Abstract
β-Asarone (1,2,4-trimethoxy-5-[(Z)-prop-1-enyl]benzene) is an essential component of Acorus tatarinowii Schott volatile oil. Previous research has observed that β-asarone effectively attenuated symptoms in parkinsonian rats and improved their performance, but the mechanism of this effect remains unclear. Other research has shown that endoplasmic reticulum (ER) stress plays an important role in the pathogenesis of Parkinson’s disease (PD). The protein kinase RNA-like endoplasmic reticulum kinase (PERK) was observed in the nigrostriatal dopaminergic neurons of patients with PD. However, our group observed that ER stress and autophagy occurred in 6-hydroxy dopamine (6-OHDA)-induced parkinsonian rats, and ER stress might induce autophagy. We assume that the protective role of β-asarone in parkinsonian rats is mediated via the ER stress-autophagy pathway. To support this hypothesis, we investigated the expressions of glucose regulated protein 78 (GRP78), PERK phosphorylation (p-PERK), C/EBP homologous binding protein (CHOP), Bcl-2 and Beclin-1 in 6-OHDA-induced parkinsonian rats after β-asarone treatment. The results showed that the β-asarone group and PERK inhibitor group had lower levels of GRP78, p-PERK, CHOP and Beclin-1 while having higher levels of Bcl-2. We deduced that β-asarone might regulate the ER stress-autophagy via inhibition of the PERK/CHOP/Bcl-2/Beclin-1 pathway in 6-OHDA-induced parkinsonian rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases [1]. The clinical manifestations of PD include tremor, slowness of movement, rigidity and postural instability [2]. The pathological manifestations of PD are widely recognized as the decrease of nigrostriatal dopaminergic neurons and the presence of Lewy bodies in neurons [3]. Meanwhile, endoplasmic reticulum (ER) stress also has an important effect on the pathological process of PD [4]. Faulty folding of proteins causes ER dysfunction, which contribute to ER stress. ER stress triggers the unfolded protein response (UPR) to maintain the steady state of the ER [5]. Studies found that the protein kinase RNA-like endoplasmic reticulum kinase (PERK) and eukaryotic initiation factor 2α (eIf2α) in the nigrostriatal dopaminergic neurons of patients with PD, and the PERK phosphorylation (p-PERK) and α-synuclein (α-syn) increased synchronously [6]. Furthermore, the target proteins of the UPR signal pathway changed after neurons were stimulated with, for instance, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxy dopamine (6-OHDA), and rotenone, leading to upregulation of such proteins as the transcription index C/EBP homologous binding protein (CHOP), glucose regulated protein 78 (GRP78), cut X-box-binding protein (XBP1) and PERK [7, 8].
β-Asarone (1,2,4-trimethoxy-5-[(Z)-prop-1-enyl]benzene) is an essential component of Acorus tatarinowii Schott volatile oil, which can easily pass through the blood–brain barrier [9]. Our previous studies showed that β-asarone could improve the behavioral symptoms of parkinsonian rats [10], and β-asarone and levodopa co-administration could protect the parkinsonian rats from further symptoms [11]. Thus, β-asarone might have a good therapeutic effect on PD. Meanwhile, β-asarone could also inhibit ER stress in 6-OHDA-induced parkinsonian rats [12]. The purpose of this study was to clarify whether β-asarone may inhibit the PERK pathway in 6-OHDA-induced parkinsonian rats.
Previous studies showed that autophagy could clear the abnormal concentrations of α-syn in neurons, indicating that autophagy is involved in the pathological process of PD [13]. Recent studies suggested that ER stress could induce autophagy [14, 15]. Our previous study demonstrated that ER stress and autophagy occurred in 6-OHDA-induced parkinsonian rats, and indicated that ER stress might induce autophagy. Our group observed that β-asarone had a neural protective effect on 6-OHDA-induced parkinsonian rats by regulating the JNK/Bcl-2/Beclin-1 autophagy pathway [10]. Other groups have shown that β-asarone can inhibit neuronal apoptosis via the CaMKII/CREB/Bcl-2 pathway and regulate Bcl-2 family proteins [16, 17]. In addition, CHOP could decrease the expression of Bcl-2 [18]. In this study, we investigate whether β-asarone can regulate ER stress and autophagy via the PERK/CHOP/Bcl-2/Beclin-1 pathway in 6-OHDA-induced parkinsonian rats.
