Abstract
Parkinson's disease is a neurodegenerative disorder which accompanied with cognitive decline, chorei form moves and behavioral difficulties. Oxidative stress which promote the apoptotic cell death are responsible for neurodegeneration in Parkinson. The purpose of this study is to evaluate the protective effects of betanin against toxicity and oxidative damage induced by 6-hydroxydopamine (6-OHDA) and hydrogen peroxide (H2O2) in PC12 cells as an appropriate model of Parkinson's cell damage. PC12 cells pretreated with betanin (1–200 µM) for 24 h, and exposed to either 6-OHDA (100 µM) or H2O2 (150 µM) for 24 h. Cell survival and intracellular reactive oxygen species (ROS) production analyzed by resazurin and DCF-DA assay. The anti-apoptotic effects of betanin in PC12 cells were studied using flow cytometry of PI stained cells. Also, western blot analysis of survivin, Cyt c, Phospho SAPK/JNK, SAPK/JNK, Phospho-PI3 kinase P85, PI3 kinase P85 was performed for detection of apoptosis. Betanin (1–200 µM) significantly decreased the 6-OHDA and H2O2 cytotoxicity also attenuated the ROS level. Cell apoptosis significantly increased after 6-OHDA (100 µM) treatment, compared to the control. However, pretreatment with betanin (20 and 50 µM), protected against apoptosis. Western blot analysis of PC12 cells showed that 100 µM 6-OHDA could increase the proteins involved in apoptosis signaling and betanin (20 and 50 µM), could decrease the apoptosis. The results show that betanin has antioxidant and anti-apoptotic effects and may have the ability to prevent or delay the progress of neural death in Parkinson's disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson's is a progressive disease of the nervous system that affect body movement. Vibration in rest, bradykinesia, rigidity and difficulty at the start of the move are the main symptoms [1]. Signs of Parkinson's disease at the cellular level include selective deprivation of dopaminergic neurons in substantia nigra pars compacta and the presence of Lewy bodies containing α-synuclein in neuronal cytoplasm [2]. The death cause of dopaminergic neurons in Parkinson's disease has not been clearly clarified. In the last two decades, in addition to underlying genetic factors, oxidative stress and mitochondrial function impairment have been identified as the main contributors to the neuronal degeneration of Parkinson's disease [3]. 6-hydroxydopamine (6-OHDA) leads to a syndrome similar to Parkinson's in humans and rodents. Impair in the function of mitochondria through inhibition of mitochondrial complex I has been shown with 6-OHDA [4]. Oxidation of dopamine increases the amount of hydrogen peroxide (H2O2) and changes the mitochondrial function [5, 6]. Antioxidants preserve the redox/oxidation balance in central nervous system and protect against oxidative damage [7]. Over the past few years, the use of natural compounds has been noticed in protection against neurological diseases. Anthocyanin pigments are among the natural products which have attracted much attention as antioxidant [8]. Betalains which present in red beetroot (Beta vulgaris L.) are considered as hydrophilic nitrogen-based pigments [9, 10]. Betanin is one of the abundant compounds among betalains [11] with excellent antioxidant and anti-inflammatory effects [12,13,14]. Phenolic and cyclic groups within the betanin, trigger the free-radical scavenging function [15]. Interestingly, as a potent NO generator with high content of betanin, beetroot help in improvement of the cognitive responses and importantly cerebrovascular blood flow [16, 17].
Since there is not a report about the putative mechanism of betanin against neurodegeneration, in the present study, we have evaluated the protective effect of betanin on toxicity and oxidative damage induced by 6-OHDA and H2O2 in PC12 cells as an appropriate model of Parkinson's cell damage.
Materials and Methods
Materials
Betanin, Resazurin, the fluorescent probe propidium iodide (PI), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), H2O2 33%, 6-OHDA and 1640 RPMI medium and QuantiPro™ BCA Assay Kit from Sigma (Germany); rabbit anti-serum against survivin, rabbit polyclonal anti-serum against Cyt c, rabbit polyclonal Phospho SAPK/JNK, rabbit polyclonal SAPK/JNK, rabbit polyclonal Phospho-PI3 Kinase P85, rabbit polyclonal PI3 Kinase P85, β-Actin (13E5), anti-rabbit IgG HRP-linked antibody from Cell Signaling Technology (USA); fetal bovine serum (FBS) and penicillin-streptomycin (PS) from Gibco (USA); dimethyl sulfoxide (DMSO) from Merk (Germany); differentiated rat pheochromocytoma PC12 cells purchased from Pasteur Institute (Iran).
