Abstract
Epilepsy is a chronic neurological disease. Astrogliosis is an important pathological change in epileptic lesions. Studies have reported that ibuprofen can affect autophagy and/or inhibit cell proliferation in many diseases. This study investigated the effect and significance of ibuprofen on autophagy of astrocytes during pentylenetetrazol (PTZ) induced epilepsy. 60 male Sprague–Dawley (SD) rats were randomly divided into five groups: control group (received normal saline), PTZ group, 3-methyladenine (3-MA) + PTZ group, ibuprofen + PTZ group and 3-MA + ibuprofen + PTZ group. Dose of each agent was 35 mg/kg (PTZ), 10 mg/kg (3-MA) and 30 mg/kg (ibuprofen) and all drugs were administered intraperitoneally 15 times on alternate days (29 days). Human astrocytes were cultured in vitro. Behavioral performance (i.e., latency, grade and duration of seizures) and EEG of rats were observed and recorded. Proliferation of astrocytes was detected by CCK-8 method. Immunofluorescence and Western blot test were used to detect the expression of LC3 and GFAP. Mean number, grade and duration of seizures were markedly reduced in ibuprofen + PTZ group and 3-MA + ibuprofen + PTZ group (P < 0.05). Similarly, peak of EEG waves were markedly reduced in ibuprofen + PTZ group and 3-MA + ibuprofen + PTZ group (P < 0.05). Compared to the control group, the level of LC3 in ibuprofen group was significantly increased in vitro (P < 0.05). While, levels of LC3 were significantly higher and that of GFAP were significantly lower in ibuprofen + PTZ group (P < 0.05) compared to PTZ group in vivo. Ibuprofen reduces the proliferation of astrocytes by increasing autophagy, thus affecting the development of epilepsy. Therefore, ibuprofen may be used as an adjuvant to improve efficacy of treatment in epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a chronic neurological disease characterized by repeated occurrence of epileptic seizures, and affects about 50 million people worldwide [1]. It is often accompanied by psychological, behavioral, and cognitive impairments, which imposes a heavy burden on the society. Effective control of epileptic seizures is not possible with the currently available antiepileptic drugs. Around 30% of the patients are not sensitive to anticonvulsant drugs, which relieves only the symptoms, and thus, are prone to drug resistance [2]. Hence, the prevention and treatment of epilepsy still remains an unsolved global problem.
Studies have shown that astrocytes are closely related to the pathogenesis of epilepsy [3]. Astrogliosis is an important pathological change in epileptic lesions [4]. The expression level of glial fibrillary acidic protein (GFAP) can be used as a measure of astrocyte proliferation [5]. The formation of glial scars changes the structure and function of cells, thus affecting amino acid metabolism and regulation of ion concentration, thereby leading to seizures [6]. Therefore, effective inhibition of astrocyte proliferation is a unique approach to prevent epilepsy.
Autophagy is an important process by which eukaryotic proteins are degraded [7]. Microtubule-associated protein 1 light chain 3 (LC3) is a specific marker for autophagy, LC3-II/LC3-I can reflect the activation of autophagy [8]. In last few years, the focus has shifted to the role of astrocyte autophagy in epilepsy [9]. Moreover, recent studies have observed that autophagy can inhibit the progression of disease by inhibiting cell proliferation [10], and thus, studying effects of autophagy of astrocytes and reducing their proliferation can be a novel way to prevent and treat epilepsy. Studies have reported the role of ibuprofen in many diseases, where it acts by affecting autophagy [11] and/or inhibiting cell proliferation [12], but this correlation has not been documented in epilepsy. Thus, the present study was performed to evaluate the effects and significance of ibuprofen on autophagy of astrocytes during pentylenetetrazol (PTZ)-induced epilepsy.
Materials and Methods
Experimental Animals
Sixty male Sprague–Dawley (SD) rats (250–300 g), 6–8 weeks-old, were provided by Shandong Jinan Pengyue Experimental Animal Breeding Co. Ltd. They were raised in individual cages with a controlled environment set at a temperature of 22 ± 2 °C and humidity of 50–60% and a strict 12 h light/dark cycle was maintained. They were provided with a liberal amount drinking water and standard feeds. All animals were acclimated for 1 week before performing experiments. All experiments were approved by the Ethics Committee of the Binzhou Medical University Hospital.
