Abstract
Inflammatory responses play a major role in the pathophysiology of cerebral ischemia. Mesenchymal stem cell-derived exosomes (MSC-exos) have important anti-inflammatory effects on the treatment of organ injury. This study aimed to determine the anti-inflammatory effect and furtherly investigate the potential mechanism of MSC-exos on acute cerebral ischemia. MSC-exos were isolated by ultracentrifugation, characterized by transmission electron microscopy and FACS. Rats subjected to middle cerebral artery occlusion/reperfusion (MCAO/R) surgery were administered MSC-exos through the tail vein. In vitro, microglia exposed to oxygen- and glucose-deprivation (OGD) and leukotrienes were used to study the protective mechanism of exosomes against ischemia/reperfusion-induced inflammation. The intake of exosomes into microglia was visualized through immunofluorescence staining. The results showed that MSC-exos treatment significantly improved motor, learning and memory abilities of MCAO/R rats 7 days later. The production of pro-inflammatory factors decreased, while the anti-inflammatory cytokines and neurotrophic factors increased both in the cortex and hippocampus of ischemic hemisphere as well as in the culture supernatant of microglia treated with OGD and NMLTC4. MSC-exos treatment also significantly inhibited M1 microglia polarization and increased M2 microglia cells. Furthermore, western blot analysis demonstrated that CysLT2R expression and ERK1/2 phosphorylation were downregulated both in vivo and in vitro. Thus, MSC-exos attenuated brain injury and inhibited microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia are the main inherent immune cells in the brain and play a central role in regulating the immune and inflammatory responses after various injuries. Brain injuries trigger microglia activation within minutes after cerebral ischemia [1]. Persistent microglia activation and M1 polarization finally lead to severe cerebral inflammation [2]. As known to us all, microglia have two different phenotypes. During the course of cerebral ischemia, they function completely contrary to each other. M1 microglia aggravate ischemic injury to the brain by releasing pro-inflammatory and neurotoxic factors, highly expressing IL-1β, TNF-α, IFN-γ, and CD86. However, M2 microglia protect nervous tissues by secreting anti-inflammatory and neurotrophic mediators to promote brain repairment. Its polarization markers include Arg-1, CD206, TGF-β, and IL-10 [3]. Therefore, microglia M1/M2 polarization is closely associated with the prognosis of brain injuries. To reach a good balance between M1 and M2 phenotypes may represent a prospective therapeutic strategy for ischemic injury.
Cysteinyl leukotrienes (CysLTs) are largely produced with the decomposition of necrotic cells. As potent inflammatory mediators, they accelerated the development of several central nervous diseases, including epilepsy, depression, cerebral ischemia, and brain trauma. Up to now, CysLT1R and CysLT2R are two well-studied CysLT receptors to induce inflammatory responses [4]. N-methyl LTC4 (NMLTC4) was a selective agonist of CysLT2R and LTD4 was a non-selective agonist of CysLT1R and CysLT2R [5]. It has been reported that CysLT1R has no effect on early microglia activation and proliferation. While CysLT2R is mainly related to both post-ischemic inflammation in vivo and in vitro. HAMI3379, a selective antagonist of CysLT2R, protects neurons against ischemic injury through inhibiting IL-1β, IFN-γ, TNF-α expression and ameliorating microglia activation [6].
Recently mesenchymal stem cells (MSCs) transplantation has been widely investigated in the treatment of various organ injuries for their immunosuppressive and anti-inflammatory properties. MSCs-derived exosomes are membranous structures that come from the cytoplasm, characterized by the expression of specific marker proteins from the tetraspanin superfamily such as CD9, CD63, and CD81. Exosomes are one of the main effectors of paracrine function. These markers are inevitable in the formation and transportation of exosomes within the cells as well as the recognition of target cells, which represent a novel cell-to-cell interaction mode and play an important role in regulating signal pathways [7]. They deliver their content, such as protein, mRNA and miRNA to recipient cells and regulate occurrence, progression and prognosis of many diseases. In the current study, we evaluated the effect of MSC-exos on OGD and CysLTs induced microglia activation as well as middle cerebral artery occlusion (MCAO) induced cerebral ischemia via CysLT2R-ERK1/2 mediated pathway.
