Abstract
Oxidative stress and cytotoxic damage induced by amyloid beta (Aβ) have been considered pivotal in the pathogenesis of Alzheimer’s disease (AD) and may represent a target for treatment. The phosphatidylinositol 3-kinase (PI3K)/Akt pathway elicits a survival signal to protect against multiple injuries, and the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), a downstream target of the PI3K/Akt pathway, can bind to HO-1. Resveratrol, a natural polyphenol derived from grapes, has been widely reported to have diverse antioxidative effects against AD, but the mechanisms have not been fully elucidated. The present study aims to investigate the effects of resveratrol on Aβ1–42-induced cytotoxicity in PC12 cells and to explore the potential mechanisms of these effects. PC12 cells were cultured and treated with Aβ1–42. Oxidative stress was assessed by measuring malondialdehyde (MDA), glutathione (GSH), and superoxide dismutase (SOD) levels. After treating with resveratrol at different concentrations (0, 10, 20, 40 μM) and for different durations (24, 48, 72 h), the generation of MDA, GSH, and SOD were detected; cell viability was assessed by MTT assay. The production of reactive oxygen species (ROS) was determined using an ROS Assay Kit. Western blotting was used to detect the protein expression. Our studies showed that pretreatment with resveratrol could reduce Aβ1–42-induced oxidative stress in PC12 cells by inhibiting the generation of MDA and ROS and increasing the production of SOD and GSH. Resveratrol markedly attenuated the Aβ1–42-induced loss in cell viability in PC12 cells in both a dose- and time-dependent manner. More importantly, resveratrol stimulated the activation of HO-1, Nrf2, PI3K, and phosphorylated Akt. Notably, the neuroprotective effects of resveratrol were eliminated by the HO-1 inhibitor zinc protoporphyrin IX (ZnPP), Nrf2 small interfering RNA (siRNA), and the PI3K/Akt inhibitor LY294002. Taken together, the findings suggest that the cytoprotection of resveratrol against the cytotoxicity induced by Aβ1–42 in PC12 cells is through the upregulation of HO-1 expression via the activation of the PI3K/AKT/Nrf2 intracellular signaling pathway, which might provide novel insights for understanding the mechanism of the neuroprotective effect of resveratrol as an anti-AD drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Induction
Oxidative stress is a key mechanism of cell death; the overproduction of reactive oxygen species (ROS) generated from oxidative stresses, such as free radicals or hydrogen peroxide, can directly or indirectly damage the physiological functions of cellular proteins, lipids, nucleic acids and other macromolecules. Oxidative stress is the leading pathophysiological basis for many degenerative diseases, such as Parkinson’s disease [1], Huntington’s disease [2] and Alzheimer’s disease (AD) [3]. AD, the crucial cause of dementia in people over the age of 60, is characterized by neuronal loss, the deposition of senile plaques (SPs) and the formation of neurofibrillary tangles in circumscribed regions of the neocortex and hippocampus. Amyloid beta (Aβ), a major component of SPs, is generated from the amyloid precursor protein by β- and γ-secretases. Although the deposition mechanism of Aβ in brain degeneration has remained elusive, the oxidative stress induced by Aβ remains a dominant pathological basis for AD pathogenesis. Hence, targeting Aβ-associated oxidative stress is a promising approach for AD modification.
HO-1 is the most important member of the heme oxygenase family. It catalyzes the rate-limiting step in heme catabolism, leading to the formation of biliverdin, free iron, and carbon monoxide. In the presence of biliverdin reductase, biliverdin is further converted to bilirubin. These three byproducts have a protective role against cytotoxicity in different cell models of many diseases, including AD [4]. Therefore, looking for one drug that can activate HO-1 against oxidative stress would be beneficial for AD treatment.
Unfortunately, for the treatment of AD, few of the chemical drugs designed for clinical applications have reached the expected preventive or therapeutic effect, and these drugs also have significant side effects. Therefore, there is an urgent need for new strategies to be developed for AD treatment. Traditional Chinese Medicine has accumulated much experience in the treatment of dementia during thousands of years of practice; modern pharmacological studies have confirmed the therapeutic effects of many active components derived from Chinese herbal medicines [5].