Cell Culture
Human astrocyte cell line (HA1800) was purchased from Wuhan Boster Bioengineering Co. Ltd. The cells were cultured in H-DMEM containing 10% FBS, and incubated at 37 °C, and 5% CO2. They were passed on to the 5th generation for related experiments.
Chemicals
PTZ, 3-methyladenine (3-MA) and ibuprofen were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse anti-GFAP monoclonal antibody, rabbit anti-LC3 monoclonal antibody, Alexa Fluor® 647-labeled goat anti-mouse polyclonal IgG, Alexa Fluor® 488-labeled goat anti-rabbit polyclonal IgG, Alexa Fluor® 488-labeled goat antibody Mouse polyclonal IgG, Alexa Fluor® 647-labeled goat anti-rabbit polyclonal IgG, HRP-labeled goat anti-mouse IgG, HRP-labeled goat anti-rabbit IgG and DAPI were purchased from Abcam (San Francisco, CA, USA). RIPA lysate, PMSF protease inhibitor, BCA protein quantification kit, polyacrylamide gel preparation kit and chemiluminescence reagent were purchased from Wuhan Dr. Biological Engineering Co. Ltd., (China); RM2245 paraffin slicer and SP5II laser confocal microscope were purchased from Leica (Germany); Nicolet EEG V32 recorder was purchased from CareFusion (USA).
Animals Inducing Procedure
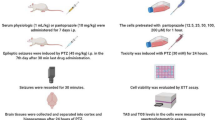
PTZ was used to induce epilepsy in SD rats [13]. Using a random number table, SD rats were randomly divided into five groups (n = 12/group): (i) Control group—rats received normal saline (NS) administered intraperitoneally (i.p.); (ii) PTZ group—rats received PTZ (35 mg/kg i.p.); (iii) 3-MA + PTZ group—rats received 3-MA (10 mg/kg i.p.) [14] 30 min before PTZ injection; (iv) Ibuprofen + PTZ group—rats received ibuprofen (30 mg/kg i.p.) [15] 30 min before PTZ injection; (v) 3-MA + Ibuprofen + PTZ group—rats received 3-MA [14] and ibuprofen [15] 30 min before PTZ injection. NS and all other drugs were administered 15 times on alternate days (29 days).
After i.p. administration of PTZ, rats were observed for 30 min, and rats with at least three epileptic seizures of grade 4 or above were considered to be fully induced [16]. The seizures were graded based on the following criteria [17]: grade 0: no seizure response; grade 1: rhythmic twitching of mouth, ear or facial muscles; grade 2: nodding with more severe twitching of facial muscles; grade 3: forelimb clonic but not with standing; grade 4: forelimb clonic accompanied by standing; grade 5: generalized tonic–clonic seizures with posture out of control.
Behavioral Observation and EEG Recording
Initially, the latency of seizures (the time from administration of PTZ to induction of seizures), the grade of seizures (grade 4 or above), and the duration of seizures (within 30 min after administration of PTZ)in each group of rats were recorded. Finally, the number of fully induced seizures after 15 drug injections in each group of rats was calculated.
Each group of rats were monitored by EEG and recordings were recorded. Light anesthesia was induced by 4% chloral hydrate (0.4 ml/100 g i.p.) and fixed on the brain stereotaxic instrument. The subcutaneous needle electrodes were used and the implantation point was centered on the top. The binaural electrode was used as the reference electrode, the filter was set to 40 Hz, the sensitivity was 70 μV/mm, the paper speed was maintained at 30 mm/s [18].
Organizational Materials and Processing
Within 4 h of the last drug administration, each group of rats were anesthetized by 10% chloral hydrate (0.4 ml/100 g i.p.). After the disappearance of the blink reflex, the each rat was decapitated, the brain tissue was dissected and placed in an ice tray along the sagittal suture. The brain tissue was then cut into two halfs. The right brain tissue was removed and quickly stored at − 80 °C in a refrigerator for subsequent protein extraction, while the left brain tissue was fixed in a neutral formalin buffer, and subsequently dehydrated, dipped in wax and sliced after embedding.
Cell Grouping and Drug Intervention
Astrocytes were randomly divided into four groups: control group, 3-MA group, ibuprofen group and 3-MA + ibuprofen group. The control group was administered with medium containing NS. While, each of the remaining groups were administered with medium containing 10 uM 3-MA [19], 100 uM ibuprofen [20], and 10 uM 3-MA + 100 uM ibuprofen, respectively. Astrocytes were cultured for 24 h and relevant tests were performed.