Cell Culture and Treatment
Differentiated rat pheochromocytoma PC12 cells maintained in RPMI-1640 medium with 1% penicillin and streptomycin, 10% (v/v) FBS. Cells incubated at temperature of 37 °C, a relative humidity of about 95% and 5% CO2 concentration. 6-OHDA and betanin were dissolved in DMSO to obtain a 40 mM stock solution. PC12 cells pretreated with betanin (1–200 µM) for 24 h. Then exposed to either 6-OHDA (100 µM) or H2O2 (150 µM) for the next 24 h. The optimum time and concentration points were used according to the pre-test evaluation with 6-OHDA (12.5, 25, 50, 100, 200 and 50) for 24 h (Data not shown) and previous studies [18].
Analysis of Cell Viability
Resazurin reduces to resorufin and the change in the color is proportional to metabolic activity of the cell which is measured by colorimetric or fluorometric methods [19]. 1 × 104 PC12 cells seeded in each well of 96-well culture plates. Betanin was added 24 h before 6-OHDA and H2O2 exposure. After 24 h, 20 µl of resazurin was added to each well and incubated at 37 °C for 4–6 h. Cellular viability was determined by Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA) and the absorbance intensity measured in 570 and 600 nm.
ROS Generation
To determine the amount of reactive oxygen production, DCFH-DA is added to the cells. Lipophilic and non-fluorescent DCFH-DA passes through the cell membrane, de acetylated with intracellular esterase and finally converted to the fluorescent DCF via interaction with intracellular ROS [20]. 1 × 104 PC12 cells per well were seeded in 96-well culture plates. Betanin (1–200 µM) was added to the cultures 24 h before 6-OHDA (100 µM) and H2O2 (150 µM) exposure. After 24 h, DCFH-DA was added to the cultured cells. Then, the cells were exited at 485 nm and the emission at 538 nm was plotted with a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA). Data expressed as proportional to the amount of active species of mitochondrial oxygen.
Flowcytometric Apoptosis Assay
To sub G1 peak in the flow cytometry histogram of PI stained cells determine the amount of apoptosis in cells [21, 22]. 105 PC12 were seeded in each well of a 12-well plates and treated with betanin (20 and 50 µM) for 24 h before 6-OHDA (100 µM). In the following, cells were harvested and 400 µl of a hypotonic buffer containing 50 µg/mL PI in 0.1% sodium citrate plus 0.1% triton X-100 was added to each sample. Then, analyzed by FACS Scan flow cytometer (BD Biosciences, CA, USA).
Western Blotting
About 106 PC12 cells were treated with betanin (20 and 50 µM) for 24 h before 6-OHDA (100 µM). The cells were harvested and washed with cold PBS, then the western blot test performed according to the instructions previously published [23]. The membrane exposed to rabbit monoclonal survivin, polyclonal Cyt c, rabbit polyclonal Phospho SAPK/JNK, rabbit polyclonal SAPK/JNK, polyclonal Phospho-PI3 kinase P85, polyclonal PI3 kinase P85 and β-actin (13E5) as primary antibodies and anti-rabbit IgG, a HRP-linked antibody as secondary antibody. The values obtained from each sample were divided into its respective β-actin content. In this way, we calculated how much bandwidth has changed over control (intensity of the related β-Actin band) using Gel-pro Analyzer V.6.0 Gel Analysis Software. (Media Cybernetics, InG, Bethesda, MD).
Statistical Analysis
All data were expressed as Mean ± SD compared with the respective control using one-way ANOVA, followed by Dunnett's post hoc test in Graph Pad Prism 5 software. For all the findings differences in levels p < 0.05 are considered as significant levels.
