Abstract
Under pathological conditions, nitric oxide can become a mediator of oxidative cellular damage, generating an unbalance between oxidant and antioxidant systems. The participation of neuronal nitric oxide synthase (nNOS) in the neurodegeneration mechanism has been reported; the activation of N-methyl-d-aspartate (NMDA) receptors by agonist quinolinic acid (QUIN) triggers an increase in nNOS function and promotes oxidative stress. The aim of the present work was to elucidate the participation of nNOS in QUIN-induced oxidative stress in knock-out mice (nNOS−/−). To do so, we microinjected saline solution or QUIN in the striatum of wild-type (nNOS +/+), heterozygote (nNOS+/−), and knock-out (nNOS−/−) mice, and measured circling behavior, GABA content levels, oxidative stress, and NOS expression and activity. We found that the absence of nNOS provides a protection against striatal oxidative damage induced by QUIN, resulting in decreased circling behavior, oxidative stress, and a partial protection reflected in GABA depletion. We have shown that nNOS-derived NO is involved in neurological damage induced by oxidative stress in a QUIN-excitotoxic model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is involved in many physiological processes like synaptic plasticity, neuromodulation, and control of smooth muscle relaxation [1]. Under pathological conditions, NO has been reported as a probable promoter of oxidative stress and cell damage by inhibition of oxidative phosphorylation, triggering mitochondrial permeability transition [2, 3]. Transgenic Huntington’s disease (HD) models have reported the relationship between excitotoxic striatal damage and the increase in nitric oxide synthase (NOS) expression and activity [4, 5]. This suggests that neuronal damage is associated with excessive NO synthesis due to the increasing activity and expression of neuronal nitric oxide synthase (nNOS), triggering an excitotoxic cascade. Excitotoxicity has been demonstrated to be an important mechanism of damage in HD and several studies have pointed out that the overactivation of N-methyl-d-aspartate receptor (NMDAR) plays a key role in the selective neuronal loss found in patients suffering this disease [6]. Among the most common excitotoxicity animal models, the administration of quinolinic acid (QUIN), on NMDAR agonist, reproduces several biochemical and histopathological characteristics of HD.
Given that NO has been involved in oxidative stress induced by QUIN neurotoxicity in transgenic HD mice models [5], it is relevant to characterize the direct consequences of nNOS absence in oxidative damage induced by QUIN. This study aimed to analyze the important participation of nNOS in QUIN-induced oxidative stress in nNOS−/− knock-out (KO) mice.
Materials and Methods
Animal Handling
All animal procedures were approved by the Institutional Review Committee and were in accordance with the current Mexican legislation, NOM-062-ZOO-1999, and the Guide for the care and use of laboratory animals of the National Institutes of Health (NIH, Bethesda, MD). Mice were maintained under conditions previously described [7].
Mouse Genotypes
Male homozygous nNOS KO mice from B6 129-NOS1tmP1h line (nNOS−/−) [8] were purchased from Jackson Laboratory (Bar Harbor, ME) and were backcrossed with wild-type, nNOS+/+, and B6CBA female mice to start our colony. The genotype of each mouse was identified as previously described [9]. DNA was obtained from ear tissue of 8-week-old mice. Our colony of nNOS KO mice was housed under the environmental protocolized conditions of the vivarium of the National Institute of Neurology and Neurosurgery, MVS. For all behavioral tests and measurements, we used 13–19 animals with their corresponding genotype: wild, heterozygous, and homozygous. We noted that the number of wild mice in the colony was always higher, followed by heterozygotes and fewer homozygous mice.
The sequences of primers that recognized exon 2 of nNOS were the following: IMR 0406 (sense) TCAGATCTGATCCGAGGAGG and IMR 0407 (antisense) TTCCAGAGCGCTGTCATAGC, while IMR 0013 (sense) CTTGGGTGGAGAGGCTATTC and IMR 0014 (antisense) AGGTGAGATGACAGGAGATC recognized NEO gene. This analysis allowed us to identify KO mice (nNOS−/−) with the NEO gene introduced, the heterozygous mutation (nNOS+/−), and the wild-type mice (nNOS+/+).
