Abstract
Oxidative stress caused by mitochondrial dysfunction during reperfusion is a key pathogenic mechanism in cerebral ischemia–reperfusion (IR) injury. Propofol (2,6-diisopropylphenol) has been proven to attenuate mitochondrial dysfunction and reperfusion injury. The current study reveals that propofol decreases oxidative stress injury by preventing succinate accumulation in focal cerebral IR injury. We evaluated whether propofol could attenuate ischemic accumulation of succinate in transient middle cerebral artery occlusion in vivo. By isolating mitochondria from cortical tissue, we also examined the in vitro effects of propofol on succinate dehydrogenase (SDH) activity and various mitochondrial bioenergetic parameters related to oxidative stress injury, such as the production of reactive oxidative species, membrane potential, Ca2+-induced mitochondrial swelling, and morphology via electron microscopy. Propofol significantly decreased the ischemic accumulation of succinate by inhibiting SDH activity and inhibited the oxidation of succinate in mitochondria. Propofol can decrease membrane potential in normal mitochondria but not in ischemic mitochondria. Propofol prevents Ca2+-induced mitochondrial swelling and ultrastructural changes to mitochondria. The protective effect of propofol appears to act, at least in part, by limiting oxidative stress injury by preventing the ischemic accumulation of succinate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemia–reperfusion (IR) injury is the primary health issue that occurs after events such as stroke, cardiac arrest and resuscitation, and organ transplantation [1]. Although several strategies to protect against IR injury have been discovered in experimental studies, no effective protective therapy is currently used in clinical IR injury [2]. Oxidative stress, caused by mitochondrial dysfunction during reperfusion, is a key pathogenic mechanism in cerebral IR injury [3, 4]. In a recent study, Chouchani and colleagues presented a new mechanistic concept that could inspire novel therapeutic approaches: they revealed that succinate, a citric acid cycle intermediate molecule in mitochondria, is consistently elevated in ischemic tissues, and abnormally high levels of succinate are responsible for mitochondrial reactive oxygen species (ROS) production during reperfusion [5, 6]. It has also been reported that SDH inhibitor decreases succinate accumulation during ischemia and reduces tissue damage in both the brain and heart after IR injury [5,6,7]. Propofol, a widely used intravenous anesthetic agent, has been associated with reduced damage and apoptosis in cerebral IR injury [8,9,10,11,12,13]. In our recent study, we found that propofol may attenuates mitochondrial dysfunction by reducing the production of ROS in mitochondria [14]. In another study, propofol inhibited SDH activity in immature swine cerebral cortex [15]. Thus, we designed the present study to examine whether propofol prevents oxidative stress by limiting ischemic accumulation of succinate in focal cerebral IR injury.
Materials and Methods
Animals and Experimental Groups
This study was conducted in accordance with the Harbin Medical University Committee Guidelines issued for animal experiments. All efforts were made to minimize both discomfort and the number of animals used. This study was performed using male Wistar rats (8 weeks old; body weight 260–280 g; Harbin Medical University 2nd Affiliated Hospital Laboratories, Harbin, China). Rats were housed under controlled conditions (temperature of 24 ± 2 °C, humidity of 60–70% and under a 12 h light–dark cycle).
The experiment was divided into two parts. For the in vivo experiments, rats were divided into three groups: (1) a sham group, wherein rats were subjected to a sham operation with intravenous administration of vehicle (equal volume of saline); (2) an MCAO group, wherein rats underwent MCAO and received intravenous administration of vehicle; and (3) a propofol group, wherein rats underwent MCAO and received an intravenous administration of propofol (25 mg·kg−1·h−1). Propofol or vehicle administration began 10 min before MCAO and continued for 45 min after MCAO completion. For the in vitro experiments, mitochondria isolated from the 45 min MCAO or sham operation groups were randomly assigned to one of three groups: 200 μM propofol, 400 μM propofol, or vehicle treatment. Mitochondria were post-treated with propofol or vehicle in vitro for 15 min at 37 °C.