Cell Proliferation Assay
The proliferation of astrocytes in each group was detected by CCK-8 method. Each group of cells was digested and seeded in a 96-well cell culture plate at 5 × 103/well, and the corresponding medium was separately added. After 24 h of culture, 10 μL of CCK-8 reagent was added to each well of the cells, and incubation was continued for 2 h. The optical density of each well was measured at 450 nm. Finally, the proliferation of astrocytes in each group was analyzed.
Immunofluorescence
Rat brain tissue was serially sectioned, routinely dewaxed, rehydrated, and repaired. After the intervention, the astrocytes were placed in a laser confocal small dish and fixed with ice methanol for 15 min. After serum blocking, mouse anti-GFAP monoclonal antibody (1:75) and rabbit anti-LC3 monoclonal antibody (1:200) were added and incubated overnight at 4 °C. After the next day PBS rinse, the secondary antibody was added (the rat brain tissue section was supplemented with 647-labeled goat anti-mouse IgG 1:400 and 488-labeled goat anti-rabbit IgG 1:400, and 488-labeled goat anti-mouse IgG 1:400 and 647-labeled goat anti-rabbit IgG 1:400 was added to the laser confocal small dish). This was incubated for 1 h at 37 °C, then DAPI solution was added, and let to stand for 5 min at room temperature. The PBS was rinsed again and the film was observed under a laser confocal microscope. Fluorecent intensity of LC3 and GFAP was calculated.
Western Blotting
The grounded brain tissue and digested centrifuged astrocytes were fully lysed by adding RIPA containing 0.1 mmol/L PMSF cell lysate. After centrifugation, the supernatant was taken, and the BCA protein was quantified and loaded. 50 μg of each group was subjected to SDS-PAGE electrophoresis, transferred to a nitrocellulose membrane, and PBST containing 5% skimmed milk powder was added and then incubated for 1 h at room temperature. Mouse anti-GFAP monoclonal antibody (1:1000) and rabbit anti-LC3 monoclonal antibody (1:2000) were incubated for 1 h and overnight at 4 °C. Next day HRP-labeled goat anti-mouse lgG (1:8000) and goat anti-rabbit lgG (1:15000) were added and incubated for 2 h at 37 °C. The dark room was developed and imaged, and each strip was quantitatively analyzed to obtain a gray value.
Statistical Analysis
The qualitative data was compared by Fisher’s exact test. The quantitative data were expressed as mean ± standard deviation. One way analysis of variance (ANOVA) was used to compare differences between groups, and then LSD-t test was used to compare the specific two groups.Statistical analysis was performed using SPSS Version 24.0 software. P < 0.05 indicated that the difference is statistically significant.
Results
Effect of Ibuprofen on Rats with PTZ-Induced Epilepsy
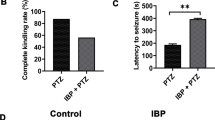
After 15 NS injections, the control rats had no epileptic seizures.The mean seizure grades of the PTZ group and the 3-MA + PTZ group were 4.17 ± 0.75 and 4.83 ± 0.16, respectively. However, the mean seizure grades of the ibuprofen + PTZ group and the 3-MA + ibuprofen + PTZ group were reduced to 2.67 ± 0.52 and 3.67 ± 0.82, respectively (P < 0.05) (Fig. 1a). In addition, the mean number of the fully induced seizures in the PTZ group and the 3-MA + PTZ group reached 83.33% and 91.67%, respectively. However, administration of ibuprofen + PTZ and 3-MA + ibuprofen + PTZ reduced the mean number of fully induced seizure to 33.33% and 50.00% (P < 0.05) (Table 1). The ibuprofen + PTZ had significantly increased latency and shortened duration of seizures (P < 0.05) (Fig. 1b, c).
Effect of Ibuprofen on EEG in Rats with PTZ-Induced Epilepsy
The EEG waveform of the control group was normal, and that of the PTZ group and the 3-MA + PTZ group revealed frequent spikes. The peaks of the EEG waves in the ibuprofen + PTZ group and the 3-MA + ibuprofen + PTZ group were significantly reduced, revealing small amplitude spikes and spine slow waves, and this was more obvious in ibuprofen + PTZ group (Fig. 2).