Resveratrol, 3,5,4′-trihydroxy-trans-stilbene (C14H12O3), is a natural polyphenol derived from plants such as Morus alba and grapes. It has been reported that resveratrol has a wide range of pharmacological properties, including anti-inflammation [6], antioxidant [7], wound healing [8], anti-infective [9], and anti-cancer properties [10]; it is also used to ameliorate defects associated with cystic fibrosis [11]. In addition, resveratrol could activate a xenobiotic response in the target cells, affecting the expression of phase II enzymes, such as NAD(P)H:quinone oxidoreductase, aldoketoreductase, glutathione (GSH) S-transferase, gamma-glutamylcysteine synthetase, GSH synthetase, and HO-1 [12, 13]. At present, it has been reported that resveratrol exerts its cytoprotective properties by inducing the protective protein HO-1 against oxidative stress in AD [14, 15], but the mechanisms are not fully known. Therefore, there has been great interest in understanding the cellular mechanisms through which resveratrol acts, not only to improve the understanding of the molecular mechanisms that activate HO-1 but also to facilitate the design of safe clinical agents that can regulate the HO-1 inducing properties of resveratrol.
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway plays a crucial role in regulating cell differentiation, proliferation, survival, and phagocytosis. PI3K catalyzes the phosphorylation of IP2 (phosphatidylinositol 4,5-diphosphate) to IP3 (phosphatidylinositol 3,4,5-trisphosphate), which is an important activity regulator of various kinases involved in cell signaling pathways [16]. Nuclear factor erythroid 2-related factor (Nrf), a downstream transcription factor of the PI3K/Akt signaling pathway, controls the coordinated expression of important antioxidant and detoxification genes (phase II genes) through a promoter sequence termed the antioxidant response element (ARE). HO-1 contains an ARE, and it is a Nrf2-dependent phase enzyme. Once they are bound together, they can regulate the expression of a set of antioxidant/detoxification genes that act in synergy to remove ROS/RNS through sequential enzymatic reactions [17].
In the present study, we demonstrate the effects of resveratrol on HO-1 expression against oxidative stress in PC12 cells during incubation with Aβ and its influence on PI3K/Akt/Nrf2 signaling, which might provide novel insights into the understanding of the mechanism of resveratrol’s neuroprotective effects.
Materials and Methods
Culture of PC12 Cells
The rat pheochromocytoma cell line PC12 (Department of Pathophysiology, Chongqing Medical University, China) was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 50 U/mL penicillin G sodium, and 50 µg/mL streptomycin sulfate (Invitrogen, USA). Cells were maintained at 37 °C in an incubator containing 5% CO2.
Resveratrol Treatment
Resveratrol was obtained from Sigma-Aldrich (St. Louis, MO, USA) and dissolved with DMSO as a stock solution. The drug stock solution was further diluted with DMEM to the proper concentration before usage. PC12 cells were treated with resveratrol at 0, 10, 20, 40 μM for 24, 48 and 72 h. Then, the cells were collected for different assays. The control group was treated with DMSO (1%, v/v).
Preparation and Treatment with Aβ1–42
The Aβ1–42 peptide was purchased from Sigma-Aldrich (St. Louis, MO, USA) and was freshly prepared before each treatment at 1 mg/mL in double distilled deionized water and considered to be solubilized. The cells were then treated with the Aβ1–42 peptide in a range of 0–80 μg/mL in serum-free medium containing 1% PS for 24 h. Then, the cells were incubated at 37 °C in a humidified and sterile atmosphere containing 5% CO2 for 24, 48, and 72 h.
Nrf siRNA and PI3K Inhibitor LY294002 Treatment
PC12 cells were cultured with DMEM. For transfection, cells were grown in 75 cm2 flasks to approximately 80% confluence. Each flask was transfected with Nrf2 siRNA (Santa Cruz, USA), 15 μL, using 20 μL of Lipofectamine 2000 Reagent (Invitrogen, USA) according to the manufacturer’s instructions. The concentration of 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) in the culture medium was 5.0 µM.
Preparation and Treatment with ZnPP Solution
ZnPP was purchased from Sigma-Aldrich (St. Louis, MO, USA), and the ZnPP solution was prepared as follows. First, 25 mg ZnPP was dissolved in 3.3 mL of NaOH (0.2 M) in a dark room, and 0.2 M HCl was added to adjust the pH 7.0. Finally, saline was added to a final volume of 50 mL (0.5 mg/mL). The resulting solution was stored away from light.
MTT Assays
The effect of resveratrol on the PC cell survival rate was evaluated by the MTT assay. The PC12 cells were plated at 4 × 104 cells per well of a 96-well plate in differentiation media, and each group contained six parallel wells. The medium was then replaced with 180 μL of conditioned medium containing either resveratrol or DMSO. After being incubated for 24 h, 20 μL of the MTT solution (5 g/L) was added. Then, 4 h later, the medium and MTT were replaced with 100 µL of DMSO. After being vortexed gently for 10 min, the absorbance was measured at 490 nm using an ultraviolet spectrophotometer (Bio-Rad, USA).