Focal Cerebral Ischemia Model
Focal cerebral ischemia was induced by a transient MCAO model. All animals were anesthetized via intraperitoneal injection of pentobarbital sodium (50 mg/kg in normal saline) and then allowed to spontaneously breathe oxygen-enriched air (fraction of inspired oxygen: 30%) through a facemask. Rectal temperature was also recorded, and the rat’s temperature was maintained at 37.0 ± 0.5 °C using a heat lamp and pad. During the procedure, regional cerebral blood flow was monitored by a laser Doppler perfusion monitor (OMEGAFLO FLO-N1; OMEGAWAVE, Inc., Tokyo, Japan). The left femoral artery was cannulated with a polyethylene tube to monitor blood pressure and to collect samples, and the left femoral vein was used for drug administration. MCAO was induced with a silicone-coated monofilament (40-3734 PK 10, Doccol Corporation, Pennsylvania Ave., Redlands, CA, USA). Briefly, the occlusion was produced by advancing the filament 16–18 mm into the internal carotid artery via the carotid artery until mild resistance was encountered for 45 min [16]. In the propofol-treated group, propofol (Diprivan, AstraZeneca, Cambridge, UK) was continuously i.v. administered during ischemia. Physiological variables (arterial blood pH, PaO2, and PaCO2) were recorded 3 min before the suture was inserted (baseline) and 45 min after MCAO (ischemia) by blood gas analyzer (Blood Gas Analyzer ABL 330, Leidu, Denmark).
Measurement of Mortality and Neurobehavioral Scores
Mortality data from all of the groups were calculated 24 h after reperfusion. After the mortality calculation, an 18-point system for neurobehavioral evaluation was used by a blinded observer. Briefly, the 18-point system was based on the following six tests: (1) response to vibrissae stimulation, (2) body proprioception, (3) forepaw outstretching, (4) climbing, (5) symmetry in movement of the four limbs, and (6) spontaneous activity. The scores were summed upon completion of the evaluation (minimum score, 3; maximum score, 18).
Isolation of Mitochondria
Nonsynaptic mitochondria from the rat forebrain of the infarct core were isolated according to the Percoll gradient method in a previous study [17]. The infarct core was defined according to methods described by Ashwal et al. [18]. After decapitation, the rat brain was quickly removed and placed in ice-cold isolation buffer (IB, 320 mM sucrose, 1 mM ethylene glycol tetraacetic acid (EGTA), 10 mM Tris base, pH 7.4 at 4 °C). The tissue was manually homogenized with a 2-mL Dounce homogenizer, and the homogenate was layered on a discontinuous Percoll gradient of 40 and 26%. After centrifugation (30,700×g, 10 min, 4 °C), a dense layer band (mitochondria-rich band) located in the interface between the 40 and 26% Percoll layer was generated. The mitochondria-rich band was collected and resuspended in IB and centrifuged (16,700×g, 12 min, 4 °C). The non-synaptosomal mitochondria were added to bovine serum albumin (BSA) 1 mg/mL and centrifuged (7300×g, 5 min, 4 °C). In addition, protein content was determined using a Bradford protein assay (Thermo Scientific).
Measurements of Mitochondrial Membrane Potential (MMP) and ROS
Freshly prepared mitochondrial pellets were assessed by flow cytometry, as previously described [14]. Briefly, to exclude debris, pellets were gated based on light scattering properties in forward scattering (FSC) and side scattering (SSC) modes, and 10,000 events within this gate (R1) were collected for each sample. To quantify ROS, 50 μg of mitochondria (isolated at 2, 48 and 72 h after I/R) per sample was suspended in 300 μL of analysis buffer (20 mM MOPS, 250 mM sucrose, 100 mM Pi(K), 10 mM Tris base, 0.5 mM Mg2+, 5 mM succinate, pH 7.0). Then, 2,7-dichlorodihydrofluorescein diacetate (H2DCF-DA) (10 μM, excitation at 488 nm, emission at 525 nm) was used to measure ROS. To measure the membrane potential, 50 μg of mitochondria (isolated from the sham and 45-min MCAO groups) per sample was suspended in 300 μL of analysis buffer (20 mM MOPS, 250 mM sucrose, 100 mM Pi(K), 10 mM Tris base, 0.5 mM Mg2+, 5 mM succinate, pH 7.0). Tetramethylrhodamine methyl ester (TMRM) was used to measure membrane potential using an excitation wavelength of 488 nm and an emission wavelength of 590 nm. All samples were incubated at room temperature for 30 min in H2DCF-DA and for 10 min in NAO and TMRM. For each mitochondrial sample, the mitochondrial probe acridine orange 10-nonyl bromide (NAO) was used to verify the purity of the analyzed sample using an excitation wavelength of 488 nm and an emission wavelength 525 nm. Propofol was added to mitochondrial suspensions from the propofol treatment group at final concentrations of 200 or 400 μM. The samples were incubated at 37 °C for 15 min before probe staining.