Materials and Methods
Animals
Male SD rats (Slac, Shanghai, China) were kept in a clean room with controlled temperature (23 ± 1 ℃). All rats had access to enough food and water except for the 8 h fasting before the operation. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and the guidelines of the Ethics Committee of Hangzhou Medical College. Every effort was made to minimize animal suffering and to reduce the number of animals used. Anesthetization was induced with pentobarbital sodium (30 mg/kg) through intraperitoneal injection.
MSCs Exosomes Isolation and Characterization
MSCs were isolated from SD rats by the whole bone marrow adherence method and cultured [8]. When MSCs were nearly 80 ~ 90% confluence, they were washed with PBS, and cultured in FBS-free L-DMEM for 48 h. Then the supernatant was collected and subject to sequential centrifugation: 300 g × 10 min, 2000 g × 10 min, 10,000 g × 30 min, and 100,000 g × 120 min. The precipitated exosomes were reserved at − 80 ℃.
For transmission electron microscopy (TEM), exosomes were fixed with 1% glutaraldehyde and a drop was loaded onto a formvar/carbon-coated grid negatively stained with 3% aqueous phosphotungstic acid for 1 min and observed under TEM (Hitachi, Tokyo, Japan, SU-8010).
For flow cytometry analysis of exosomes, MSC-exos were mixed with 3 μm aldehyde/sulfate latex beads (Invitrogen, Batch Num: 979383) for 15 min with continuous rotation, then diluted to 0.4 ml with PBS and left overnight at 4 ℃. The next day 1 M glycine in PBS containing 2% BSA was added into the mixture to stop the reaction. Beads coated with exosomes were washed twice with PBS with 2% BSA and incubated with antibodies CD63-FITC (Abcam, Lot: GR320523-9), CD81-PE (Invitrogen, Cat: MA5-17941) at 37 ℃ for 30 min. After being washed the beads were resuspended in 200 μL PBS with 0.5% BSA for detection on a BD FACSalibur. The protein concentrations of MSC-exos were determined with the Bradford protein quantification kit (Beyotime Biotech, Nanjing, China).
MCAO Rats Preparation and MSC-Exos Therapy
MCAO surgery was performed on male SD rats (with bodyweight 270 ± 10 g). Under anaesthetization with pentobarbital sodium, the right cerebral artery was occluded for 90 min using a nylon filament that was coated with silica gel at one end (Jialing, Guangzhou, China). The filament was carefully withdrawn 90 min after MCAO. In sham-operated rats, the same procedure was done without advancing of the filament into the MCA. The average mortality was about 10 ~ 15%. MSC-exos (200 μL/rat) were administered through the tail vein 2 h after reperfusion. The sham and MCAO/R groups were given an equal volume of normal saline (n = 8 in each group). Rats were sacrificed 7 days after ischemia and the brain tissues were separated.
Measurement of Neurological Deficit
According to Longa’s method [9], the neurological severity scores (NSS) were assessed at 1 h, 1 days, 3 days, and 7 days after ischemia (n = 8) by investigators blind to the groups, which implied motor, sensory, reflex and balance abilities. The motor behavior and memory function were also assessed by the shuttle box test. In the boxes, light, a sound from the loudspeaker or an electric shock from the floor grid incentivized the rats to move to the opposite end of the box. The latency and frequency of active avoidance were recorded for further analysis.
OGD Treatment and MTT Assay of Microglia
To simulate an in vivo environment of MCAO, microglia cell line BV-2 (Bioleaf, Shanghai, China) cultured in glucose-free Earle's balanced salt solution (EBSS), was placed in an incubator with 95% N2, 5% CO2 for 1–5 h, then under normal condition for an additional 24 h. MTT assay aimed to find an appropriate OGD time with no cell activity reduction. In the following experiments, microglia cells were firstly subject to OGD for the given time, then incubated with NMLTC4 or LTD4 (0.1 nM/mL). Meanwhile, MSC-exos (5 μL/mL, l-Exos group; 10 μL/mL, H-Exos group) were furtherly added into the culture medium to explore the effect of MSC-exos on microglia polarization phenotypes and inflammation status. In order to confirm whether MSC-exos entered microglia, MSC-exos labeled with CD81-PE monoclonal antibody (Invitrogen, Cat: MA5-17941) were used to incubate with microglia cells.