Oxidative Stress Assays
Oxidative stress was assessed by measuring malondialdehyde (MDA), GSH, and superoxide dismutase (SOD) levels. PC12 cells cultured in 6-well plates (4 × 104 cells/well) for 24 h were treated with various concentrations of resveratrol for 3 h before the addition of 40 μM Aβ1–42 and further incubation for 24 h. Cells were then digested with trypsin and washed twice in phosphate buffered solution (PBS). Thereafter, cells were suspended in 500 μL of PBS and lysed by ultrasonication in the presence of a protease inhibitor before centrifugation at 4000 rpm for 5 min. The supernatant was collected for analysis. Supernatant protein concentrations were measured using a Bradford protein assay kit from Key Gen Biotech (Nanjing, China). The levels of MDA, GSH, and SOD were measured using appropriate kits purchased from KeyGen Biotech following the manufacturer’s instructions.
ROS Measurement
To measure cellular ROS, we used the molecular probe H2DCFDA. PC12 cells were seeded in 96-well plates at a density of 2 × 104 cells per well. The next day, the cells were washed three times with HBSS and then treated with resveratrol, as required, in the presence of H2DCFDA at a final concentration of 10 μM, which diffuses through the cell membrane and is hydrolyzed by intracellular esterases to the nonfluorescent form, dichlorofluorescein (DCFH). DCFH reacts with intracellular H2O2 to form a green fluorescent dye. Fluorescence was measured using a fluorescence microplate reader. Wavelengths of excitation and emission were of 485 and 520 nm, respectively. The fluorescence after a 30-min exposure to the ROS generator (H2O2) was normalized to 1; the remaining variables were expressed in relation to this value.
Western Blotting
The PC12 cells were lysed in PRO-PREP™ liquid (0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 0.15 M NaCl, 0.05 M Tris–HCl, pH 7.2; Intron Biotechnology, Kyungi-Do, Korea) supplemented with a protease inhibitor cocktail (Roche, Welwyn Garden City, UK). The lysates were collected by centrifugation (13,000×g, 10 min, 4 °C), and the protein concentrations were determined by the Bradford method employing a Universal Microplate Reader (Gene Company, Hong Kong) at 595 nm. After gel electrophoresis (15% polyacrylamide in a Tris–glycine buffer system) in the presence of SDS, the proteins were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After blocking, the membranes were incubated with primary antibodies, including the anti-rat HO-1 polyclonal antibody (Santa Cruz Biotechnology, California, USA; 1:1000 dilution) and the anti-rat polyclonal PI3K, (p)Akt and Nrf2 antibodies (Santa Cruz Biotechnology, California, USA; 1:1000 dilution), diluted in 0.1% (w/v) non-fat dry milk and incubated overnight at 4 °C. After washing, the bound antibodies were detected by incubation for 1–2 h at room temperature with secondary, peroxidase-conjugated anti-mouse or anti-rabbit antibodies (Zhong Shan Golden Bridge Biotech Company, China). The membranes were exposed on X-ray film in a darkroom with a commercial enhanced chemiluminescence system (Bio-Rad, Hercules, CA, USA). The relative optical density of each target of the digitized images were analyzed by Image J software. All the procedures were carried out in accordance with the tutorials.
Statistical Analysis
Data were expressed as the mean ± standard error of the mean; Student’s t-test was applied to comparisons between two groups. Multiple comparisons between the group models and the different concentrations of resveratrol-treated groups were analyzed by one-way ANOVA, followed by Dunnett’s test. Differences were considered significant at P < 0.05.
Results
Determination of Aβ1–42 Cytotoxicity in PC12 Cells
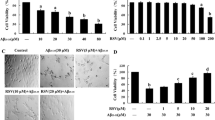
The relative survival rate of PC12 cells was inhibited after treatment with Aβ1–42 at different concentrations along the time course. As shown in Fig. 1, the relative survival rate of PC12 cells was 96.4% with 1.25 μg/mL Aβ1–42 and 37.8% with 80 μg/mL Aβ1–42 after 24 h. The survival rate was approximately 50% with exposure to 40 μg/mL Aβ1–42 for 24 h. Hence, in the following experiments for the determination of survival in response to different treatments, the optimum concentration and incubation time with Aβ1–42 was 40 μg/mL for 24 h.