Effect of Ibuprofen on the Proliferation of Astrocytes
The cell viability in the control group was normal. After administration of 3-MA (3-MA group), the cell viability was significantly higher than that in the control group (P < 0.01). There was no significant difference in cell viability between the ibuprofen group and the control group (P > 0.05). However, after administration of 3-MA (3-MA + ibuprofen group), the cell viability was significantly higher than that in the ibuprofen group (P < 0.05), and after administration of ibuprofen (3-MA + ibuprofen group), the cell viability was significantly lower than that in the 3-MA group (P < 0.05). (Fig. 3).
Effect of Ibuprofen on the Expression of LC3 and GFAP in Rats with PTZ-Induced Epilepsy
Immunofluorescence and Western blot demonstrated that the expression of LC3 and GFAP in rats was lower in the control group. The expression of LC3 and GFAP in PTZ group was significantly higher than that in the control group (P < 0.01). After administration of 3-MA (3-MA + PTZ group), the expression of LC3 significantly decreased and that of GFAP significantly increased further when compared with the PTZ group (P < 0.05). Compared with the PTZ group, the level of LC3 in the ibuprofen + PTZ group was significantly higher and the GFAP level was significantly lower (P < 0.05). However, after administration of 3-MA (3-MA + ibuprofen + PTZ group), LC3 expression decreased significantly and GFAP expression increased significantly as compared to ibuprofen + PTZ group (P < 0.05) (Figs. 4, 5, 6).
Expression of GFAP and LC3 in the brain tissue of rats in each group. a Strip diagram of GFAP obtained by Western blot and the comparison of GFAP and β-actin gray values in each group of rats; b strip diagram of LC3 obtained by Western blot and the comparison of LC3-II and LC3-I gray values in each group of rats. ***P < 0.001, **P < 0.01, *P < 0.05
Effect of Ibuprofen on the Expression of LC3 and GFAP in the Astrocytes
The expression of astrocytes by immunofluorescence and Western blot test revealed that the expression of LC3 and GFAP in control group was normal. Compared with the control groups, the expression of LC3 significantly decreased and that of GFAP significantly increased further in the 3-MA groups (P < 0.01). Compared with the control group, the level of LC3 in the ibuprofen group was significantly higher and the GFAP level was significantly lower (P < 0.05). However, the expression of LC3 decreased significantly and that of GFAP increased significantly in the 3-MA + ibuprofen group compared with the ibuprofen group (P < 0.05). And the expression of LC3 increased significantly and that of GFAP decreased significantly in the 3-MA + ibuprofen group compared with the 3-MA group (P < 0.05) (Figs. 7 and 8).
Expression of GFAP and LC3 in each group of astrocytes. a Strip diagram of GFAP obtained by Western blot and the comparison of GFAP and β-actin gray values in each group of astrocytes. b Strip diagram of LC3 obtained by Western blot and the comparison of LC3-II and LC3-I gray values in each group of astrocytes. **P < 0.01, *P < 0.05
Discussion
Astrocytes are important glial cells of the central nervous system (CNS) [21]. The development of the nervous system, formation of synapse, neuroimmunity and regulation of ion concentration are inseparable from astrocytes [22]. Activation of astrocytes is a specific manifestation of its plasticity, also known as reactive gliosis [23]. It is often characterized by shortened cell division cycle, swelling of cell body, prolongation of protuberance, and enhanced expression of GFAP. Studies have shown that astrocyte proliferation is closely related to the onset of epilepsy. There is an obvious astrogliosis in epileptic lesions [24]. In the present study, astrocyte proliferation of PTZ group in vivo was obvious, and the results were consistent with the above studies. Various membrane channels, receptors and transporters on the membrane of proliferating astrocytes undergo significant changes, resulting in imbalance of K+ concentration inside and outside the cells, a decrease in the threshold of nerve excitation, and excessive excitation of the nerves leading to epilepsy [25]. Moreover, the activity of glutamine synthetase in proliferating astrocytes is reduced, leading to abnormal metabolism of glutamate and an increase in extracellular glutamate levels, further aggravating seizures [26]. Therefore, effective inhibition of astrocyte proliferation is a novel mechanism of preventing epilepsy.