Inflammatory Factors Detection
Cytokines production was detected in the hippocampus and cortex homogenate of the ischemic hemisphere as well as the supernatant collected from microglia culture medium 24 h after treatment with cysLTs (0.1 nM/mL) and MSC-exos by commercial ELISA kits according to the instructions (Elisa, Biotech, China). The content of NO was detected through the Griess method (Beyotime, Nanjing, China).
Multiple Immunofluorescence Staining
Multiple indirect immunofluorescence staining was performed on microglia cells seeded on coverslips and frozen sections of brains. The antibodies were as follows: Iba-1 (Gene Tex, Cat: GTX100042), CD86 (BD, Cat: 551396), CD206 (Origene, Cat: TA326270S), FITC (Biowestern, Cat: ISH8004-1), Cy3 (Beyotime, Lot: 032218180628). At last, nuclei were labeled with DAPI (Beyotime, Cat: C1005) for 15 min in the dark. The fluorescence was observed under fluorescence microscopy (Zeiss Scope A1, Germany).
Flow Cytometric Analysis
Microglia were harvested and incubated with CD86, CD206 (two characteristic makers of different microglial phenotypes) and fluorescence antibodies. Flow cytometric analysis (Cytoflex, Beckman Coulter) was performed to quantify the percentage of M1/M2 microglia.
Western Blotting
Protein was isolated from cultured microglia, cortex and hippocampus tissues of the ischemic hemisphere, respectively. Samples were separated using the standard SDS–polyacrylamide gel electrophoresis. Bands were visualized with enhanced chemiluminescence and images were captured with the image analysis system (Bio-Rad, CA, USA). Antibodies rabbit anti-cysLT2R (1:1000, Bioworld, Cat: BS2614), p-ERK1/2 (1:2000, Huabio, Cat: ET1610-13) and ERK1/2 (1:5000, Huabio, Cat: ET1601-29) were used for detection.
Statistical Analysis
Data were reported as mean ± SD and analyzed with GraphPad Prism (GraphPad 6.0, San Diego, CA). All the data were checked for normality and variance homogeneity by F test or Bartlett’s test. The comparison between two groups was determined by an independent sample t-test. Significant differences among multiple groups were determined by one-way analysis of variances (ANOVA) followed by Tukey’s post hoc test. The repeated-measures analysis was conducted on the behavior scores. P ≤ 0.05 was considered statistically significant.
Results
Characterization of MSC-Exos
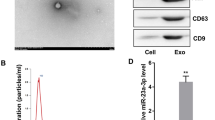
Under the transmission electronic microscope, MSC-exos presented widespread, derangement distribution, and conglobation in some areas. They were membrane-encapsulated vesicles with round or cup-like shapes. As MSC-exos were isolated through ultracentrifugation and 0.22 μm filtration, these vesicles exhibited typical exosome morphology with a dimension varying from 30 to 150 nm (Fig. 1a). FACS indicated MSC-exos isolated through ultracentrifugation were of certain purity. The CD63 and CD81 positive rates were 30.84% and 43.75%, respectively (Fig. 1b). They were characteristic protein markers of exosomes. According to the Bradford quantification, the protein concentration of the MSC-exos was 0.6034 mg/mL.