Materials and Methods
β-Asarone Preparation
β-Asarone was extracted from Acorus tatarinowii Schott according to the procedure that we previously established [19]. The purity of β-asarone reached 99.55%, as confirmed by GC-MS spectrometry, infrared spectrum and nuclear magnetic resonance detection.
Animal Experiments
Forty Sprague Dawley rats (20 males and 20 females) each weighing 220–250 g, were taken from Guangzhou University of Chinese Medicine. All procedures were carried out upon institutional ethical approval and in accordance with the Ethics Committee of Guangzhou University of Chinese Medicine (TCMF1-2015024). During the experiment, all rats were fed with Specific Pathogen Free (SPF) food by the laboratory technician, and unlimited food and water were available. The room was moderately ventilated, illuminated for 12 h, and kept at 25 °C and with humidity 55 ± 5%. The rats were fed for 3 days before the experiment. The experiment was performed at the SPF animal laboratory of the first affiliated hospital of Guangzhou University of Chinese Medicine, certificate number: SYXK (Guangdong) 2013-0092. The 40 rats were randomly and evenly divided into four groups: the sham-operated group was given normal saline, the model group (6-OHDA) was given by 6-OHDA lesion followed by normal saline through daily intragastric administration 30 days after modeling, the β-asarone group (6-OHDA + β-asarone) was given by 6-OHDA lesion followed by 15 mg/kg of β-asarone through intragastric administration per day 30 days after modeling, and the PERK inhibitor group (6-OHDA + ISRIB) was given by 6-OHDA lesion followed by 0.125 mg/kg of ISRIB through intraperitoneal injection per day 30 days after modeling. All groups were continuously administered for 30 days.
6-OHDA Lesion
Rats were anesthetized with intraperitoneal injection of 20% urethane (1.5 g/kg, i.p.) and were placed in the flat skull position on a cotton bed on a stereotaxic frame (Stoelting, USA) with the incisor bar fixed at 4.5 mm below the interaural line. Severe unilateral lesion of the nigrostriatal pathway was obtained by micro-injection (SGE, Australia) of 6 µl of 6-OHDA (4 µg/µl, free based dissolved in a solution of 0.2 mg/ml L-ascorbic acid in 0.9% normal saline, Shanghai Yuan Ye Biological Technology Co., Ltd., YY12246, China) at a rate of 0.2 µl/min into the medial forebrain bundle at the following coordinates according to the coordinates of Paxinos and Watson [20] (flat skull, as above): tooth bar: − 2.3 mm, anteroposterior: − 4.4 mm, medio-lateral: 1.2 mm, dorso-ventral: − 7.8 mm. The rats in the sham-operated group received the same volume of normal saline according to the same procedure except for the 6-OHDA lesions. The injection speed was 0.5 µL/min, and after that the needle was left for 15 min. The rats were injected intraperitoneally with 0.5 mg/kg apomorphine 30 days after modeling. The successful PD model rotated to the healthy side more than seven cycles/min.
Sample Collection
At 1 h after the last administration, rats were anesthetized with intraperitoneal injection of 20% urethane (1.5 g/kg). Rats were perfused with normal saline instead, and perfusion ended only when colorless liquid outflow was observed from the right atrial appendage. Subsequently, rats were sacrificed after perfusion. The mesencephalons and striatums of the injured hemisphere (left cerebral hemisphere) were harvested for flow cytometry, RT-PCR and western blot analysis.