Autophagy involves degradation of eukaryotic protein and includes macromolecular substances such as organelles and proteins in the cytoplasm that are encapsulated by bilayered vesicles forming autophagosomes. The lysosome forms an autophagolysin and is degraded by a hydrolase [7]. Autophagy is a bidirectional process: during starvation and hypoxia, it can provide energy to the cells; while, during disease processes such as infections and neurodegeneration, it can prevent the occurrence of related diseases by degrading the pathogens, damaged proteins or organelles present in the cells. However, excessive autophagy causes cell degeneration, leading to autophagic death [27].
Studies have demonstrated that autophagy occurs in astrocytes and plays a role in a variety of diseases. In neurodegenerative diseases, due to defects in autophagy of astrocytes, abnormal glycogen metabolism leads to accumulation of glycogen and induces neurodegeneration [28]. Duringacute cerebral ischemia, astrocyte autophagy can reduce the concentration of extracellular K+ and prevent neuronal damage, thereby improving the prognosis of patients [29]. Tripathi et al. used a co-cultured model of astrocytes and neurons and demonstrated that astrocyte autophagy affects the release of TGF-β1 and induces the protein aggregation in amyotrophic lateral sclerosis [30]. Furthermore, autophagy of astrocytes in epilepsy has also been reported. Kim et al. observed that P2RX7 plays an important role in epilepsy by regulating the expression of HSP-B1 and affecting astrocyte autophagy [31]. However, the specific mechanism of astrocyte autophagy involved in the pathogenesis of epilepsy needs further evaluation.
The relationship between astrocyte autophagy and its proliferation has been explored. Cocaine can induce astrocyte autophagy to promote its proliferation, causing an inflammatory response, and as a result resulting in neurodegenerative diseases to some extent [32]. However, on the contrary, Pereira et al. concluded that in Huntington’s disease, pretreatment with autophagy activator can reduce astrocyte proliferation and have a protective effect on nerves [10]. Thus, it can be stated that astrocyte autophagy affects its proliferation and plays a different role in different diseases. However, this relationship between autophagy and proliferation of astrocytes has not been reported in epilepsy. In the present study, autophagy and proliferation of astrocytes in the PTZ group were significantly higher than those in the control group in vivo. Epileptic seizures were observed in both the behavioral and EEG patterns in rats. Compared with the PTZ group, the autophagy of astrocytes in the 3-MA + PTZ group had decreased, the proliferation was further aggravated, and the epileptic seizures were more obvious. Similarly, in vitro, astrocytes were reduced in autophagy and increased in proliferation in the 3-MA group compared with the control group. Based on these findings, we speculate that seizures can moderately activate autophagy in astrocytes and reduce its proliferation through the physiological properties of autophagy, and the use of 3-MA to inhibit astrocyte autophagy leads to more obvious astrocyte proliferation and aggravates seizures. Therefore, affecting autophagy of astrocytes and reducing their proliferation is an effective way to prevent and treat epilepsy.
Ibuprofen is a classic non-steroidal anti-inflammatory drug (NASIDs) with antipyretic, analgesic and anti-inflammatory effects [33]. As its chemical structure contains carboxyl groups, the rate of passage through the blood–brain barrier is relatively faster than other NSAIDs [20]. Its primary mechanism of action is to inhibit the activity of cyclooxygenase, thereby reducing the biosynthesis of local tissue prostaglandins and thus exerting pharmacological effects. Various studies have reported that ibuprofen inhibits the proliferation and fibrosis. Lands et al. documented that high doses of ibuprofen can slow the progression of cystic fibrosis [34]. Further, Carlile et al. confirmed this finding in a mouse model [35]. In CNS disorders, ibuprofen inhibits epidural fibrosis, reduces adhesions and pain, and contributes to the prognosis of patients [36]. Sekiyama et al. reported that long-term application of ibuprofen can improve cognitive dysfunction in mice with Alzheimer’s disease, and significantly reduces the protein aggregation and astrocyte proliferation [12]. However, it has not been documented that ibuprofen can inhibit astrocyte proliferation and affect seizures in patients with epilepsy. In the present study, astrocyte proliferation was significantly reduced in the ibuprofen + PTZ group compared to the PTZ group and the behavioral and EEG findings revealed significant improvement in seizures in rats. Based on these findings, we speculate that ibuprofen can affect the seizures by inhibiting astrocyte proliferation.