Intrastriatal QUIN Microinjection
Male mice (10–12 weeks old) were anesthetized with sodium pentobarbital (70 mg/kg, i.p.) and later infused in the right striatum with a single unilateral injection of 1 µL over 2 min of sterile saline solution (SS; 0.9% NaCl) as a control or QUIN (30 nmol/µL) dissolved in SS. The stereotaxic coordinates were established according to the [10] mouse brain atlas: 0.6 mm anterior to bregma, − 2.2 mm lateral to bregma, and − 2.6 mm ventral to dura [11]. The experimental groups were sacrificed by decapitation 120 min or 4 days after QUIN injection, depending on the experimental purpose, and the corpora striata were dissected out on ice and stored at − 75 °C until analysis.
Circling Behavior Counting
Rotational behavior was evaluated according to a previous report [12]. Briefly, 3 days after the QUIN-induced striatal lesion was performed, animals from all groups were injected with apomorphine hydrochloride (1 mg/kg, s.c.) dissolved in SS containing 1% ascorbic acid to prevent oxidation. Mice were placed individually into acrylic cages, and the number of ipsilateral rotations to the lesioned striatum was recorded for 30 min. Results were expressed as ipsilateral turns/30 min.
Analysis of Striatal GABA Levels
Striatal content of gamma-aminobutyric acid (GABA) was determined 24 h after the circling behavior (CB) evaluation by high-performance liquid chromatography (HPLC) with fluorometric detection, using an elution gradient as previously described [13]. Each mouse was injected with 3-mercaptopropionic acid (0.25 mmol/kg, i.p.), a glutamate decarboxylase inhibitor, to prevent post-mortem GABA formation. The mice were sacrificed by cervical dislocation, and the striata were dissected out and kept at − 70 °C until analysis. The striatal tissue was homogenized in 1 mL methanol (85% HPLC-grade), using an ultrasonic processor (130 W, 40% amplitude, 10–15 s), and then centrifuged (18,500×g, 15 min, 4 °C). The supernatants obtained were derivatized with o-phthalaldehyde/mercaptoethanol prior to HPLC analysis with fluorescence detection (Series 1100, Agilent). Results were expressed as µmol/mg tissue.
Oxidative Stress Measurement
Lipid Peroxidation
The lipid-soluble fluorescent compounds in the striatum were measured according to [14], as previously reported by [7]. Briefly, 120 min after QUIN injection, mice were sacrificed, and the striata were obtained. The sectioned striata were homogenized in 2 mL SS. 1 mL of this homogenate was added to 4 mL chloroform–methanol (2:1 v/v). Samples were vortex-mixed and placed on ice for 30 min in the dark, and then the upper phase was removed. The lipid peroxidation (LP) of each sample was determined in a PerkinElmer LS50B luminescence spectrophotometer at excitation/emission wavelengths 350/430 nm respectively. The spectrophotometer sensitivity was adjusted to 140 fluorescence units using a calibrating solution (0.001 mg/mL quinine in 0.05 M H2SO4 solution). Protein content was determined according to Lowry assay [15]. Results were expressed as relative fluorescence/mg prot.
Reactive Oxygen Species
Reactive oxygen species (ROS) formation was evaluated in the same striatal homogenate obtained in the LP method, as previously described [7], by measuring the oxidation and deacetylation of 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA; Molecular Probes, USA), which is converted to the fluorescent compound dichlorofluorescein (DCF) by ROS activity. Briefly, striatal tissue samples were homogenized in 20 volumes of SS and a 1:10 dilution was made to a final volume of 50 µL with Tris:HEPES buffer (18:1). 5 µL of this dilution were incubated with 145 µL of Tris:HEPES buffer (18:1) and 50 µL of DCFH-DA (50 µM) at 37 °C for 60 min in a microplate. A standard calibration curve was constructed using increasing concentrations of DCF (100 nM) with Tris:HEPES buffer (9:1) incubated in parallel. Fluorescent signals at excitation/emission wavelengths 488/525 nm in a microplate reader were detected at the end of the incubation using FLx800 (Biotek, Winoosky, VT). Results were expressed as nmoles DCF/mg prot/min.
NOS Activity
NOS activity was measured 120 min after QUIN lesion, based on the stoichiometric conversion of l-arginine to NO and l-citrulline [16], as we have previously described in brain tissue [9]. Results were expressed as nanograms (ng) of l-citrulline/250 µg prot/min.