Results
Effects of Betanin on Cell Viability
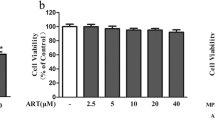
To determine the optimal protective concentration of betanin, the cell cytotoxicity of the betanin was measured. Betanin did not show cytotoxic effects in any concentration compared to the control group (Fig. 1a).
a Effects of betanin on cell viability. The viability of PC12 cells determined after treatment with betanin (1–200 µM) for 24 h. The data presented as the mean ± SD (n = 9) of three independent experiments. b, c Effects of betanin on 6-OHDA and H2O2 induced PC12 on cell viability. PC12 cells were pretreated with betanin (1–200 µM) 24 h before treatment with 100 µM 6-OHDA and 150 µM H2O2 for 24 h. The data presented as the mean ± SD (n = 9) of three independent experiments. *p < 0.05, ***p < 0.001 and **p < 0.01 compared with 6-OHDA and H2O2 group (n = 3) in triplicate. d, e Effects of betanin on 6-OHDA and H2O2 induced ROS production. The PC12 cells pretreated with betanin (1–200 µM) for 24 h before treatment with 100 µM 6-OHDA and 150 µM H2O2 for 24. The data presented as the mean ± SD (n = 9) of three independent experiments. ***p < 0.001 compared with 6-OHDA and H2O2 group
Effects of Betanin on 6-OHDA and H2O2 Induced PC12 on Cell Viability
6-OHDA (100 µM) significantly reduced the cell viability compared with the control group ( p < 0.001) while pretreatment with betanin (5–200 µM) showed significantly higher cell survival rates compared with the 100 µM 6-OHDA (p < 0.05, p < 0.01 and p < 0.001) (Fig. 1b). H2O2 (150 µM) led to significant reduction in cell viability compared with the control group (p < 0.001) while pretreatment with betanin (5–200 µM) exhibited significantly higher cell survival rates compared with the 150 µM H2O2 (p < 0.001) (Fig. 1c). This finding show that betanin potentially can protect PC12 cells from 6-OHDA and H2O2 induced cell death.
Effects of Betanin on 6-OHDA and H2O2 Induced ROS Production
Treatment with 6-OHDA (100 µM) for 24 h induced a significant elevation in the cell fluorescence intensity compared with the control group (p < 0.001). After pretreatment with betanin (1–200 µM); however, the fluorescence intensity decreased significantly (p < 0.001) (Fig. 1d). H2O2 (150 µM) significantly increased cell fluorescence intensity compared with the control group (p < 0.001). After pretreatment with betanin (5–200 µM); however, the fluorescence intensity decreased significantly (p < 0.001) (Fig. 1e). This results can indicate that betanin reduces the production of 6-OHDA and H2O2 induced ROS.
Effects of Betanin on 6-OHDA Induced Apoptosis by Flow Cytometry
Effects of betanin on apoptosis induced by 6-OHDA in PC12 cells were examined using flow cytometry after PI staining. Cell apoptosis was significantly increased to 75.9% after treatment with 6-OHDA (100 µM) compared to control (1.9%) (p < 0.001). After pretreatment with betanin (20 and 50 µM); however, apoptosis was significantly reduced to 15.3% and 31.3% (p < 0.001). The sub-G1 peak in flow cytometry histograms of PC12 cells showed that betanin could reduce the amount of apoptosis compared to 6-OHDA (100 µM) (Fig. 2a, b).
Effect of Betanin and 6-OHDA on Apoptosis Signaling Proteins
To determine the mechanism of protective effects of betanin versus 6-OHDA, the amount of apoptotic proteins (survivin, Cyt c, phospho SAPK/JNK46/54, SAPK/JNK46/54, PI3 Kinase P85, Phospho-PI3 Kinase P85) were compared in the PC12 cells treated with betanin and 6-OHDA. Our results showed that treatment with 6-OHDA (100 µM) for 24 h reduced survivin (p < 0.05) and significantly increased Cyt c (p < 0.01) whereas pretreatment with betanin (20 and 50 µM) markedly decreased Cyt c (p < 0.01). Also, 6-OHDA (100 µM) increased Phospho SAPK/JNK46/54 to SAPK/JNK46/54 compared with the control group (p < 0.05) (p < 0.001), and pretreatment with betanin (20 and 50 µM) inverted the 6-OHDA induced apoptosis (p < 0.05) (p < 0.01). In addition, 6-OHDA (100 µM) reduced the ratio of Phospho-PI3 kinase p85/p55 to PI3 kinase p85/p55 (p < 0.01) compared with the control group, and betanin (20 and 50 µM) protected the cells against apoptosis (p < 0.01) (Fig. 3).