Measurement of SDH Activity
To measure SDH activity, 50 μg of mitochondria (isolated from the vehicle and 45-min MCAO groups) per sample was suspended in 100 μL of IB. Propofol or equal volume of saline was added to mitochondrial suspensions from the two groups at a final concentration of 200 μM. Samples were incubated at 37 °C for 15 min prior to measurement. SDH activity was defined by spectrophotometric measurement with a commercially available kit (No. A022, Nanjing Jiancheng Bio. Inst., China) following the manufacturer’s instructions, and all tests were performed in triplicate.
Measurement of Succinate
Succinate from the rat forebrain of the infarct core was measured by high-performance liquid chromatography (HPLC). After decapitation, the rat brain was quickly removed, and 10 mg wet weight of the infarct core tissue was lysed in 250 μL extraction solution (50% methanol, 30% acetonitrile and 20% water) in Precellys-24 vials. The homogenate was collected and centrifuged (16,000×g, 15 min, 0 °C), and 1000 μL supernatant was removed, dried under nitrogen, and then reconstituted in 500 μL of water. For HPLC separation, a Shim-pack VP-ODS (4.6 mm × 250 mm, 5 μm; Shimadzu, Kyoto, Japan) column was coupled to a Shimadzu HPLC system under the following conditions: column temperature of 40 °C, injection volume of 50 μL, flow rate of 500 μL/min, and mobile phases of formic acid (≥ 99.9%) in KH2PO4 (0.02%) (v/v, 7/93). All samples were determined under the HPLC conditions described above, and the external standard method was used to calculate the contents of succinate.
Ca2+-Induced Mitochondrial Swelling
To quantify the resistance against Ca2+, mitochondria isolated from the 45-min MCAO group were resuspended in assay buffer (20 mM MOPS, 10 mM Tris base, 250 mM sucrose, 100 mM Pi(K), 0.5 mM Mg2+, pH 7.0), and 100 μg of mitochondria per sample was diluted in 1 mL assay buffer at 25 °C. After adding 200 μM CaCl2, swelling of mitochondria was monitored with a spectrophotometer as the decrease in light scattering at 540 nm for 5 min. This method was similar to that used in our previous studies [14].
Electron Microscopy
The changes in propofol on the mitochondrial ultrastructural evoked by Ca2+-induced swelling were analyzed by transmission electron microscopy. Mitochondrial suspensions for each sample were recentrifuged (10,000×g, 5 min, 4 °C). The mitochondria were gently removed and fixed in 4% glutaraldehyde in 0.1 mol/L cacodylate buffer (pH 7.4), and then the mitochondria were post-fixed with 1% osmium tetroxide. All samples were dehydrated through an ethanol gradient, embedded in Epon 812, and sectioned with an 80-nm rotary microtome. The slices were stained with uranyl acetate and viewed under a Tecnai G2 (FEI, USA) electron microscope.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 18.0 (IBM Corp., Armonk, NY). The normally distributed data are presented as the mean ± standard deviations (SD), and Independent Student’s t tests or one-way analysis of variance for parametric data were performed. Mortality rates were compared using Chi square test. The results were considered statistically significant when P was less than 0.05.
Results
Physiological Parameters
The physiological parameters are shown in Table 1. There were no significant differences between the ischemic group and propofol group. Propofol treatment did not influence the baseline values of rectal temperature or mean arterial pressure. Transient ischemia had no effects on arterial pH, PaCO2, or PaO2.