a Morphological observation of exosomes under transmission electron microscopy. b FACS analysis of exosome surface markers CD63 and CD81. NSS assessment (c) and shuttle box avoidance test (d) of MCAO rats after MSC-exos treatment (n = 8). Data were expressed as mean ± SD, #P < 0.05, ##P < 0.01, ###P < 0.001 vs sham; *P < 0.05, **P < 0.01, ***P < 0.001 vs MCAO. NSS was analyzed by repeated-measures method and data of shuttle box test by one-way ANOVA
MSC-Exos Improved Neurological Deficit
In this study, we applied NSS to assess the neurological function of MCAO rats after MSC-exos treatment. MCAO rats exhibited severe behavior deficits after ischemia. In the following days, there was a persistent improvement in both MCAO/R group and MSC-exos treated group (0.4 mg/kg) in a time-dependent manner. The difference between the two groups increased progressively over time. On the 3rd (F(2,21) = 171.5, P < 0.05) and 7th day (F(2,21) = 95.15, P < 0.001), there was significant difference in NSS scores between the Exo group and the MCAO/R group (Fig. 1c).
Frequency and latency of active avoidance are the most important indicators of the shuttle box experiment. Compared with the sham group, the frequency of active avoidance responses of MCAO/R groups was significantly reduced (F(2,21) = 48.02, P < 0.001), and latency increased (F(2,21) = 20.43, P < 0.001, Fig. 1d). After MSC-exos therapy, there was a markedly increase in frequency (P < 0.05) and a decrease in latency of active avoidance (P < 0.01). It showed a great improvement in fear memory and locomotor ability, which was completely consistent with NSS.
Microglia Activity After OGD and Phagocytosis of MSC-Exos
Microglia activity changed in different OGD period. MTT showed that OGD more than 4 h greatly decreased cell activity and promoted cell death (t = 8.924, P < 0.001, Fig. 2a). We selected 2 h as the optimal OGD time for the subsequent experiments to investigate the effect of MSC-exos on microglia in vitro.
After incubation with MSC-exos labeled with CD81-PE antibody, immunofluorescence staining showed unevenly distributed orange fluorescence spots. They all closely surrounded the microglia cell nuclei (Fig. 2b). It demonstrated that MSC-exos had been efficiently taken into microglia cells. Whether NMLTC4 (t = 9.009, P < 0.001) or LTD4 (t = 7.455, P < 0.001) pre-treatment, the number of CD81 positive cells was much higher in H-Exo group than L-Exo (Fig. 2c). Besides, LTD4 triggered phagocytosis more extensively than NMLTC4.
MSC-Exos Mitigated MCAO and cysLTs Induced Microglia M1 Polarization
Microglia cells subject to OGD for 2 h were treated with NMLTC4/LTD4 (0.1 nM/mL) and MSC-exos (5 μg/mL and 10 μg/mL, respectively) for 24 h, then harvested for flow cytometric analysis (Fig. 3). The results demonstrated that there was a sharp rise in CD86+ microglia after treatment with NMLTC4 (F(3,32) = 548.7, P < 0.001) or LTD4 (F(3,32) = 1433, P < 0.001) compared with the control. But MSC-exos co-incubation only significantly reversed NMLTC4 induced M1 microglia conversion in a concentration dependent manner (F(3,32) = 548.7, P < 0.001). As for the surface marker of the M2 phenotype, CD206, there was statistical significance (F(3,32) = 129.8, P < 0.001) only in the NMLTC4-H-Exo group. Therefore, exosomes from mesenchymal stem cells significantly mitigated microglial M1 polarization induced by OGD and NMLTC4 treatment and promoted microglial conversion into M2 phenotype.
Immunofluorescence further illustrated the effect of exosomes on microglia phenotype after OGD and NMLTC4 treatment as well as in the brain tissues 7 days after MCAO/R (Fig. 4). Exos co-incubation reduced of CD86 expression in BV-2 cells induced by OGD and NMLTC4 treatment (F(3,16) = 99.09, P < 0.001). The higher the exosome concentration was, the less the CD86 + antigen expressed. It was in complete consistency with that obtained through flow cytometry. The expression of CD206 in BV-2 was absolutely contrary to CD86 (F(3,16) = 167, P < 0.001). In the brain tissue around the infarction site, there was more CD206 (F(2,21) = 54.20, P < 0.01) and less CD86 (F(2,21) = 2018, P < 0.001) expression in microglia in the Exos group than in the MCAO.