Effect of betanin and 6-OHDA on apoptosis signaling proteins. About 106 PC12 cells were treated with betanin (20 and 50 µM) 24 h before 6-OHDA (100 µM) exposure. Images were quantified using Gel-pro Analyzer V.6.0 Gel Analysis Software. The data are presented as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 compared to the 6-OHDA
Discussion
In this study, we examined the possible protective effects of betanin on the toxicity of 6-OHDA in PC12 cells. The findings of this study showed the protective effects of betanin as a potent antioxidant on PC12 cells against 6-OHDA and H2O2 toxicity. In addition, we examined the effects of betanin on apoptosis also related molecular mechanisms against 6-OHDA toxicity in PC12 cells. Previously it was shown that 6-OHDA produces hydrogen peroxide and hydroxyl radicals leading to the impairment of mitochondrial function [24]. Types of reactive oxygen are collectively referred to as reactive oxygen species (ROS) which many of them are free radicals. ROS is not always harmful. For example, they play a vital role in the killing of pathogenic microbes by phagocytes also they have other beneficial effects. Fortunately, ROS is naturally inhibited in the body by a variety of complex defense systems. These systems are perfect because they work on different ROSs or on different cellular constituents. Oxidative stress is a mechanism involved in the patogenesis of Parkinson's disease and may attribute to the apoptosis of dopaminergic neurons through mitochondrion defect. In the cellular model of Parkinson's disease with 6-hydroxy dopamine (6-OHDA) on PC12 cells, oxidative stress is increased which leads to inhibition of Sirtuin1 function and cell death [25]. Investigations have focused on the highly complex and complementary relationships between oxidative stress and genes involved in Parkinson's disease. It is also shown that dopamine metabolism in the brain spontaneously leads to oxidative stress and this will cause changes in intracellular macromolecules which their performance is naturally essential for cell survival. In addition, activated microglia produc nitric oxide and superoxide during the inflammatory responses of neurons and this will be worse with the release of molecules such as α-synuclein, neuromelanin and matrix metalloproteinase-3 from the damaged dopaminergic neurons [26]. Polyphenolic compounds as unique antioxidants, regardless of their role to combat ROS, also may have synergistic effect with other natural compounds [27]. Red beetroot has high level of betalain pigments [28]. Recent studies show that betalain pigments in red beetroot extract has free radical-scavenging properties and as natural antioxidants, they have the ability to prevent and treat oxidative stress related diseases [9]. One of the reasons for the high antioxidant property of betanin is due to its high electron-donor properties [29]. Studies have shown betalains induce glutathione formation in human erythrocytes [30]. Also, betalain keeps the LDL (low density lipoprotein) particle safe from oxidation [29, 31] and reduce biomarkers of lipid oxidation [30]. Furthermore, in mice fed with red beet extract, betanin exert antioxidant activity [32] and has anti-inflammatory effects by inhibiting cyclooxygenase-2 [13]. There is a report implying that betanin may have protective effect on kidney of parquet treated rats by reducing oxidative stress and inflammatory reactions [33]. Another study has shown that betanin protects against parquet-induced acute lung injury and interstitial pneumonia in rats as a natural antioxidant through antioxidant and anti-inflammatory mechanisms [34]. Also, studies indicate that the presence of betanin in beetroot leads to reduction in ROS production, DNA damage and modify the neutrophil oxidative metabolism [14]. In human lymphocytes, betalains decrease the H2O2 induced damage of DNA [35]. Furthermore, in human hepatocytes, treatment with betanin activated the nuclear factor erythroid 2 related factor 2(Nrf2) dependent signaling pathway (NRF2-ARE) [36]. Treatment of Huh7 cells with betanin increased the expression of heme oxygenase 1(HO1) and paraoxonase 1 (PON1) genes [37]. In addition, treatment of rat liver cells with betanin in the red beetroot extract increased the quinone reductase [38].