Mortality and Neurobehavioral Scores
The overall mortality at 24 h was 2.6% (1/38), and no significant differences among the groups were observed. According to an 18-point scoring system, rats from the MCAO group presented neurological deficits, and rats from the propofol group showed higher neurological scores than those of the MCAO group (Table 2).
Effect of Propofol on ROS Production
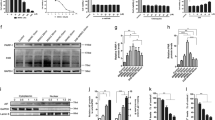
Before analysis of ROS and MMP by flow cytometry, mitochondrial purity was confirmed by NAO. Mitochondria were collected from the background based on light scattering properties (SSC and FSC). Gating was verified by selective NAO-stained mitochondria. Almost all the events within R1 were NAO-positive compared with control samples without staining (M1), confirming the mitochondrial fraction (Fig. 1a, b).
Propofol reduced ROS generation in mitochondria. a A total of 10,000 events representing mitochondria were collected within the R1 gate based on light scattering properties. b Samples were stained with 100 nM NAO, a fluorophore that can selectively bind to mitochondrial cardiolipin, to assess their purity. The purple area (background fluorescence) is an unstained control sample, and the green line represents a NAO-stained sample. M2 is a NAO-stained positive area, and almost all of the particles were positive for NAO. c Relative changes in DCF fluorescence intensity with propofol treatment. Each value is expressed as the mean (SD), and P values were obtained by one-way analysis of variance. ※P < 0.05 versus the IR 2 h, IR 24 h, and IR 72 h MCAO groups; #P < 0.05 versus the IR 2 h and IR 72 h MCAO groups; ▽P < 0.05 versus the IR 2 h and IR 24 h MCAO groups (n = 6 for each group). P propofol
In this study, we investigated the effects of propofol on the production of ROS as indicated by the DCF fluorescence intensity generated from the mitochondria. DCF fluorescence intensity markedly reduced at 2, 24, and 72 h with propofol (200, 400 μM) pretreatment (P < 0.05). The overall DCF fluorescence intensity was highest at 2 h and decreased thereafter. DCF fluorescence intensity was similar between propofol 200 μM and propofol 400 μM, corresponding to 60 versus 56 at 2 h, 46 versus 42 at 24 h, and 38 versus 39 at 72 h, respectively (Fig. 1c).
Effect of Propofol on Succinate Production
Figure 2a shows representative succinate spectra obtained from the standard preparation of succinate dissolved in H2O, wherein the succinate peak occurred at approximately 8.5 min. The ischemic cortex showed a significant increase in succinate compared with corresponding regions of the cortex taken from the contralateral side during MCAO. Importantly, brains treated with propofol under ischemic conditions but not normoxic conditions had significantly decreased succinate concentrations (P < 0.05) (Fig. 2b–d). Succinate levels quickly recovered during reperfusion from the high level observed at the end of ischemia, and there were no significant differences in succinate levels between the groups (Fig. 2d). In addition, propofol pretreatment significantly decreased the SDH activity of normal and ischemic mitochondria (Fig. 3).
Propofol prevented succinate accumulation during ischemia. a Representative succinate spectra obtained from a standard by HPLC; the area around the succinate peak occurred at approximately 8.5 min. Succinate levels in extracts from brains with (b) or without (c) propofol treatment. d Normal brain tissue was taken from corresponding regions on the contralateral side. Brains treated with propofol under ischemic conditions but not normoxic conditions had significantly decreased succinate concentrations. Each value is expressed as the mean (SD), and P values were obtained by one-way analysis of variance. ※P < 0.05 versus the MCAO group at 45 min of ischemia (n = 6 in each group)
Effects of propofol on mitochondrial SDH activity. Propofol significantly decreased SDH activity of mitochondria in normal and ischemic condition. Each value is expressed as the mean (SD), and P values were obtained by Independent Student’s t tests. ※P < 0.05 versus the MCAO group at 45 min of ischemia (n = 6 in each group)
Propofol Prevented Mitochondrial Swelling Induced by Ca2+
Compared with the sham group, mitochondria from MCAO cortexes treated with 200 μM CaCl2 demonstrated severe swelling with decreasing matrix density and poorly defined cristae. Mitochondria showed fragmented internal membranes and dilated intracristal spaces (Fig. 4a, b). After administration of 200 μM propofol, the mitochondria showed only minimal swelling, and the basic integrity of the mitochondrial membranes was maintained. Cristae and uniform intracristal spaces were nearly intact in most mitochondria. The morphological changes observed upon treatment with 1 μM cyclosporin A, an inhibitor of MPTP, were comparable with those resulting from propofol treatment (Fig. 4c, d). The mitochondrial sample treated with 200 μM CaCl2 showed slowly decreasing transmission values. Compared with the MCAO group, the group treated with 200 μM propofol or 1 μM cyclosporin A exhibited slightly reduced transmission (P < 0.001) (Fig. 4e).