Western Blot Evaluation of GRP78, p-PERK, CHOP, Bcl-2 and Beclin-1
Striatums were homogenized at 1:5 (wt:vol) in an ice-cold lysis buffer and were centrifuged at 14,027 g at 4 °C for 15 min to obtain the cellular proteins in the supernatant. The concentration of total protein extracted was determined by the bicinchoninic acid (BCA) Protein Assay (Beyotime, China). Proteins (30 µg) were separated by 10% SDS–polyacrylamide gel electrophoresis (Beyotime, China) and transferred to a PVDF membrane (0.22 m, Bio-Rad, USA). The membranes were blocked with 5% skim milk in TBS containing 0.1% Tween-20 for 1 h. Next, the membranes were incubated overnight at 4 °C with the following primary antibodies: rabbit Anti-GRP78 Bip (1:800, Abcam, ab21685, USA), rabbit Anti-PERK (phospho T980) (1:500, Abcam, ab156919, USA), rabbit Anti-DDIT3 (1:2000, Abcam, ab10444, USA), mouse Anti-Bcl-2 (1:200, Santa Cruz Biotechnology, sc-7382, USA), rabbit Anti-Beclin-1 (H-300) (1:200, Santa Cru Biotechnology, sc-11427, USA) and rabbit Anti-GAPDH (1:10000, Abcam, ab181602, USA). The blots were then incubated with horseradish peroxidase-conjugated secondary antibodies (rabbit, 1:2000, Beijing ComWin Biotech Co., Ltd., CW0103, China; mouse, 1:2000, Beijing ComWin Biotech Co., Ltd., China) at room temperature for 1 h. Bound secondary antibodies were visualized using an enhanced chemiluminescence kit (Bio-Rad, USA) and ChemDoc XRS with Quantity One software (Bio-Rad, Hercules, CA). Blots were repeated at least three times for every condition. After development, the band intensities were quantified with Image-pro Plus 5.0 analysis software.
RT-PCR Analysis of the mRNA Levels of GRP78, PERK and CHOP
Quantitative analysis of the mRNA levels of GRP78, PERK and CHOP in mesencephalons was performed by RT-PCR using 96-well optical reaction plates from CFX96™ RT-PCR (BioRad, USA). The PCR was performed under the following conditions: initial denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s, and annealing and extension at 60 °C for 30 s. All experiments were performed four times. The following primers were used in this study: GAPDH (forward, 5′-GGCTCTCTGCTCCTCCC-3′; reverse, 5′-CCGTTCACACCGACCTT-3′); GRP78 (forward, 5′-CACTTGGTATTGAAACTGTGGG-3′; reverse, 5′-TGTTACGGTGGGCTGATTAT-3′); PERK (forward, 5′- TCCTGTCTTGGTTGGGTCTG-3′; reverse, 5′-TGCGTGCTCCGCTTATTC-3′); CHOP (forward, 5′-GTCACAAGCACCTCCCAAAG-3′; reverse, 5′-TCCTGCTCCTTCTCCTTCAT-3′). The primers used in the current study were selected from the PubMed database and synthesized by Shanghai Jierui Biological Engineering Co., LTD. The RT-PCR data were analyzed using the relative gene expression (2−∆∆Ct) method.
Flow Cytometry Evaluation of Bcl-2 and Beclin-1
Expression of Bcl-2 and Beclin-1 were quantitatively detected by our previously published method [21, 22]. The cells of mesencephalons were counted and adjusted to a density of 106 cells/ml. The cells of each sample were divided into two parts, and subsequently they were used to evaluate the expressions of Bcl-2 and Beclin-1 respectively.
Permeabilization of the cells was achieved with a fixation and permeabilization kit (Invitrogen, USA) according to the manufacturer’s instructions. Cells were incubated in for 30 min in the dark at room temperature with a mouse Anti-Bcl-2 antibody (diluted 1:20, Santa Cruz Biotechnology, sc-7382, USA) and a rabbit Anti-Beclin-1 antibody (diluted 1:50, Santa Cruz Biotechnology, sc-11427, USA), respectively. After incubation, cells were washed twice in PBS, and then incubated for 20 min in the dark at room temperature with goat Anti-mouse IgG-FITC or goat Anti-rabbit IgG-FITC (diluted 1:25, Beijing ComWin Biotech Co., Ltd., China). After incubation, cells were washed twice in PBS. Labeled cells were fixed in 1% paraformaldehyde and prepared for flow cytometric analysis. The control cells were incubated with the secondary antibody alone. Flow cytometric analysis was performed using a flow cytometer ALTRA (Beckman Coulter FC-500, USA) equipped with an argon laser set at 488 nm. The cytometer was interfaced with the EXP032 data analysis system (Beckman Coulter, USA). Data were collected from 10,000 events. Non-specific binding was detected by the control cells.