In current study, betanin (1–200 µM) does not show any toxicity compared to the control group (Fig. 1). While 6-OHDA (100 µM) and H2O2 (150 µM) decreased the cellular viability, betanin (5–200 µM) increased the cell survival. Pre-treatment of cells with betanin decreased the toxicity of 6-OHDA (Fig. 1). DCFH-DA fluorescence intensity reflect the ability of compounds to act as hydrogen atom donors. Treatment with 6-OHDA (100 µM) and H2O2 (150 µM) for 24 h induced a significant increase in the DCFH-DA fluorescence intensity in cells compared with the control group which inhibited with betanin (1–200 µM) (Fig. 1). It seems that betanin reduces the production of free radicals and protects the PC12 cells against 6-OHDA and H2O2 toxicity. So, one of proposed the mechanisms of betanin is to enhance the activity of intracellular antioxidant enzymes. Betanin (20 and 50 µM) decreased cell apoptosis induced by 6-OHDA (100 µM) which indicates the anti-apoptotic properties of betanin (Fig. 2).
To determine the mechanism of inhibition of apoptosis, expression of Cyt c, Phospho SAPK/JNK, SAPK/JNK, Phospho-PI3 kinase P85, PI3 kinase P85 protein was evaluated after 24 h pretreatment with 20 and 50 µM betanin and after treatment with 6-OHDA. Survivin (16 kD) is a member of the family of apoptosis inhibitors that inhibit the caspases, and negatively regulate the apoptosis [39]. Our results showed that betanin (20 and 50 µM) increased survivin rate relative to 6-OHDA (100 µM) and betanin have a protective effect against 6-OHDA induced-toxicity (Fig. 3). During apoptosis, Cyt c (14 kD) is released from mitochondria and activate caspase 9 [40]. The results of this study show that 6-OHDA (100 µM) increased the amount of Cyt c protein while betanin (20 and 50 µM) reduced the release of Cyt c (Fig. 3). MAPK (mitogen-activated protein kinase) is a type of protein kinase with amino acids serine and threonine that regulate cell activity including cell viability and apoptosis [41]. Studies have shown that blocking the transfer of SAPK/JNK (46/54 kD) to mitochondria prevents the toxic effect of 6-OHDA [42]. It seems when the cascade of 6-OHDA neural degeneration initiated, betanin reduces phosphorylation of SAPK/JNK and induction of apoptosis. Our results showed that 6-OHDA (100 µM) activated JNK, while betanin (20 and 50 µM) decreased the ratio of Phospho SAPK/JNK to SAPK/JNK and reduced the cell apoptosis (Fig. 3). The PI3K pathway regulates cell activity including survival. When PI3K (85 kD) is activated, phosphorylation of the Akt (protein kinase B or PKB) protein occurs. The phosphorylated Akt, in turn, inhibit the pro-apoptotic family of protein including Bad, Bax, caspase-9, GSK-3, and FoxO1 [43]. Our results showed that 6-OHDA (100 µM) reduces the ratio of Phospho-PI3 kinase p85/p55 to PI3 kinase p85/p55 while betanin (20 and 50 µM) reduces the cell death (Fig. 3). So, probably betanin inhibits apoptosis by reducing phosphorylation of SAPK/JNK and PI3K pathways.
The possible mechanism of the protective effect of betanin to attenuate the oxidative stress induced by 6-OHDA in PC12 cells appear to be through reduction in phosphorylation of SAPK/JNK and increase in phosphorylation of PI3K. As mentioned, phosphorylation of PI3K let to inhibition of the pro-apoptotic family of protein including Bax and activation of the anti-apoptotic Bcl-2 protein. Subsequently the increase in the amount of survivin and reduction in the release of Cyt c from mitochondria, in turn inhabit the activation caspase 9 and caspase 3. Inhabiting caspase 3 activation reduces the cleavage of PARP and finally decrease apoptosis induced by 6-OHDA (Fig. 4). Of course, more research is needed to clarify the mechanism of the effect of betanin.