NOS Protein Expression
The striatal expression of the three NOS isoforms was evaluated 120 min after QUIN lesion by Western blot assays, as previously described [9]. The nNOS monoclonal antibody (sc-5302, Santa Cruz Biotechnology, Santa Cruz, CA) and the polyclonal antibodies for eNOS and iNOS (sc-654 and sc-7271, Santa Cruz Biotechnology, Santa Cruz, CA) were used at a final dilution of 1:500. After incubation with primary antibodies, membranes were incubated with either a secondary goat anti-mouse peroxidase-labeled antibody or a secondary goat anti-rabbit peroxidase-labeled antibody (Zymed, South San Francisco, CA) in a 1:6000 dilution for 1 h at room temperature. Protein blot was developed with the enhanced chemiluminescence (ECL) detection system (PerkinElmer, Norwalk, CT). Blots were stripped, and β-actin levels were determined using a monoclonal antibody, 1:5000 dilution [17]. Images from films were digitally acquired with a BioDoc-It System (UVP), and densitometry analysis was performed in Lab Works 4.0. Densitometric results were normalized by β-actin. Data were expressed as optical density (OD) normalized arbitrary units.
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). Mean differences between genotypes in CB were tested by Kruskal–Wallis test followed by Mann–Whitney U-test. Differences between genotypes in GABA levels, LP, and ROS and NOS expression were analyzed by two-way ANOVA. Differences between genotypes in NOS activity were analyzed by one-way ANOVA, followed by Tukey test. Significance was considered when p < 0.05. All the analyses were performed using Prism 6.0 software (GraphPad).
Results
Striatal Protection in nNOS KO Mice After QUIN-Induced Oxidative Damage
The systemic administration of apomorphine to mice striatally injected with QUIN caused characteristic ipsilateral turning toward the lesioned striatum [18]. The nNOS−/− showed a significant reduction in ipsilateral turning (80%) in comparison to nNOS+/+ and nNOS+/− mice. Interestingly, no differences were found between nNOS+/+ and heterozygous nNOS+/− mice (Fig. 1). These results suggest that neuronal protection against QUIN insult is due to the lack of both alleles of nNOS since heterozygous mice did not show a decrease in CB.
Evaluation of apomorphine-induced circling behavior in knock-out nNOS−/− mice. 3 days after the intrastriatal administration of QUIN (30 nmol/µL) wild-type nNOS+/+, heterozygous nNOS+/−, and knock-out nNOS−/− mice were challenged with apomorphine (1 mg/kg). Circling behavior was significantly lower in knock-out nNOS (−/−) compared with control. Data are mean ± SEM (n = 3–6). *p < 0.05
To corroborate the protection conferred by nNOS ablation, GABA levels were determined as a neurochemical marker of QUIN-induced neurotoxicity. The intrastriatal microinjection of QUIN decreased GABA levels, in comparison with SS control from wild-type nNOS+/+, heterozygous nNOS+/−, and nNOS−/− KO mice; however, in the KO mice lesioned with QUIN, the GABA reduction was slightly prevented (p < 0.05) in comparison with the other genotypes lesioned with the NMDAR agonist (Fig. 2). These results suggest that nNOS ablation partially protects against QUIN-induced GABA depletion.
Effect of the absence of nNOS on striatal GABA content after the unilateral administration of QUIN. GABA content was determined by HPLC in the right striatum of wild-type nNOS+/+, heterozygous nNOS+/−, and knock-out nNOS−/− mice, 4 days after intrastriatal administration of QUIN (30 nmol/µL) or saline solution (SS). Each bar represents the mean ± SEM (n = 3–6). *p < 0.05
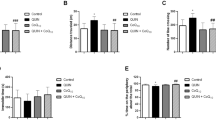
Since NO derived from nNOS after NMDAR overactivation promotes oxidative damage, we determined whether the absence of nNOS provides a protective effect against excitotoxic insult. To do so, we analyzed oxidative stress in the striatum of QUIN-administered mice. We observed that intrastriatal injection of QUIN induced a significant increase in both LP and ROS formation in striatum dissected from the lesioned side of both wild-type nNOS+/+ and heterozygous nNOS+/− animals in comparison with their control groups, whereas no differences were observed between lesioned and control striata obtained from nNOS−/− KO mice. Interestingly, nNOS−/− KO mice lesioned with QUIN showed a significant reduction in LP (about 60%) when compared to wild-type nNOS+/+ or heterozygous nNOS+/− mice (Fig. 3A). Similar results were obtained in ROS formation, in which nNOS−/− KO mice displayed a decreased production of ROS (4.513 ± 0.366) in comparison to wild-type nNOS+/+ (8.789 ± 0.707) and heterozygous nNOS+/− mice (8.093 ± 0.519) (Fig. 3B). Altogether, these results indicate that NO derived from nNOS promotes oxidative damage after QUIN insult.