Propofol prevented mitochondrial swelling induced by Ca2+. The ultrastructure of the mitochondria was observed by electron microscopy. a Sham group mitochondria displayed a regularly shaped appearance and intramitochondrial matrix. b Forty-five minutes of cerebral ischemia induced dramatic ultrastructural alterations and mitochondrial swelling, including fragmentation of the internal membranes and disruption of the cristae. c, d Treatment with 100 μM propofol or 1 μM cyclosporin A markedly prevented the mitochondrial ultrastructural alterations caused by focal cerebral ischemia–reperfusion injury (n = 4 in each group). e Treatment with 200 or 1 μM cyclosporin A prevented a Ca2+-induced decrease in light transmission (n = 6 in each group). Each value is expressed as the mean (SD), and P values were obtained by one-way analysis of variance. ★P < 0.05, versus the MCAO group. P propofol, Csa cyclosporin A
Effect of Propofol on MMP
Mitochondria were confirmed by NAO within the R1 gate, and the MMP was determined according to the TMRM fluorescence intensity. Fluorescence intensity of TMRM was high in the sham group, which demonstrated a high MMP. In the MCAO group, 45 min of ischemia significantly reduced the MMP. Importantly, propofol pretreatment significantly decreased the membrane potential in normal mitochondria but not in ischemic mitochondria (Fig. 5).
Effects of propofol on the mitochondrial membrane potential. Propofol pretreatment significantly decreased the membrane potential in normal mitochondria but not in ischemic mitochondria. Each value is expressed as the mean (SD), and P values were obtained by one-way analysis of variance (n = 6 in each group). ※P < 0.05 versus the sham control group. #P < 0.01 versus the sham propofol group
Discussion
The neuroprotective effects of propofol are well known to protect against pathological states characterized by an increase in the basal rate of ROS production [19,20,21,22]. However, to our knowledge, little is known regarding the neuroprotective mechanisms that fight ROS generation during cerebral IR injury. Propofol is chemically similar to phenol-based free-radical scavengers such as alpha tocopherol, and it was previously considered to be an antioxidant that protects against pathological states characterized by an increase of ROS production in IR [9]. It should be noted that alpha tocopherol, despite reducing ROS release from polymorphonuclear cells, cannot prevent ROS generation [23]. In the present study, we demonstrated that propofol can markedly decrease succinate accumulation during ischemia and reduce the extent of ROS generation in a MCAO model.
Ischemic succinate accumulation develops from the reversal of SDH, which in turn is driven by fumarate overflow from purine nucleotide breakdown and partial reversal of the malate/aspartate shuttle [5]. The succinate accumulated during ischemia is rapidly re-oxidized by SDH within 5 min of reperfusion in vivo in the heart [5], and reversed transport through mitochondrial complex I drives reperfusion-related ROS formation [24, 25]. In our study, we observed that accumulated succinate is restored to normoxic levels 10 min after reperfusion in vivo in the brain, and propofol pretreatment significantly decreased mitochondrial SDH activity under ischemic conditions. To prevent ROS generation caused by succinate accumulation, the propofol delivery time is important, and it might have no effect on post-conditioning after more than 10 min. In addition, propofol intravenously infused into the lateral cerebral ventricle had little effect on inhibiting ischemic succinate accumulation in our preliminary experiments.