Schematic representation of the protective role of betanin on 6-OHDA cytotoxicity. 6-OHDA induce apoptosis through SAPK/JNK and PI3 K pathways. Betanin decreased phosphorylation of SAPK/JNK and increased phosphorylation of PI3 K. Also, 6-OHDA activated the intrinsic pathway of apoptosis through reduction of Cyt c and increase survivin
Conclusion
The results of our study show that 6-OHDA leads to apoptosis via PI3K/AKT and MAPK pathways. Also, it can activate the internal pathway of apoptosis. While betanin prevents 6-OHDA-induced apoptosis and may able to prevent the neural degeneration in Parkinson's disease as a potential treatment.
References
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry79:368–376
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD. Hasan K, Laurie C, Gendelman HE (2006) Neuroinflammation, oxidative stress, and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6:261–281
de Vries HE, Kooij G, Frenkel D, Georgopoulos S, Monsonego A, Janigro D (2012) Inflammatory events at blood–brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia 53:45–52
Singh S, Ahmad R, Mathur D, Sagar RK, Krishana B, Arora R, Sharma RK (2006) Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease
Hernandez-Baltazar D, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and parkinsonian pathophysiology: novel findings in an older model. Neurología (English Edition) 32:533–539
Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909
Ebadi M, Srinivasan SK, Baxi MD (1996) Oxidative stress and antioxidant therapy in Parkinson's disease. Prog Neurobiol 48:1–19
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Aspects Med 24:345–351
Azeredo HM (2009) Betalains: properties, sources, applications, and stability—a review. Int J Food Sci Technol 44:2365–2376
Nemzer B, Pietrzkowski Z, Spórna A, Stalica P, Thresher W, Michałowski T, Wybraniec S (2001) Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem 127:42–53
Gliszczyńska-Świgło A, Szymusiak H, Malinowska P (2006) Betanin, the main pigment of red beet: molecular origin of its exceptionally high free radical-scavenging activity. Food Addit Contam 23:1079–1087
Kujawska M, Ignatowicz E, Murias M, Ewertowska M, Mikołajczyk K, Jodynis-Liebert J (2009) Protective effect of red beetroot against carbon tetrachloride-and N-nitrosodiethylamine-induced oxidative stress in rats. J Agric Food Chem 27:2570–2575
Reddy MK, Alexander-Lindo RL, Nair MG (2005) Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J Agric Food Chem 53:9268–9273
Zielińska-Przyjemska M, Olejnik A, Kostrzewa A, Łuczak M, Jagodziński PP, Baer-Dubowska W (2012) The beetroot component betanin modulates ROS production, DNA damage and apoptosis in human polymorphonuclear neutrophils. Phytother Res 26:845–852
Han J, Zhang Z, Yang S, Wang J, Yang X, Tan D (2014) Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem Toxicol 70:100–106
Thompson KG, Turner L, Prichard J, Dodd F, Kennedy DO, Haskell C, Blackwell JR, Jones AM (2014) Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir Physiol Neurobiol 193:11–20
Clifford T, Howatson G, West DJ, Stevenson EJ (2015) The potential benefits of red beetroot supplementation in health and disease. Nutrients 7:2801–2822
Liu H, Mao P, Wang J, Wang T, Xie CH (2015) Allicin protects PC12 cells against 6-OHDA-induced oxidative stress and mitochondrial dysfunction via regulating mitochondrial dynamics. Cell Physiol Biochem 36:966–979
O'Brien J, Wilson I, Orton T, Pognan F (2000) Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–5426
Liu RT, Zou LB, Lü QL (2009) Liquiritigenin inhibits Aβ 25–35-induced neurotoxicity and secretion of Aβ 1–40 in rat hippocampal neurons. Acta Pharmacol Sin 30:899
Zhang H, Wang X, You M, Liu C (1999) Water-yield relations and water-use efficiency of winter wheat in the North China Plain. Irrig Sci 19:37–45
Rahiman N, Akaberi M, Sahebkar A, Emami SA, Tayarani-Najaran Z (2018) Protective effects of saffron and its active components against oxidative stress and apoptosis in endothelial cells. Microvasc Res 118:82–89