Recent studies have reported that ibuprofen can also play a role in affecting autophagy. Moon et al. documented that ibuprofen promotes the sensitivity of tumor-resistant cells to HSP-90 inhibitors by promoting autophagy, and thus is used as an effective treatment of tumor resistance [11]. The mechanism of ibuprofen affecting autophagy has been reported, and ibuprofen’s anti-inflammatory effect can significantly reduce NLRP activity, thereby activating Beclin-1 and promoting autophagy [37, 38]. However, whether ibuprofen can exert anti-epileptic effects by affecting autophagy during the onset of epilepsy needs to be explored. In the present study, in vitro, compared with the 3-MA + ibuprofen group, the autophagy decreased and the astrocyte proliferation increased in the 3-MA group; in contrast, autophagy increased and astrocyte proliferation decreased in the ibuprofen group. Similarly, when 3-MA + ibuprofen + PTZ group was compared with ibuprofen + PTZ group, the astrocyte proliferation was observed following the 3-MA-induced inhibition of autophagy in vivo, and the behavior and EEG pattern of the rats revealed worsening of epileptic seizures. Based on these findings, we further speculate that ibuprofen can inhibit the onset of epilepsy by promoting astrocyte autophagy.
In summary, we conclude that ibuprofen reduces the proliferation of astrocytes, by promoting its autophagy, which affects the development of epilepsy. Thus, ibuprofen may be used as an adjuvant to improve the efficacy of other anti-epileptic agents. However, the specific mechanism by which ibuprofen affects autophagy and exerts its maximal efficacy remains to be further explored.
References
Xiao Y, Luo M, Wang J (2018) Losigamone add-on therapy for focal epilepsy. Cochrane Database Syst Rev 1:CD009324
Panebianco M, Prabhakar H (2018) Rufinamide add-on therapy for refractory epilepsy. Cochrane Database Syst Rev 4:CD011772
Robel S (2017) Astroglial scarring and seizures: a cell biological perspective on epilepsy. Neuroscientist 23(2):152–168
Thom M (2014) Review: hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol 40(5):520–543
Wilcox KS, Gee JM, Gibbons MB et al (2015) Altered structure and function of astrocytes following status epilepticus. Epilepsy Behav 49:17–19
Pekny M, Pekna M, Messing A et al (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131(3):323–345
Hurley JH (2017) Mechanisms of autophagy initiation. Annu Rev Biochem 86:225–244
Schille S, Crauwels P, Bohn R et al (2017) LC3-associated phagocytosis in microbial pathogenesis. Int J Med Microbiol 308(1):228–236
Kim JE, Hyun HW, Min SJ (2017) Sustained HSP25 expression induces clasmatodendrosis via ER stress in the rat hippocampus. Front Cell Neurosci 11:47
Pereira GJS, Tressoldi N, Hirata H et al (2013) Autophagy as a neuroprotective mechanism against 3-Nitropropionic acid-induced murine astrocyte cell death. Neurochem Res 38(11):2418–2426
Moon HJ, Kim HB, Lee SH et al (2018) Sensitization of multidrug-resistant cancer cells to Hsp90 inhibitors by NSAIDs-induced apoptotic and autophagic cell death. Oncotarget 9(13):11303–11321
Sekiyama K, Fujita M, Sekigawa A et al (2012) Ibuprofen ameliorates protein aggregation and astrocytic gliosis, but not cognitive dysfunction, in a transgenic mouse expressing dementia with Lewy bodies-linked P123H β-synuclein. Neurosci Lett 515(1):97–101
Gao B, Wu Y, Yang YJ et al (2018) Sinomenine exerts anticonvulsant profile and neuroprotective activity in pentylenetetrazole kindled rats: involvement of inhibition of NLRP1 inflammasome. J Neuroinflammation 15(1):152
Wang H, Huo X, Chen H et al (2018) Hydrogen-rich saline activated autophagy via HIF-1 pathways in neuropathic pain model. BioMed Res Int 17:4670834
Wallenstein MC (1991) Attenuation of epileptogenesis by nonsteroidal anti-inflammatory drugs in the rat. Neuropharmacology 30(6):657–663
Liu Y, Wang T, Liu X et al (2018) Overexpression of zinc-α2-glycoprotein suppressed seizures and seizure-related neuroflammation in pentylenetetrazol-kindled rats. J Neuroinflammation 15(1):92