Mitochondria, as major sources of ROS production, can also be major targets of ROS attack. Early studies have suggested that the initial burst of ROS upon reperfusion may cause mitochondrial lipid peroxidation [26] and oxidative changes in protein structure/function, which lead to mitochondrial dysfunction of mitochondrial respiratory chain complexes [27]. This dysfunction might result in further ROS generation, and develop into a vicious cycle of ROS–cardiolipin-complexes of the respiratory chain [9, 28]. In our study, propofol pretreatment appears to, at least in part, inhibiting ischemic succinate accumulation, thus interrupting the cycle. In addition, the ROS production was at a peak level during the initial period of reperfusion and gradually decreased through 72 h of reperfusion. Pretreatment of propofol significantly decreased ROS generation at three selected reperfusion time points. As no difference in ROS generation was found between the 200 mM propofol group and the 400 mM propofol group, the lower concentration of clinically effective propofol may be preferred for testing oxidative stress damage.
Strong evidence has shown that mitochondrial permeability transition pore (mPTP) opening is enhanced by oxidative stress [29,30,31,32], and some studies even suggest that mPTP opening can be induced by oxidant generation itself [33, 34]. Mice with deletion of the gene encoding cyclophilin D, a regulator of the mPTP, are more resistant to exogenous oxidative stress and are protected from IR injury [35,36,37,38]. It has been suggested that the mPTP can mediate cell death induced by oxidative challenge. In our study, propofol prevented Ca2+-induced mPTP opening and mitochondrial swelling by inhibiting ROS generation, and this hypothesis was also supported by morphological swelling changes revealed via electron microscopy.
In addition, ROS in combination with elevated calcium concentrations not only lead to opening of mPTPs but also the consequent loss of MMP during IR [39, 40]. The subsequent release of cytochrome c from mitochondria leads to the execution of cell death processes [41]. Although propofol inhibited SDH activity in immature swine cerebral cortex [15], pretreatment with propofol dose-dependently attenuated the decline in both complex I and complex III activity during cardiac ischemia and reperfusion injury [9]. To measure the membrane potential in our study, mitochondria were suspended in analysis buffer containing 5 mM succinate. Succinate enters the mitochondrial matrix via an electroneutral exchange for inorganic phosphate [42]. Within the mitochondrion, succinate is rapidly oxidized by succinate dehydrogenase to fumarate and, finally, the membrane potential evaluated. There is no doubt that if propofol inhibited SDH activity, the MMP would decrease. In fact, propofol pretreatment significantly decreased membrane potential in normal mitochondria but not in ischemic mitochondria in our study. Explanations regarding why the membrane potential in ischemic mitochondria did not decrease could be as follows: First, propofol can reduce ROS generation and restore complex I and complex III activity, which are the proton exchangers that generate MMP by transferring electrons to molecular oxygen or to the subsequent complex, with a concomitant transfer of protons out of the mitochondrial matrix [43]. Second, propofol can exert its action at the mitochondrial level through interrupting mPTP opening and Ca2+-induced mitochondrial swelling during IR [10, 14].
In summary, the neuroprotective effect of propofol appears to, at least in part, interrupt the vicious cycle of ROS—mitochondrial dysfunction—ROS by reducing the production of ROS. This protective effect may be mediated by inhibiting ischemic accumulation of succinate. These findings may explain the neuroprotective mechanism of propofol and will help in the exploration of available antioxidants.