Tayarani-Najaran Z, Makki F. Alamolhodaei NS, Mojarrab M, Emami SA (2017) Cytotoxic and apoptotic effects of different extracts of Artemisia biennis Willd. on K562 and HL-60 cell lines. IJBMS 20:166
Blum D, Torch S, Lambeng N, Nissou MF, Benabid AL, Sadoul R, Verna. JM (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol 65:135–172
Ramazani E, Fereidoni M, Tayarani-Najaran Z (2019) Protective effects of vitamin K2 on 6-OHDA-induced apoptosis in PC12 cells through modulation bax and caspase-3 activation. Mol Biol Rep 12:1–7
Keum JW, Shin A, Gillis T, Mysore JS, Elneel KA, Lucente D, Hadzi T, Holmans P, Jones L, Orth M, Kwak S (2016) The HTT CAG-expansion mutation determines age at death but not disease duration in Huntington disease. Am J Hum Genet 98(2):287–298
Gilgun-Sherki Y, Melamed E, Offen D (2003) Antioxidant treatment in Alzheimer’s disease. J Mol Neurosci 21(1):1–1
Strack D, Vogt T, Schliemann W (2003) Recent advances in betalain research. Phytochemistry 62(3):247–269
Kanner J, Harel S, Granit R (2001) Betalains a new class of dietary cationized antioxidants. J Agric Food Chem 4 9(11):5178–5185
Tesoriere L, Butera D, Pintaudi AM, Allegra M, Livrea MA (2004) Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: a comparative study with vitamin C. Am J Clin Nutr 80:391–395
Tesoriere L, Butera D, D'arpa D, Di Gaudio F, Allegra M, Gentile C, Livrea MA (2003) Increased resistance to oxidetion of betalain-enriched human low density lipoproteins. Free Radic Res 37:689–696
Lee JH, Son CW, Kim MY, Kim MH, Kim HR, Kwak ES, Kim S, Kim MR (2009) Red beet (Beta vulgaris L.) leaf supplementation improves antioxidant status in C57BL/6J mice fed high fat high cholesterol diet. Nutr Res Pract 3:114–121
Tan D, Wang Y, Bai B, Yang X, Han J (2015) Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem Toxicol 78:141–146
Han J, Ma D, Zhang M, Yang X, Tan D (2015) Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. Biomed Res Int 2015:608174
Siriwardhana N, Shahidi F, Jeon YJ (2006) Potential antioxidative effects of cactus pear fruit (Opuntia ficus-indica) extract on radical scavenging and DNA damage reduction in human peripheral lymphocytes. J Food Lipids 13:445–458
Krajka-Kuźniak V, Paluszczak J, Szaefer H, Baer-Dubowska W (2013) Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br J Nutr 110(12):2138–2149
Esatbeyoglu T, Wagner AE, Motafakkerazad R, Nakajima Y, Matsugo S, Rimbach G (2014) Free radical scavenging and antioxidant activity of betanin: electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem Toxicol 73:119–126
Wettasinghe M, Bolling B, Plhak L, Xiao H, Parkin K (2002) Phase II enzyme-inducing and antioxidant activities of beetroot (Beta vulgaris L.) extracts from phenotypes of different pigmentation. J Agric Food Chem 50(23):6704–6709
Wang TT, Qian XP, Liu BR (2007) Survivin: potential role in diagnosis, prognosis and targeted therapy of gastric cancer. World J Gastroenterol 13:2784
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22:153–183
Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol 10:205–219
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’connor R, O’neill C (2005) Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J Neurochem 93:105–117
Acknowledgements
The authors would like to thank Mr. Malaeke for assistance in the flow cytometry of samples.
Funding
This work has been supported by Grant No. 3/42879 from Ferdowsi University of Mashhad, Mashhad, Iran and Grant No. 951766 from Research Affairs of Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Contributions
EH performed the experiments and wrote the manuscript. MF and ZT-N conceived, designed, and supervised the project, wrote the manuscript nd provided financial support.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Ethics Approval
As this work is carried out in PC12 cells, there is no need for ethical clearance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hadipour, E., Fereidoni, M. & Tayarani-Najaran, Z. Betanin Attenuates Oxidative Stress Induced by 6-OHDA in PC12 Cells via SAPK/JNK and PI3 K Pathways. Neurochem Res 45, 395–403 (2020). https://doi.org/10.1007/s11064-019-02927-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02927-w