Zhu X, Shen K, Bai Y et al (2016) NADPH oxidase activation is required for pentylenetetrazole kindling-induced hippocampal autophagy. Free Radic Biol Med 94:230–242
Altwegg-Boussac T, Schramm AE, Ballestero J et al (2017) Cortical neurons and networks are dormant but fully responsive during isoelectric brain state. Brain 140(9):2381–2398
Wang JL, Wang JJ, Cai ZN (2018) The effect of curcumin on the differentiation, apoptosis and cell cycle of neural stem cells is mediated through inhibiting autophagy by the modulation of Atg7 and p62. Int J Mol Med 42(5):2481–2488
Novakova I, Subileau EA, Toegel S et al (2014) Transport rankings of non-steroidal antiinflammatory drugs across blood-brain barrier in vitro models. PLoS One 9(1):e86806
Liddelow SA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967
Khakh BS, Beaumont V, Cachope R et al (2017) Unravelling and exploiting astrocyte dysfunction in huntington’s disease. Trends Neurosci 40(7):422–437
Burda JE (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81(2):229–248
Seifert G, Steinhäuser C (2013) Neuron–astrocyte signaling and epilepsy. Exp Neurol 244:4–10
Devinsky O, Vezzani A, Najjar S et al (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36(3):174–184
Papageorgiou IE, Valous NA, Lahrmann B et al (2018) Astrocytic glutamine synthetase is expressed in the neuronal somatic layers and down-regulated proportionally to neuronal loss in the human epileptic hippocampus. Glia 66(5):920–933
Servante J, Estranero J, Meijer L et al (2018) Chemical modulation of autophagy as an adjunct to chemotherapy in childhood and adolescent brain tumors. Oncotarget 9(81):35266–35277
Singh PK (2015) Changing shapes of glycogen-autophagy nexus in neurons: perspective from a rare epilepsy. Front Neurol 6:14
Milton M (2018) It’s all about timing: the involvement of Kir41 channel regulation in acute ischemic stroke pathology. Front Cell Neurosci 12:36
Tripathi P, Rodriguez-Muela N, Klim JR et al (2017) Reactive astrocytes promote ALS-like degeneration and intracellular protein aggregation in human motor neurons by disrupting autophagy through TGF-β1. Stem Cell Rep 9(2):667–680
Kim JE, Ko AR, Hyun HW et al (2018) P2RX7-MAPK1/2-SP1 axis inhibits MTOR independent HSPB1-mediated astroglial autophagy. Cell Death Dis 9(5):546
Periyasamy P, Guo ML (2016) Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy 12(8):1310–1329
Irvine J, Afrose A (2018) Formulation and delivery strategies of ibuprofen: challenges and opportunities. Drug Dev Ind Pharm 44(2):173–183
Lands LC (2016) Oral non-steroidal anti-inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database Syst Rev 4:CD001505
Carlile GW, Robert R, Goepp J et al (2015) Ibuprofen rescues mutant cystic fibrosis transmembrane conductance regulator trafficking. J Cyst Fibros 14(1):16–25
Liu S, Pan G, Liu G et al (2017) Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J Controll Release 264:1–13
Salminen A, Kaarniranta K, Kauppinen A et al (2013) Impaired autophagy and APP processing in Alzheimer’s disease: the potential role of Beclin 1 interactome. Prog Neurobiol 106:33–54
Cosin-Roger J, Simmen S, Melhem H et al (2017) Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun 8(1):98
Acknowledgements
This work was supported by grants from Shandong Medical and Health Technology Development Plan (to Zhongbo Hu, NO. 2017WS553), Binzhou Medical University Science and Technology Plan Project (to Chong Guo, NO. BY2015KJ13).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All protocols were approved by the Institutional Animal Ethical Committee of Affiliated Hospital of Binzhou Medical University (China), and experiments were performed in accordance to the CPCSEA guidelines for ethical use of animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, J., Wu, S., Guo, C. et al. Effect of Ibuprofen on Autophagy of Astrocytes During Pentylenetetrazol-Induced Epilepsy and its Significance: An Experimental Study. Neurochem Res 44, 2566–2576 (2019). https://doi.org/10.1007/s11064-019-02875-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02875-5