MSC-Exos Affected MCAO and NMLTC4 Induced Cytokines Secretion
In the culture supernatant of microglia after treatment with OGD and NMLTC4, the production of pro-inflammatory mediators such as NO (F(3,32) = 18.9, P < 0.001), IL-1β (F(3,32) = 8.9, P < 0.01), IL-12 (F(3,32) = 25.63, P < 0.05) and TNF-α (F(3,32) = 16.23, P < 0.001) was greatly increased in the model group than in the control. However, they were significantly decreased in the MSC-exos co-incubation groups, especially in the H-Exo group (P < 0.05). On the contrary, the secretion of anti-inflammatory and neurotrophic factors, including TGF-β (F(3,32) = 23.37, P < 0.001), IL-10 (F(3,32) = 22.42, P < 0.001) and BDNF (F(3,32) = 19.75, P < 0.001), was remarkably upregulated in the MSC-exos groups. Their expression level rose with the increase of MSC-exos concentration. Interestingly, there was no statistical difference in GDNF content among these groups (F(3,32) = 0.4271, P > 0.05, Fig. 5a).
In the hippocampus of right hemisphere, MSC-exos significantly reversed the increased expression of NO (F(2,33) = 248.9, P < 0.001), IL-1β (F(2,33) = 9.059, P < 0.01), TNF-α (F(2,33) = 37.64, P < 0.001) and IL-12 (F(2,33) = 12.96, P < 0.01) induced by MCAO/R injury. Meanwhile, MSC-exos evidently increased the secretion of IL-10 (F(2,33) = 21.3, P < 0.001), TGF-β (F(2,33) = 87.28, P < 0.001) and BDNF(F(2,33) = 12.07, P < 0.01), but there was no distinct change on GDNF level (Fig. 5b). The trend of these cytokines expression was generally consistent with that in vitro. In the cortex of right hemisphere, there was no significant difference in the content of NO (F(2,33) = 19.24, P > 0.05), IL-1β (F(2,33) = 1.516, P > 0.05), IL-10 (F(2,33) = 0.6516, P > 0.05), IL-12 (F(2,33) = 1.294, P > 0.05) and GDNF (F(2,33) = 0.08491, P > 0.05) after MSC-exo therapy. Only TNF-α remarkably decreased after MSC-exos therapy compared with the ischemia–reperfusion group (F(2,33) = 6.329, P < 0.01). On the contrary, TGF-β (F(2,33) = 72.96, P < 0.001) and BDNF (F(2,33) = 169.3, P < 0.001) significantly increased after ischemia reperfusion and furtherly increased after MSC-exos injection (P < 0.01, Fig. 5b).
MSC-Exos Suppressed cysLT2R Expression and ERK1/2 Phosphorylation
It is well known that NMLTC4 is the selective receptor agonist of cysLT2R. MSC-exos significantly inhibited NMLTC4 induced microglia activation and inflammatory cytokines secretion. Here we made a further investigation on the effect of MSC-exos on microglial cysLT2R expression. As the results showed (Fig. 6a), cysLT2R expression in NMLTC4 treated microglia was more than twice the level of the control ((F(5,48) = 36.03, P < 0.001). MSC-exos co-incubation greatly reversed the up-regulated expression of cysLT2R (P < 0.001). In the NMLTC4 + H-Exo group, it was decreased nearly to the level of the control. In MCAO/R rats (Fig. 6b, c), MSC-exos significantly reduced cysLT2R expression both in the hippocampus (F(2,33) = 157.4, P < 0.001) and cortex of the ischemic side (F(2,33) = 47.37, P < 0.001). The results in vivo were in good agreement with that in vitro.