Effect of the absence on nNOS on LP and ROS formation after a unilateral striatal QUIN lesion in wild-type nNOS+/+, heterozygous nNOS+/−, and knock-out nNOS−/− mice. Oxidative damage was measured 2 h after intrastriatal administration of saline solution (SS) or QUIN (30 nmol/µL). A Measurement of LP. B Measurement of ROS. Wild-type nNOS+/+, heterozygous nNOS+/−, and knock-out nNOS−/− mice showed a significant oxidative damage after QUIN administration compared to their respective controls; however, knock-out nNOS−/− mice did not show any differences between the QUIN-lesioned side and the SS-side. Data are mean ± SEM. (n = 3–6 per group). *p < 0.05
NOS Expression and Activity in nNOS KO Mice After Administration of QUIN
NO is produced by three NOS enzymes, however, NO is produced in the neurons mainly by nNOS. To evaluate the role of NO in the QUIN-induced neurotoxicity, we determined NOS enzyme activity in striata from the different genotypes of mice, 120 min after QUIN lesion. There was a significant decrease in total NOS activity in striata from nNOS−/− KO mice in comparison with wild-type nNOS+/+ and heterozygous nNOS+/− mice (Fig. 4A). Regarding the constitutive Ca2+-dependent NOS activity (cNOS), it is evident that nNOS−/− KO mice presented very low levels of this parameter in comparison with wild-type nNOS+/+ and heterozygous nNOS+/− mice (Fig. 4B). On the other hand, the activity of the Ca2+-independent NOS in the striatum 2 h after QUIN lesion did not participate in neuronal damage (Fig. 4C). Moreover, we evaluated the striatal expression of the three protein isoforms of NOS by Western blot after QUIN insult. We could not detect nNOS expression in the striatum of nNOS−/− KO mice whereas expression was readily observed in wild-type nNOS+/+ and heterozygous nNOS+/− mice. In nNOS+/+ and nNOS+/− mice, the nNOS isoform showed a non-significant mild increase with the QUIN insult in comparison to SS controls, indicating a partial participation of this isoform in the neuronal damage. However, endothelial NOS (eNOS) expression did not show any significant differences between genotypes and/or treatments (Fig. 5A, B). The expression of inducible NOS (iNOS) could not be detected in either genotype (data not shown). These results suggest that the lack of nNOS resulted in decreased total NOS and constitutive cNOS activity in response to QUIN insult while QUIN lesion promotes the increase of nNOS expression. These events could lead to early oxidative damage.
Discussion
In the present work, we demonstrated that the absence of NOS decreases circling behavior (Fig. 1), a result that is associated with the prevention of oxidative stress induced by striatal microinjection of QUIN (Fig. 3). However, it is evident that the results in circling behavior are not associated with a recovery in GABA neurotransmitter levels, which suggests that other pathways may be participating in neurological behavioral prevention. In this regard, our results from genetically modified animals (knock-out nNOS) are in agreement with other studies that emphasize that the strategy of inhibiting the activity of nNOS in a non-specific [4, 5] or specific [19] prevents circling behavior. The apparent discrepancy between the circling behavior and the GABA content could be explained by the BDNF–TrkB pathway, which is feedback between nNOS and BDNF. Cheng et al., describe the positive BDNF–nNOS feedback in some regions [20]. However, this regulation can be decoupled in cases of nNOS deficiency [21] or increase [22], producing surges of nNOs-independent BDNF in some cases. In turn, BDNF activates TrkB receptor, promoting its phosphorylation and modulating the triggering of signaling pathways, which are involved in motor behavior. Similarly, neuroprotective effects have been reported in mice and cortical neuron cultures of nNOS KO mice exposed to NMDA [23, 24]. In addition, the inhibition of nNOS also exerts protection against MPTP toxin in Parkinson’s disease model [25].