Statistical Analysis
Data were expressed as the mean ± standard deviation (SD), and significant differences among different groups were determined by One-Way ANOVA followed by the Bonferroni post hoc test for multiple comparisons. P < 0.05 was considered to be significantly different. All statistical analyses were performed with SPSS 17.0 statistical software.
Results
Changes in GRP78, p-PERK and CHOP Protein Levels in the Striatum
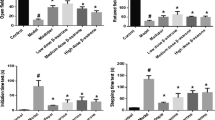
A significant increase in the expression of GRP78, p-PERK and CHOP proteins was found in the 6-OHDA model group compared with that in the sham-operated group (P < 0.05). In contrast, GRP78, p-PERK and CHOP levels significantly decreased in the β-asarone group and PERK inhibitor group compared with that in the 6-OHDA model group (P < 0.05) (Fig. 1).
Changes in GRP78, p-PERK and CHOP protein levels in the striatum. Effects of β-asarone on GRP78, p-PERK and CHOP levels were analyzed by western blot in striatums of 6-OHDA-induced rats. β-asarone group and PERK inhibitor group treatments followed 6-OHDA lesions. Values are expressed as the means ± SD of ten rats. *P < 0.05 indicates a significant difference compared to the sham-operated group; &P < 0.05 indicates a significant difference compared to the model group. There was no significant difference between the β-asarone group and PERK inhibitor group
Changes in GRP78, PERK and CHOP mRNA Levels in the Mesencephalons
A significant increase in GRP78, PERK and CHOP mRNA levels was found in the 6-OHDA model group compared with that in the sham-operated group (P < 0.05). In contrast, GRP78, PERK and CHOP mRNA levels significantly decreased in the β-asarone group and PERK inhibitor group compared with that in the 6-OHDA model group (P < 0.05) (Fig. 2).
Changes in GRP78, PERK and CHOP mRNA levels in the mesencephalons. Effects of β-asarone on GRP78, PERK and CHOP mRNA levels were analyzed by RT-PCR in mesencephalons of 6-OHDA-induced rats. β-asarone group and PERK inhibitor group treatments followed 6-OHDA lesions. Values are expressed as the means ± SD of ten rats. *P < 0.05 indicates a significant difference compared to the sham-operated group; &P < 0.05 indicates a significant difference compared to the model group. There was no significant difference between the β-asarone group and PERK inhibitor group
Changes in Bcl-2 and Beclin-1 Protein Levels in the Striatums
A significant increase in Beclin-1 expression but a decrease of Bcl-2 were found in the 6-OHDA model group compared with that in the sham-operated group (P < 0.05). In contrast, Beclin-1 expression showed a significant decrease in the β-asarone group and PERK inhibitor group compared with that in the 6-OHDA model group (P < 0.05). Whereas the β-asarone group and PERK inhibitor group rats showed a significant increase in Bcl-2 expression compared with that in the 6-OHDA model group (P < 0.05) (Fig. 3).
Changes in Bcl-2 and Beclin-1 protein levels in the striatums. Effects of β-asarone on Bcl-2 and Beclin-1 levels were analyzed by western blot in striatums of 6-OHDA-induced rats. β-asarone group and PERK inhibitor group treatments followed 6-OHDA lesions. Values are expressed as the means ± SD of ten rats. *P < 0.05 indicates a significant difference compared to the sham-operated group; &P < 0.05 indicates a significant difference compared to the model group. There was no significant difference between the β-asarone group and PERK inhibitor group
Changes in Bcl-2 and Beclin-1 Levels in the Mesencephalons
A significant increase in Beclin-1 expression but a decrease in Bcl-2 were observed in the 6-OHDA model group compared with that in the sham-operated group (P < 0.05). In contrast, Beclin-1 expression significantly decreased in the β-asarone group and PERK inhibitor group compared with that in the 6-OHDA model group (P < 0.05). Whereas rats in the β-asarone group and PERK inhibitor group showed a significant increase in Bcl-2 expression compared with that in the 6-OHDA model group (P < 0.05) (Table 1).