Western-blot analysis of cysLT2R and ERK1/2 expression in microglia (a, n = 3) and the brain tissues (b, n = 4). Each sample was tested in triplicate. Data were expressed as mean ± SD, #P < 0.05, ##P < 0.01, ###P < 0.001 vs control/sham, *P < 0.05, **P < 0.01, ***P < 0.001 vs NMLTC4/MCAO, analyzed by one-way ANOVA
ERK1/2 is the key mitogen kinase in the ERK signaling pathway involved in inflammation. We found that in rats, MSC-exos therapy obviously decreased the p-ERK1/2(42 KD) (F(2,33) = 34.70, P < 0.001) and p-ERK1/2 (44 KD) (F(2,33) = 4.603, P < 0.05) production induced by MCAO in the cortex. ERK1/2 (42 KD/44 KD) phosphorylation also significantly decreased (F(2,33) = 21.54, P < 0.001; F(2,33) = 8.401, P < 0.01, respectively). Only the phosphorylation of ERK1/2(44kd) was of statistical significance in the hippocampus (F(2,33) = 11.62, P < 0.001). All these changes were entirely consistent with that of cysLT2R and inflammatory factors expression (Fig. 6b, c). In vitro, ERK1/2 (42 KD/44 KD) phosphorylation was dramatically diminished in high MSC-exos concentration group (F(3,32) = 47.37, P < 0.001; F(3,32) = 39.15, P < 0.0 01, respectively. Figure 6a). In the low MSC-exos concentration group, there was a marked rise in ERK1/2 (42 KD) phosphorylation (F(3,32) = 47.37, P < 0.01).The content of p-ERK1/2 (42 KD) was much less than p-ERK1/2 (44 KD).
Discussion
In the present study, we administered MSC-exos to MCAO rats through tail vein injection at 2 h after ischemia/reperfusion at the dose of 120 μg /rat. Our results showed MSC-exos significantly reduced neurological severity score, improved spatial learning and memory ability. Both In vivo and in vitro experiments demonstrated the effect of MSC-exos might be CysLT2R dependent. MSC-exo markedly inhibited the expression of CysLT2R in the MCAO injured brain and NMLTC4 treated microglia, modulated the balance between M1 and M2 microglia, decreased pro-inflammatory cytokines secretion, increased anti-inflammatory and neurotrophic factors production.
Inflammation is widely implicated in the pathogenesis of various central nervous system disorders, such as cerebral ischemia, intracerebral hemorrhage, brain tumor, epilepsy, and Parkinson’s disease [10, 11], resulting in the secondary insult to the brain. CysLTs are potent inflammatory mediators, including LTC4, LTD4, LTE4. CysLT2R is highly expressed in hypertrophic microglia in the ischemic core zone of rats with MCAO/R [12]. Our results showed that MSC-exos significantly decreased CysLT2R expression in microglia after OGD and CysLTs treatment and in focal cerebral ischemia rats. They had a much stronger reversing effect on NMLTC4 treated M1 microglia polarization than LTD4. Therefore, MSC-exos have a stronger antagonistic effect against CysLT2R than other CysLTs receptors to inhibit cerebral inflammation.
Microglia activation is one of the characteristics of central nervous system inflammation. Microglia have two completely opposite functions with different phenotypes: M1 /M2 paradigm [13]. Generally, the former predominates at the injury site and the latter promotes repairment, so it is of clinically benefit to switch microglia phenotype from cytotoxic to neuroprotective by drug treatment or genetic modification. In our experiment in vitro, MSC-exos were effectively internalized by microglia, which promoted the transformation of microglia from M1 into M2 phenotype. A downregulation of pro-inflammatory factors accompanied by M2 microglia activation with an upregulation of CD206 + in vivo and in vitro was clearly proved. The improvement in NSS and shuttle box test showed the central nervous system had benefited from the anti-inflammatory property of MSC-exos.