References
Hausenloy DJ, Yellon DM (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123(1):92–100. https://doi.org/10.1172/JCI62874
Hausenloy DJ, Yellon DM (2016) Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 13(4):193–209. https://doi.org/10.1038/nrcardio.2016.5
Suliman HB, Piantadosi CA (2016) Mitochondrial quality control as a therapeutic target. Pharmacol Rev 68(1):20–48. https://doi.org/10.1124/pr.115.011502
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med 17(11):1391–1401. https://doi.org/10.1038/nm.2507
Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515(7527):431–435. https://doi.org/10.1038/nature13909
Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP (2016) A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab 23(2):254–263. https://doi.org/10.1016/j.cmet.2015.12.009
Dhingra R, Kirshenbaum LA (2015) Succinate dehydrogenase/complex II activity obligatorily links mitochondrial reserve respiratory capacity to cell survival in cardiac myocytes. Cell Death Dis 6:e1956. https://doi.org/10.1038/cddis.2015.310
Iijima T, Mishima T, Akagawa K, Iwao Y (2006) Neuroprotective effect of propofol on necrosis and apoptosis following oxygen-glucose deprivation–relationship between mitochondrial membrane potential and mode of death. Brain Res 1099(1):25–32. https://doi.org/10.1016/j.brainres.2006.04.117
Shao H, Li J, Zhou Y, Ge Z, Fan J, Shao Z, Zeng Y (2008) Dose-dependent protective effect of propofol against mitochondrial dysfunction in ischaemic/reperfused rat heart: role of cardiolipin. Br J Pharmacol 153(8):1641–1649. https://doi.org/10.1038/bjp.2008.45
Adembri C, Venturi L, Tani A, Chiarugi A, Gramigni E, Cozzi A, Pancani T, De Gaudio RA, Pellegrini-Giampietro DE (2006) Neuroprotective effects of propofol in models of cerebral ischemia: inhibition of mitochondrial swelling as a possible mechanism. Anesthesiology 104(1):80–89
Li J, Han B, Ma X, Qi S (2010) The effects of propofol on hippocampal caspase-3 and Bcl-2 expression following forebrain ischemia-reperfusion in rats. Brain Res 1356:11–23. https://doi.org/10.1016/j.brainres.2010.08.012
Wang HY, Wang GL, Yu YH, Wang Y (2009) The role of phosphoinositide-3-kinase/Akt pathway in propofol-induced postconditioning against focal cerebral ischemia-reperfusion injury in rats. Brain Res 1297:177–184. https://doi.org/10.1016/j.brainres.2009.08.054
Yano T, Nakayama R, Ushijima K (2000) Intracerebroventricular propofol is neuroprotective against transient global ischemia in rats: extracellular glutamate level is not a major determinant. Brain Res 883(1):69–76
Li J, Yu W, Li XT, Qi SH, Li B (2014) The effects of propofol on mitochondrial dysfunction following focal cerebral ischemia-reperfusion in rats. Neuropharmacology 77:358–368. https://doi.org/10.1016/j.neuropharm.2013.08.029
Kajimoto M, Atkinson DB, Ledee DR, Kayser EB, Morgan PG, Sedensky MM, Isern NG, Des Rosiers C, Portman MA (2014) Propofol compared with isoflurane inhibits mitochondrial metabolism in immature swine cerebral cortex. J Cereb Blood Flow Metab 34(3):514–521. https://doi.org/10.1038/jcbfm.2013.229
Kotani Y, Nakajima Y, Hasegawa T, Satoh M, Nagase H, Shimazawa M, Yoshimura S, Iwama T, Hara H (2008) Propofol exerts greater neuroprotection with disodium edetate than without it. J Cereb Blood Flow Metab 28(2):354–366. https://doi.org/10.1038/sj.jcbfm.9600532
Sims NR, Anderson MF (2008) Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc 3(7):1228–1239. https://doi.org/10.1038/nprot.2008.105
Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ (1998) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 29(5):1037–1046 (discussion 1047)
Lee H, Jang YH, Lee SR (2005) Protective effect of propofol against kainic acid-induced lipid peroxidation in mouse brain homogenates: comparison with trolox and melatonin. J Neurosurg Anesthesiol 17(3):144–148
Yumoto M, Nishida O, Nakamura F, Katsuya H (2005) Propofol attenuates oxidant-induced acute lung injury in an isolated perfused rabbit-lung model. J Anesth 19(4):287–294. https://doi.org/10.1007/s00540-005-0338-9
Tsao CM, Ho ST, Chen A, Wang JJ, Tsai SK, Wu CC (2003) Propofol ameliorates liver dysfunction and inhibits aortic superoxide level in conscious rats with endotoxic shock. Eur J Pharmacol 477(2):183–193