Discussion
PD is an age-related neurodegenerative disease [1], the incidence of which is increasing gradually. In the substantia nigra of patients with PD, the degeneration of dopaminergic neurons and Lewy bodies was found, and the Lewy bodies were created by the abnormal accumulation of α-syn in neurons [23]. In our previous studies, β-asarone improved the behavioral symptoms of parkinsonian rats, increased the PD-associated neurotransmitters, elevated the level of tyrosine hydroxylase (TH) and lowered the level of α-syn [10]. Thus, β-asarone has positive therapeutic effects on parkinsonian rats, and in this study, we investigated the mechanism by which β-asarone affects PD.
The ER is an important organelle in eukaryotic cells. When protein folding is disturbed, the UPR will start. GRP78 is an essential regulator of ER stress [24]. Three pathways are activated to resolve ER stress, including IRE1, PERK and ATF6. When a cell is not under stress, the three proteins are bound to the ER chaperone GRP78, which maintains them in the inactive state [25]. When unfolded or misfolded proteins accumulate, GRP78 preferentially binds to these abnormal proteins and releases its inhibitory hold on PERK, ATF6 and IRE1 [26]. The PERK pathway is a major branch of the UPR. Activated PERK phosphorylates eIF2α [27], eIF2α promotes activating transcription factor-4 (ATF4), and the elevated ATF4 enhances transcription of CHOP [28]. In this study, we observed that β-asarone might downregulate the expression of GRP78, p-PERK and CHOP. This result showed no significant difference with that in the PERK inhibitor group, which used ISRIB, a potent and commonly used selective PERK inhibitor [29]. This result indicates that β-asarone could inhibit PERK and then reduce the expression levels of GRP78 and CHOP. Thus, β-asarone might inhibit PERK in 6-OHDA-induced parkinsonian rats. Other studies observed that inhibition of ER stress was involved in the neuroprotective effects in parkinsonian rats [30]. Moreover, oral administration of a PERK inhibitor targeting the UPR prevented neurodegeneration and clinical disease in prion-infected mice [31].
Recent studies suggested that ER stress could induce autophagy [32, 33]. Autophagy was a likely downstream mediator of ER stress [34]. Studies showed that there was a correlation between PD and the PERK/eIF2α/ATF4 pathway [35] and that the eIf2α/ATF4 pathway could induce the expression of autophagy genes [36]. In addition, CHOP could decrease the expression of Bcl-2 [18]. Our results suggest that β-asarone increases the expression of Bcl-2, indicating that CHOP might decrease the expression of Bcl-2. Early stages of our study suggested that β-asarone had neural protective effects on parkinsonian rats via the JNK/Bcl-2/Beclin-1 autophagy pathway [10]. Beclin-1 is involved in autophagosome formation, and it is one of the essential components in the initial stage of autophagy. The expression level of Beclin-1 usually increases during autophagy. However, the Beclin-1 proautophagic function could be inhibited by interaction with Bcl-2 family proteins to form Beclin-1-Bcl-2/BclxL complexes [37, 38]. Besides, the interactions between Beclin-1 and Bcl-2 could be disrupted by Bcl-2 phosphorylation, which releases Beclin-1 to promote autophagy [39]. In this study, we show that β-asarone downregulates the expression of Beclin-1, indicating that β-asarone might inhibit autophagy by increasing Bcl-2 and that Bcl-2 might be the link between ER stress and autophagy. Therefore, we deduce that β-asarone may regulate ER stress and autophagy via the PERK/CHOP/Bcl-2/Beclin-1 pathway in 6-OHDA-induced parkinsonian rats.
