Clinical Neurosciences, 26(2), 114–125. https://doi.org/10.1176/appi.neuropsych.12070176
Patrick, C. J., & Hajcak, G. (2016). RDoC: Translating promise into progress. Psychophysiology, 53(3), 415–424. https://doi.org/10.1111/psyp.12612
Poldrack, R. A., Fletcher, P. C., Henson, R. N., Worsley, K. J., Brett, M., & Nichols, T. E. (2008). Guidelines for reporting an fMRI study. Neuroimage, 40(2), 409–414. https://doi.org/10.1016/j.neuroimage.2007.11.048
Pope, P. A., & Miall, R. C. (2012). Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimulation, 5(2), 84–94. https://doi.org/10.1016/j.brs.2012.03.006
Ramnani, N. (2006). The primate cortico-cerebellar system: Anatomy and function. Nature Reviews Neuroscience, 7(7), 511–522. https://doi.org/10.1038/nrn1953
Ramnani, N. (2014). Chapter 10 - automatic and controlled processing in the corticocerebellar system. In N. Ramnani (Ed.), Progress in Brain Research (pp. 255–285). https://doi.org/10.1016/B978-0-444-63356-9.00010-8
Rubia, K., Smith, A. B., Taylor, E., & Brammer, M. (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping, 28(11), 1163–1177
Schlerf, J., Wiestler, T., Verstynen, T., & Diedrichsen, J. (2014). Big challenges from the little brain — Imaging the cerebellum. In T. D. Papageorgiou, G. I. Christopoulos, & S. M. Smirnakis (Eds.), Advanced brain neuroimaging topics in health and disease-methods and applications (pp. 199–223). Rijeka: IntechOpen. https://doi.org/10.5772/58266
Schmahmann, J. (2004). Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. Journal of Neuropsychiatry and Clinical Neuroscience, 16(3), 367–378. https://doi.org/10.1212/WNL.32.7.790
Schmahmann, J. D., & Sherman, J. C. (1998). The cerebellar cognitive affective syndrome. Brain, 121(4), 561–579. https://doi.org/10.1093/brain/121.4.561
Sebastian, A., Jung, P., Neuhoff, J., Wibral, M., Fox, P. T., & Lieb, K. (2016). Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Structure and Function, 221(3), 1635–1651. https://doi.org/10.1007/s00429-015-0994-y
Spunt, R. P., Lieberman, M. D., Cohen, J. R., & Eisenberger, N. I. (2012). The phenomenology of error processing: The dorsal ACC response to stop-signal errors tracks reports of negative affect. Journal of Cognitive Neuroscience, 24(8), 1753–1765. https://doi.org/10.1162/jocn_a_00242
Stalnaker, T. A., Cooch, N. K., & Schoenbaum, G. (2015). What the orbitofrontal cortex does not do. Nature Neuroscience, 18(5), 620–627. https://doi.org/10.1038/nn.3982
Stoodley, C. J. (2015). The cerebellum and neurodevelopmental disorders. The Cerebellum, 15(1), 34–37. https://doi.org/10.1007/s12311-015-0715-3
Stoodley, C. J., & Schmahmann, J. D. (2009). Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage, 44(2), 489–501. https://doi.org/10.1016/j.neuroimage.2008.08.039
Stoodley, C. J., & Schmahmann, J. D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex, 46(7), 831–844. https://doi.org/10.1016/j.cortex.2009.11.008
Swick, D., Ashley, V., & Turken, A. U. (2008). Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience, 9, 102. https://doi.org/10.1186/1471-2202-9-102
Swick, D., Ashley, V., & Turken, U. (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage, 56(3), 1655–1665. https://doi.org/10.1016/j.neuroimage.2011.02.070
Turkeltaub, P. E., Eickhoff, S. B., Laird, A. R., Fox, M., Wiener, M., & Fox P. (2012). Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping, 33(1), 1–13
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., & Delcroix, N. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. https://doi.org/10.1006/nimg.2001.0978
van Gaal, S., Ridderinkhof, K. R., van den Wildenberg, W. P. M., & Lamme, V. A. F. (2009). Dissociating consciousness from inhibitory control: Evidence for unconsciously triggered response inhibition in the stop-signal task. Journal of Experimental Psychology: Human Perception and Performance, 35(4), 1129–1139. https://doi.org/10.1037/a0013551
Verbruggen, F., & Logan, G. D. (2008a). Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. Journal of Experimental Psychology. General, 137(4), 649–672. https://doi.org/10.1037/a0013170
Verbruggen, F., & Logan, G. D. (2008b). Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences, 12(11), 418–424. https://doi.org/10.1016/j.tics.2008.07.005
Verbruggen, F., & Logan, G. D. (2009). Models of response inhibition in the stop-signal and stop-change paradigms. Neuroscience & Biobehavioral Reviews, 33(5), 647–661. https://doi.org/10.1016/j.neubiorev.2008.08.014
Wilbertz, T., Deserno, L., Horstmann, A., Neumann, J., Villringer, A., & Heinze, H.-J. (2014). Response inhibition and its relation to multidimensional impulsivity. NeuroImage, 103, 241–248. https://doi.org/10.1016/j.neuroimage.2014.09.021
Wilcox, C. E., Dekonenko, C. J., Mayer, A. R., Bogenschutz, M. P., & Turner, J. A. (2014). Cognitive control in alcohol use disorder: Deficits and clinical relevance. Reviews in the Neurosciences, 25(1), 1–24. https://doi.org/10.1515/revneuro-2013-0054
Wolpert, D. M., & Ghahramani, Z. (2000). Computational principles of movement neuroscience. Nature Neuroscience, 3, 1212–1217.
Wolpert, D. M., & Kawato, M. (1998). Multiple paired forward and inverse models for motor control. Neural Networks, 11(7), 1317–1329. https://doi.org/10.1016/S0893-6080(98)00066-5
Zhang, S., & Li, C. R. (2012). Functional networks for cognitive control in a stop signal task: Independent component analysis. Human Brain Mapping, 33(1), 89–104. https://doi.org/10.1002/hbm.21197
Zhang, S., Tsai, S.-J., Hu, S., Xu, J., Chao, H. H., Calhoun, V. D., & Li, C.-S. R. (2015). Independent component analysis of functional networks for response inhibition: Inter-subject variation in stop signal reaction time. Human Brain Mapping, 36(9), 3289–3302. https://doi.org/10.1002/hbm.22819
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This research was funded by a Georgia State University Second Century Initiative (2CI) Neurogenomics fellowship to S.V. Clark.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Clark, S.V., King, T.Z. & Turner, J.A. Cerebellar Contributions to Proactive and Reactive Control in the Stop Signal Task: A Systematic Review and Meta-Analysis of Functional Magnetic Resonance Imaging Studies. Neuropsychol Rev 30, 362–385 (2020). https://doi.org/10.1007/s11065-020-09432-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-020-09432-w