The absence of nNOS did not prevent the reduction of GABA levels (Fig. 2). However, the lack of nNOS attenuated the decrease of GABA content and protected against QUIN-induced oxidative damage (Fig. 3), suggesting that oxidative damage induced by nNOS-derived NO overproduction is an early event in neurodegeneration and contributes to enhance neuronal death. In addition to the damage induced by the NOS, activation of ionotropic receptors as the NMDAR leads to enhanced permeability to Na+ and Ca2+ through the associated ion channel. Additionally, increases in cytoplasmic Ca2+ concentrations can trigger a range of downstream neurotoxic cascades, including the uncoupling of mitochondrial electron transfer from ATP synthesis and the activation and overstimulation of enzymes such as calpains and other proteases, protein kinases, calcineurin, and endonucleases [3, 26]. Supporting this idea, Qin et al. [27] reported that caspase-3 activation contributes to apoptotic neuronal death 12 h after striatal QUIN injection.
In agreement with [8], we could not detect the presence of nNOS by Western blot assays in striata from nNOS−/− KO mice. However, the lesion with QUIN increased nNOS expression in heterozygous nNOS+/− and wild-type nNOS+/+ mice in comparison with the controls, indicating the participation of this enzyme in neuronal damage. Nevertheless, we detected eNOS expression in the three genotypes of mice as previously reported [9]. Furthermore, this enzyme expression did not show any significant differences between genotypes and/or treatments. Regarding iNOS expression, we could not detect any signal in the QUIN-lesioned or saline-injected striatum of all the genotypes of mice. This result is consistent with the activity of Ca2+-independent iNOS since it does not participate in neuronal damage. Besides, previous reports indicate that iNOS probably does not contribute to the progression of motor damage in a double transgenic mouse (R6/1; nNOS−/−) [28]. This excitotoxic effect could be mediated by NMDA receptors containing the NR2B subunit [29]. Huntingtin associated protein-1 (HAP-1) displays colocalization with nNOS, resulting in an aberrant interaction that could be responsible for increased NO synthesis and leading to neuronal damage [30]. Given that NO has been involved in oxidative stress induced by QUIN neurotoxicity and transgenic HD mice models [5], in the future, it will be relevant to characterize the direct consequences of the lack of nNOS in oxidative damage induced by QUIN.
Conclusion
In summary, nNOS-deficient mice showed a decrease in QUIN-induced neurotoxicity, which correlates with the decrease in the activity of the constitutive NOS isoforms (nNOS and eNOS). However, further nNOS activity inhibition experiments will be necessary to demonstrate the exact percentage of nNOS participation.
Abbreviations
- CB:
-
Circling behavior
- DCF:
-
Dichlorofluorescein
- DCFH-DA:
-
2’,7’-Dichlorodihydrofluorescein diacetate
- ECL:
-
Enhanced chemiluminescence
- HD:
-
Huntington’s disease
- HPLC:
-
High-performance liquid chromatography
- LP:
-
Lipid peroxidation
- NEO:
-
Neomycin
- NO:
-
Nitric oxide
- nNOS:
-
Neuronal nitric oxide synthase
- OD:
-
Optical density
- QUIN:
-
Quinolinic acid
- ROS:
-
Reactive oxygen species
- SEM:
-
Standard error of the mean
- SS:
-
Saline solution
References
Calabrese V, Mancuso C, Calvani M et al (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8:766–775. https://doi.org/10.1038/nrn2214
Vieira H, Kroemer G (2004) Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life 55:613–616. https://doi.org/10.1080/15216540310001639652
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47:122–129. https://doi.org/10.1016/J.CECA.2010.01.003
Pérez-Severiano F, Escalante B, Ríos C (1998) Nitric oxide synthase inhibition prevents acute quinolinate-induced striatal neurotoxicity. Neurochem Res 23:1297–1302
Perez-Severiano F, Escalante B, Vergara P et al (2002) Age-dependent changes in nitric oxide synthase activity and protein expression in striata of mice transgenic for the Huntington’s disease mutation. Brain Res 951:36–42. https://doi.org/10.1016/S0006-8993(02)03102-5
Canzoniero LMT, Granzotto A, Turetsky DM et al (2013) nNOS(+) striatal neurons, a subpopulation spared in Huntington’s disease, possess functional NMDA receptors but fail to generate mitochondrial ROS in response to an excitotoxic challenge. Front Physiol 4:112. https://doi.org/10.3389/fphys.2013.00112