MSC-exos are important components taking part in the paracrine effects of MSCs in brain injuries. MSC-exos are small vesicles budding off the plasma membrane of MSCs with size between 30 and 150 nm [14]. They could pass through the blood–brain barrier in various cerebral diseases [15, 16]. Exosomes express several specific cell surface markers, such as tetraspanins CD9, CD63, and CD81, which give them a high affinity for target tissues [17]. They could be selectively taken up by brain microglia [15, 16]. Besides, exosomes provide a protective and controlled internal microenvironment, allowing the content to travel long distance within tissues without degradation until harvested by recipient cells [18]. In vivo, we injected MSC-exos through the tail vein far from the ischemic brain. The plasma membrane and cell affinity of exosomes facilitated the transportation in the blood and transferring to microglia in the brain greatly. As confirmed in immunofluorescence staining of BV-2, exosomes were really distributed round microglia nuclei after treatment with OGD and NMLTC4. They specifically acted on their target cells via either ligand-receptor signaling pathways or internalization by phagocytosis, endocytosis, and direct membrane fusion [19, 20].
Exosomes played a connective role in intercellular communication. When exosomes released their content (proteins, mRNA, non-coding RNAs) inside recipient cells, they may change their biological properties and functions [20]. MiRNAs, marker signatures in MSC-exos, regulate gene expression through inhibiting mRNA transcription, accelerating mRNA degradation or disturbing protein translation. Up to now, mi-223 and miR-146a have been two well-studied anti-inflammatory miRNAs in MSCs exosomes [21, 22]. MicroRNA-27a targeting TLR4 negatively modulates inflammation [23]. MiRNA-21 activating TLR7 promotes neurotoxicity [24]. We are interested in which miRNAs in MSC-exos inhibit CysLT2R expression and will make further study. In the downstream of CysLT2R, the expression and phosphorylation of ERK1/2 were remarkably downregulated, which resulted in the decreased secretion of pro-inflammatory factors and increased production of anti-inflammatory and neurotrophic factors. They protected the brain from neuroinflammation synergistically. With more and more studies carried out on the profiles and specific functions of miRNAs in MSC-exos, the cell-free therapy mediated by exosomes will make further development in the field of inflammation.
Conclusion
MSC-exos significantly improved cerebral inflammatory response and neurological syndrome after acute cerebral ischemic injury through tail intravenous injection. 120 μg /rat MSC-exos within 2 h after ischemia is an effective dose and time in vivo. MSC-exos remarkably inhibited microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglial M1 polarization and promoting M2 polarization. For acute ischemic injury, MSC-exos may be a potential therapeutic choice to improve nervous system functions through suppressing neuroinflammation.
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- CysLTs:
-
Cysteinyl leukotrienes
- CysLT1R:
-
Cysteinyl leukotrienes receptor 1
- DMSO:
-
Dimethyl sulfoxide
- ERK:
-
Extracellular regulated protein kinases
- GDNF:
-
Glial cell-derived neurotrophic factor
- IFN:
-
Interferon
- IGF:
-
Insulin like growth factor
- IL:
-
Interleukin
- LT:
-
Leukotriene
- MSC-exos:
-
MSCs-derived exosomes
- MCAO:
-
Middle cerebral artery occlusion
- NMLTC4:
-
N-methyl leukotriene C4
- OGD:
-
Oxygen–glucose-deprivation
- TEM:
-
Transmission electron microscopy
- TGF:
-
Transforming growth factor
- TNF:
-
Tumor necrosis factor
References
Yang J, Zhao Y, Zhang L, Fan H, Qi C, Zhang K, Liu X, Fei L, Chen S, Wang M, Kuang F, Wang Y, Wu S (2018) RIPK3/MLKL-mediated neuronal necroptosis modulates the M1/M2 polarization of microglia/macrophages in the ischemic cortex. Cereb Cortex 28(7):2622–2635
Ghosh M, Xu Y, Pearse DD (2016) Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J Neuroinflamm 13(9):877
Brifault C, Gras M, Liot D, May V, Vaudry D, Wurtz O (2015) Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 46:520–528
Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, Funk CD (2004) Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation 110(21):3360–3366
Yan D, Stocco R, Sawyer N, Nesheim ME, Abramovitz M, Funk CD (2011) Differential signaling of cysteinyl leukotrienes and a novel cysteinyl leukotriene receptor 2 (CysLT2) agonist, N-methyl-leukotriene C4, in calcium reporter and β arrestin assays. Mol Pharmacol 79:270–278