In conclusion, we observed that β-asarone could improve the behavior of 6-OHDA-induced parkinsonian rats [10] and decrease the levels of GRP78, p-PERK, CHOP and Beclin-1, while increasing the level of Bcl-2. β-asarone might increase Bcl-2 by inhibiting the PERK pathway, and Bcl-2 may inhibit the expression of Beclin-1. Our data show that β-asarone regulates ER stress and autophagy via the PERK/CHOP/Bcl-2/Beclin-1 pathway, making β-asarone a potential candidate for achieving neuroprotection of organisms from the progression of PD.
Change history
18 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11064-022-03601-4
References
Oikonomou E, Paparrigopoulos T (2015) Neuropsychiatric manifestations in Parkinson’s disease. Psychiatriki 26:116–130
Rodriguez-Oroz MC, Jahanshahi M, Krack P, Litvan I, Macias R, Bezard E, Obeso JA (2009) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8:1128–1139
Koch JC, Bitow F, Haack J, d’Hedouville Z, Zhang JN, Tönges L, Michel U, Oliveira LM, Jovin TM, Liman J, Tatenhorst L, Bähr M, Lingor P (2015) Alpha-synuclein affects neurite morphology, autophagy, vesicle transport and axonal degeneration in CNS neurons. Cell Death Dis 6:e1811
Omura T, Kaneko M, Okuma Y, Matsubara K, Nomura Y (2013) Endoplasmic reticulum stress and Parkinson’s disease: the role of HRD1 in averting apoptosis in neurodegenerative disease. Oxid Med Cell Longev 2013:239854
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Hoozemans JJ, Van Haastert ES, Nijholt DA, Rozemuller AJ, Scheper W (2012) Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis 10:212–215
Yamamuro A, Yoshioka Y, Ogita K, Maeda S (2006) Involvement of endoplasmic reticulum stress on the cell death induced by 6-hydroxydopamine in human neuroblastoma SH-SY5Y cells. Neurochem Res 31:657–664
Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA (2002) Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci 22:10690–10698
Fang YQ, Shi C, Liu L, Fang RM (2012) Pharmacokinetics of beta-asarone in rabbit blood, hippocampus, cortex, brain stem, thalamus and cerebellum. Pharmazie 67:120–123
Zhang S, Gui XH, Huang LP, Deng MZ, Fang RM, Ke XH, He YP, Li L, Fang YQ (2016) Neuroprotective effects of β-asarone against 6-hydroxy dopamine-induced parkinsonism via JNK/Bcl-2/Beclin-1pathway. Mol Neurobiol 53:83–94
Huang LP, Deng MZ, He YP, Fang YQ (2015) β-asarone and levodopa coadministration protects against 6-OHDA-induced damage in parkinsonian rat mesencephalon by regulting autophagy: down-expression Beclin-1 and LC3B and up-expression p62. Clin Exp Pharmacol Physiol 42:269–277
Ning BL, Deng MZ, Zhang QX, Wang NB (2016) β-asarone inhibits IRE1/XBP1 endoplasmic reticulum stress pathway in 6-OHDA-induced parkinsonian rats. Neurochem Res 41:2097–2101
Ventruti A, Cuervo AM (2007) Autophagy and neurodegeneration. Curr Neurol Neurosci Rep 7:443–451
Jheng JR, Ho JY, Horng JT (2014) ER stress, autophagy, and RNA viruses. Front Microbiol 5:388
Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B (2012) ER stress inhibits neuronal death by promoting autophagy. Autophagy 8:915–926
Wei G, Chen YB, Chen DF, Lai XP, Liu DH, Deng RD, Zhou JH, Zhang SX, Li YW, Lii H, Liu LF, Wang Q (2013) Nie H.β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AβPP/PS1 mice. J Alzheimers Dis 33:863–880
Li C, Xing G, Dong M, Zhou L, Li J, Wang G, Zou D, Wang R, Liu J, Niu Y (2010) Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur J Pharmacol 635:96–102
Liu K, Shi Y, Guo X, Wang S, Ouyang Y, Hao M, Liu D, Qiao L, Li N, Zheng J, Chen D (2014) CHOP mediates ASPP2-induced autophagic apoptosis in hepatoma cells by releasing Beclin-1 from Bcl-2 and inducing nuclear translocation of Bcl-2. Cell Death Dis 5:e1323