Pérez-Severiano F, Montes S, Gerónimo-Olvera C, Segovia J (2013) Study of oxidative damage and antioxidant systems in two Huntington’s disease rodent models. Humana Press, Totowa, pp 177–200
Huang PL, Dawson TM, Bredt DS et al (1993) Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75(7):1273–1286
Martínez-Lazcano JC, Pérez-Severiano F, Escalante B et al (2007) Selective protection against oxidative damage in brain of mice with a targeted disruption of the neuronal nitric oxide synthase gene. J Neurosci Res 85:1391–1402. https://doi.org/10.1002/jnr.21261
Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Elsevier, Boston
Petersén A, Chase K, Puschban Z et al (2002) Maintenance of susceptibility to neurodegeneration following intrastriatal injections of quinolinic acid in a new transgenic mouse model of Huntington’s disease. Exp Neurol 175:297–300. https://doi.org/10.1006/exnr.2002.7885
García-Lara L, Pérez-Severiano F, González-Esquivel D et al (2015) Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J Neurosci Res 93:1423–1433. https://doi.org/10.1002/jnr.23595
Pérez-Neri I, Castro E, Montes S et al (2007) Arginine, citrulline and nitrate concentrations in the cerebrospinal fluid from patients with acute hydrocephalus. J Chromatogr B 851:250–256. https://doi.org/10.1016/j.jchromb.2006.10.047
Triggs WJ, Willmore LJ (1984) In vivo lipid peroxidation in rat brain following intracortical Fe2+ injection. J Neurochem 42:976–980. https://doi.org/10.1111/j.1471-4159.1984.tb12699.x
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87:682–685
García-Tovar CG, Luna J, Mena R et al (2002) Dystrophin isoform Dp7l is present in lamellipodia and focal complexes in human astrocytoma cells U-373 MG. Acta Histochem 104:245–254
Ungerstedt U (1971) Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand 82:49–68. https://doi.org/10.1111/j.1365-201X.1971.tb10999.x
Girouard H, Wang G, Gallo EF et al (2009) NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 29:2545–2552. https://doi.org/10.1523/JNEUROSCI.0133-09.2009
Cheng A, Wang S, Cai J et al (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258(2):319–333
Fritzen S, Schmitt A, Köth K et al (2007) Neuronal nitric oxide synthase (NOS-I) knockout increases the survival rate of neural cells in the hippocampus independently of BDNF. Mol Cell Neurosci 35(2):261–271. https://doi.org/10.1016/j.mcn.2007.02.021
Kolarow R, Kuhlmann CRW, Munsch T et al (2014) BDNF-induced nitric oxide signals in cultured rat hippocampal neurons: time course, mechanism of generation, and effect on neurotrophin secretion. Front Cell Neurosci 8:323. https://doi.org/10.3389/fncel.2014.00323
Dawson VL, Kizushi VM, Huang PL et al (1996) Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci 76:2479–2487
Ayata C, Ayata G, Hara H et al (1997) Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knock-out mice. J Neurosci 17:6908–6917
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neurosci 16:435–452. https://doi.org/10.1177/1073858410366481
Orrenius S, Zhivotovsky B, Nicotera P (2003) Calcium: regulation of cell death: the calcium–apoptosis link. Nat Rev Mol Cell Biol 4:552–565. https://doi.org/10.1038/nrm1150
Qin Z, Wang Y, Chasea TN (2000) A caspase-3-like protease is involved in NF-kappaB activation induced by stimulation of N-methyl-D-aspartate receptors in rat striatum. Mol Brain Res 80:111–122
Deckel AW, Tang V, Nuttal D et al (2002) Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington’s disease transgenic mice. Brain Res 939:76–86. https://doi.org/10.1016/S0006-8993(02)02550-7
Heng MY, Detloff PJ, Wang PL et al (2009) In vivo evidence for NMDA receptor-mediated excitotoxicity in a murine genetic model of Huntington disease. J Neurosci 29:3200–3205. https://doi.org/10.1523/JNEUROSCI.5599-08.2009
Li XJ, Sharp AH, Li SH, Dawson TM, Snyder SH, Ross CA (1996) Huntingtin-associated protein (HAP1): discrete neuronal localizations in the brain resemble those of neuronal nitric oxide synthase. Proc Natl Acad Sci USA 93(10):4839–4844
Funding
This work was supported by Conacyt Grant CB-2014 #241911 to F.P-S and was partially supported by Grant FOSISS-2015-2-261721 to L.T-L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerónimo-Olvera, C., Tristán-López, L., Martínez-Lazcano, J.C. et al. Striatal Protection in nNOS Knock-Out Mice After Quinolinic Acid-Induced Oxidative Damage. Neurochem Res 44, 421–427 (2019). https://doi.org/10.1007/s11064-018-2688-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2688-3