Shi QJ, Wang H, Liu ZX, Fang SH, Song XM, Lu YB, Zhang WP, Sa XY, Ying HZ, Wei EQ (2015) HAMI 3379, a CysLT2R antagonist, dose- and time-dependently attenuates brain injury and inhibits microglial inflammation after focal cerebral ischemia in rats. Neuroscience 291:53–69
Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L, Yan X (2019) Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles 9(1):1697028
Liu D, Wang Y, Ye Y, Yin G, Chen L (2014) Distinct molecular basis for endothelial differentiation: gene expression profiles of human mesenchymal stem cells versus umbilical vein endothelial cells. Cell Immunol 289(2014):7–14
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91
Rahman SO, Singh RK, Hussain S, Akhtar M, Najmi AK (2019) A novel therapeutic potential of cysteinyl leukotrienes and their receptors modulation in the neurological complications associated with Alzheimer's disease. Eur J Pharmacol 842:208–220
Gelosa P, Colazzo F, Tremoli E, Sironi L, Castiglioni L (2017) Cysteinyl leukotrienes as potential pharmacological targets for cerebral diseases. Mediat Inflamm 2017:3454212
Zhao CZ, Zhao B, Zhang XY, Huang XQ, Shi WZ, Liu HL, Fang SH, Lu YB, Zhang WP, Tang FD, Wei EQ (2011) Cysteinyl leukotriene receptor 2 is spatiotemporally involved in neuron injury, astrocytosis and microgliosis after focal cerebral ischemia in rats. Neuroscience 189:1–11
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53(2):1181–1194
Willis GR, Kourembanas S, Mitsialis SA (2017) Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med 4:63
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19:1769–1779
Jiang XC, Gao JQ (2017) Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm 521:167–175
Paolicelli RC, Bergamini G, Rajendran L (2018) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157
Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J (2018) Exosomes: new molecular targets of diseases. Acta Pharmacol Sin 39(4):501–513
Zhang D, Lee H, Wang X, Rai A, Groot M, Jin Y (2018) Exosome-mediated small RNA delivery: a novel therapeutic approach for inflammatory lung responses. Mol Ther 26(9):2119–2130
Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, Wang Y, Caldwell CC, Peng T, Zingarelli B, Fan GC (2015) Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep 5:13721
Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, Ji J, Liu F, Ding L, Ni Y, Hou Y (2017) ExosomalmiR-146a contributes to the enhanced therapeutic efficacy of interleukin-1beta-primed mesenchymal stem cells against sepsis. Stem Cells 35:1208–1221
Lv YN, Ou-Yang AJ, Fu LS (2017) MicroRNA-27a begatively modulates the inflammatory response in lipopolysaccharide-stimulated microglia by targeting TLR4 and IRAK4. Cell Mol Neurobiol 37(2):195–210
Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, Levy E, Fox HS (2018) Correction: MiR-21 in extracellular vesicles leads to neurotoxicity via TLR7 signaling in SIV neurological disease. PLoS Pathog 14(5):e1007068
Acknowledgements
Thanks to the scientific research department of Hangzhou Medical College and Pro. Xinghui Song of Zhejiang University for great help in FACS. Thanks to Pro. Yilu Ye for help in MCAO model preparing.
Funding
The project was supported by Zhejiang Provincial Natural Science Foundation of China (No. LY18H090011), TCM Administration Bureau of Zhejiang Province (No. 2014ZA018), the Natural Science Youth Foundation of China (No. 81201408) and the Science Technology Department of Zhejiang Province (No. 2012C23104).
Author information
Authors and Affiliations
Contributions
YZ was the main performer throughout the experiments, YG participated in the cell experiment, GX carried out the animal experiment, GY took part in the content and methods design, DL was responsible for the conception, interpretation, and editing of the whole study. All authors approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
Authors have declared no conflict of interest.
Ethical Approval
Experiments were approved by the Institutional Animal Care and Use Committee of Hangzhou Medical College (2017, No163). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, Y., Gan, Y., Xu, G. et al. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem Res 45, 1180–1190 (2020). https://doi.org/10.1007/s11064-020-02998-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02998-0