Unsal A, Devrim E, Guven C, Eroglu M, Durak I, Bozoklu A, Balbay MD (2004) Propofol attenuates reperfusion injury after testicular torsion and detorsion. World J Urol 22(6):461–465. https://doi.org/10.1007/s00345-004-0451-7
Gonzalez-Perez O, Moy-Lopez NA, Guzman-Muniz J (2008) Alpha-tocopherol and alpha-lipoic acid. An antioxidant synergy with potential for preventive medicine. Rev Invest Clin 60(1):58–67
Wijermars LG, Schaapherder AF, Kostidis S, Wust RC, Lindeman JH (2016) Succinate accumulation and ischemia-reperfusion injury: of mice but not men, a study in renal ischemia-reperfusion. Am J Transplant 16(9):2741–2746. https://doi.org/10.1111/ajt.13793
O’Neill LA (2014) Biochemistry: succinate strikes. Nature 515(7527):350–351. https://doi.org/10.1038/nature13941
Muralikrishna Adibhatla R, Hatcher JF (2006) Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med 40(3):376–387. https://doi.org/10.1016/j.freeradbiomed.2005.08.044
Li SY, Jia YH, Sun WG, Tang Y, An GS, Ni JH, Jia HT (2010) Stabilization of mitochondrial function by tetramethylpyrazine protects against kainate-induced oxidative lesions in the rat hippocampus. Free Radic Biol Med 48(4):597–608. https://doi.org/10.1016/j.freeradbiomed.2009.12.004
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3):909–950. https://doi.org/10.1152/physrev.00026.2013
Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P (1994) The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem 269(24):16638–16642
Kim JS, Jin Y, Lemasters JJ (2006) Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 290(5):H2024–H2034. https://doi.org/10.1152/ajpheart.00683.2005
Assaly R, de Tassigny A, Paradis S, Jacquin S, Berdeaux A, Morin D (2012) Oxidative stress, mitochondrial permeability transition pore opening and cell death during hypoxia-reoxygenation in adult cardiomyocytes. Eur J Pharmacol 675(1–3):6–14. https://doi.org/10.1016/j.ejphar.2011.11.036
Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP (2008) Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res 102(9):1082–1090. https://doi.org/10.1161/CIRCRESAHA.107.167072
Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192(7):1001–1014
Schriewer JM, Peek CB, Bass J, Schumacker PT (2013) ROS-mediated PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia-reperfusion. J Am Heart Assoc 2(2):e000159. https://doi.org/10.1161/JAHA.113.000159
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434(7033):658–662. https://doi.org/10.1038/nature03434
Halestrap AP, Connern CP, Griffiths EJ, Kerr PM (1997) Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem 174(1–2):167–172
Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y (2005) Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434(7033):652–658. https://doi.org/10.1038/nature03317
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102(34):12005–12010. https://doi.org/10.1073/pnas.0505294102
Di Lisa F, Bernardi P (2006) Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res 70(2):191–199. https://doi.org/10.1016/j.cardiores.2006.01.016
Halestrap AP (2009) Mitochondria and reperfusion injury of the heart–a holey death but not beyond salvation. J Bioenerg Biomembr 41(2):113–121. https://doi.org/10.1007/s10863-009-9206-x
Huttemann M, Pecina P, Rainbolt M, Sanderson TH, Kagan VE, Samavati L, Doan JW, Lee I (2011) The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11(3):369–381. https://doi.org/10.1016/j.mito.2011.01.010
Gullans SR, Kone BC, Avison MJ, Giebisch G (1988) Succinate alters respiration, membrane potential, and intracellular K+ in proximal tubule. Am J Physiol 255(6 Pt 2):F1170–F1177
van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW (2008) Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS ONE 3(4):e2013. https://doi.org/10.1371/journal.pone.0002013
Funding
This project was supported by a Grant from the Natural Science Foundation of China (No. 81271456).
Author information
Authors and Affiliations
Contributions
Study design: SQ, WY. Study conduct: DG, SL. Data analysis: WY, WJ. Writing paper: WY, SQ. Revising paper: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yu, W., Gao, D., Jin, W. et al. Propofol Prevents Oxidative Stress by Decreasing the Ischemic Accumulation of Succinate in Focal Cerebral Ischemia–Reperfusion Injury. Neurochem Res 43, 420–429 (2018). https://doi.org/10.1007/s11064-017-2437-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2437-z