Liu L, Fang YQ (2011) Analysis of the distribution of beta-asarone in rat hippocampus, brainstem, cortex and cerebellum with gas chromatography-mass spectrometry (GC-MS). J Med Plants Res 5:1728–1734
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, San Diego
He Y, Mo Z, Xue Z, Fang Y (2013) Establish a flow cytometric method for quantitative detection of Beclin-1 expression. Cytotechnology 65:481–489
Liu L, Fang YQ, Xue ZF, He YP, Fang RM, Li L (2012) Beta-asarone attenuates ischemia-reperfusion-induced autophagy in rat brains via modulating JNK, p-JNK, Bcl-2 and Beclin1. Eur J Pharmacol 680:34–40
Kalia LV, Kalia SK (2015) α-Synuclein and lewy pathology in Parkinson’s disease. Curr Opin Neurol 28:375–381
Hotamisligil GS (2010) Endoplasmic reticulum stress and atherosclerosis. Nat Med 16:396–399
Todd DJ, Lee AH, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8:663–674
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917
Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 18:7499–7509
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995
Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonenberg N, Walter P (2013) Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2:e00498
Wu L, Tian YY, Shi JP, Xie W, Shi JQ, Lu J, Zhang YD (2013) Inhibition of endoplasmic reticulum stress is involved in the neuroprotective effects of candesartan cilexitil in the rotenone rat model of parkinson’s disease. Neurosci Lett 548:50–55
Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, Ortori CA, Willis AE, Fischer PM, Barrett DA, Mallucci GR (2013) Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 5:206ra138
Yorimitsu T, Nair U, Yang Z, Klionsky DJ (2006) Endoplasmic reticulum stress triggers autophagy. J Biol Chem 281:30299–30304
Senft D, Ronai ZA (2015) UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40:141–148
Zhang J, Morris MW Jr, Dorsett-Martin WA, Drake LC, Anderson CD (2013) Autophagy is involved in endoplasmic reticulum stress-induced cell death of rat hepatocytes. J Surg Res 183:929–935
Jing X, Shi Q, Bi W, Zeng Z, Liang Y, Wu X, Xiao S, Liu J, Yang L, Tao E (2014) Rifampicin protects PC12 cells from rotenone-induced cytotoxicity by activating GRP78 via PERK-eIF2α-ATF4pathway. PLoS ONE 9:e92110
B’chir W, Maurin AC, Carraro V, Averous J, Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P, Bruhat A (2013) The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 41:7683–7699
Kondo Y, Kanzawa T, Sawaya R, Kondo S (2005) The role ofautophagy in cancer development and response to therapy. Nat Rev Cancer 5:726–734
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Wei Y, Pattingre S, Sinha S, Bassik M, Levine B (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30:678–688
Acknowledgements
This work was supported by the National Natural Science Funds for Distinguished Young Scholar (Grant No. 81804166), the China Postdoctoral Science Foundation (Grant No. 2018M643054), the Natural Science Foundation of Guangdong Province, China (Grant No. 2018A030310531), the Scientific Research Project of Administration of Traditional Chinese Medicine of Guangdong Province, China (Grant No. 20191129), Sanming Project of Medicine in Shenzhen (Grant No. SZSM201806077) and Shenzhen Bao’an Research Center for Acupuncture and Moxibustion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ning, B., Zhang, Q., Wang, N. et al. β-Asarone Regulates ER Stress and Autophagy Via Inhibition of the PERK/CHOP/Bcl-2/Beclin-1 Pathway in 6-OHDA-Induced Parkinsonian Rats. Neurochem Res 44, 1159–1166 (2019). https://doi.org/10.1007/s11064-019-02757-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02757-w