Effect of Aβ1–42 on cell viability by MTT assay in PC12 cells. a Cells treated with Aβ1–42 (0–80 μg/mL) for 24 h. b Cells treated with 40 μg/mL Aβ1–42 for 0, 12, 24, 36, 48, 60 and 72 h. All results are expressed as mean ± standard deviation (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 as compared with the control group
Resveratrol Alleviated the Decreased Survival Rate of PC12 Cells with Aβ1–42 in a Concentration- and Time-Dependent Manner
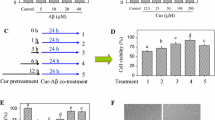
The growth of PC cells treated with 40 μg/mL Aβ1–42 was inhibited in different degrees after treatment with resveratrol at different concentrations (0, 5, 10, 20, 40 μM) for different times (24, 48 and 72 h). The results showed that the differences between the control group and the groups treated with either 5 or 10 μM resveratrol were not statistically significant (p > 0.05), while in comparison with the control group, the differences in the inhibition rates of the 20 and 40 μM groups were significant (p < 0.05). After being treated with resveratrol at 20 μM for different incubation times (24, 48, 72 h), the results showed that an evident difference existed in the survival rate between the time points (p < 0.05) (Fig. 2). Hence, the optimum concentration and acting time of resveratrol was 40 μM for 24 h in the following experiments.
Resveratrol Decreased Aβ1–42-Induced Oxidative Stress
The concentrations of MDA, GSH and SOD are indicators of oxidative stress. As shown in Fig. 3, intracellular MDA concentrations were significantly increased in Aβ1–42-treated PC12 cells compared with the negative control group cells (P < 0.05). Resveratrol decreased intracellular MDA concentrations in the PC12 cells in a dose-dependent manner. The MDA concentrations in the resveratrol-treated groups were significantly lower than the concentration in the model control group (P < 0.05). Intracellular GSH and SOD concentrations were significantly decreased in Aβ1-42-treated cells compared with the negative control group cells (P < 0.05). Resveratrol increased intracellular GSH and SOD concentrations in Aβ1–42-treated PC12 cells in a dose-dependent manner. The GSH and SOD concentrations in the resveratrol-treated groups were significantly higher than the concentrations in the model control group (P < 0.05).
Effect of resveratrol on the generation of MDA, GSH and SOD in PC12 cells treated 40 μg/mL Aβ1–42. The results are expressed as mean ± standard deviation (n = 3). *P < 0.01, **P < 0.001 as compared with the Aβ1–42-treated group; # P < 0.0 1, as compared with normal PC12 cells. MDA malondialdehyde, GSH glutathione, SOD superoxide dismutase
Resveratrol Reduced ROS Production in PC12 Cells Treated with Aβ1–42
The relative fluorescence intensity of ROS released into the extracellular medium from PC12 cell without any treatment was considered as 100%. ROS levels were significantly increased in Aβ1–42-treated PC12 cells (P < 0.001). Resveratrol significantly reduced ROS levels in a dose-dependent manner. ROS levels in the resveratrol 20 and 40 μM groups were significantly lower than the level in the model control group (P < 0.01) (Fig. 4).
PI3K/Akt/Nrf2 Signaling Pathway Was Contributed to the Induction of HO-1 by Resveratrol in PC12 Cells Treated with Aβ1–42
To gain better insight into the antioxidative property of resveratrol, we detected the protein expression level of PI3K, total-Akt (t-Akt), p-Akt, Nrf2 and HO-1 by Western blotting. As shown in Fig. 5A, the results showed that in PC12 cells Aβ1–42 decreased the expression of PI3K, p-Akt, Nrf2 and HO-1 at the protein level. After treatment with 5 µM resveratrol, the expression of PI3K, p-Akt, Nrf2 and HO-1 was not significantly increased (P > 0.05). When resveratrol was used at 20 or 40 µM, the expression of PI3K, p-Akt, Nrf2 and HO-1 at the protein level was evidently increased, and there was a significant difference compared with the PC12 cells with Aβ1–42 (P < 0.01). However, the t-Akt and β-actin protein expression had no significant changes with or without resveratrol (Fig. 5B). In order to confirm whether resveratrol-activated PI3K/Akt/Nrf2 signaling contributed to the induction of HO-1, the standalone effect of ZnPP or siRNA–Nrf2 or LY294002 was observed. As shown in Fig. 5c, d, ZnPP decreased the level of HO-1 only, siRNA-Nrf2 reduced both Nrf2 and HO-1 levels; while, LY294002 decreased the expression of PI3K, p-Akt, Nrf2 and HO-1, respectively. Furthermore, Western blotting showed that the inducing effects of resveratrol on PI3K, p-Akt, Nrf2 and HO-1 expression were reversed by the LY294002, Nrf2 siRNA or ZnPP, respectively (Fig. 5e, f).
Resveratrol-activated PI3K/Akt/Nrf2 signaling pathway contributed to the induction of HO-1 in PC12 cells treated with Aβ1-42. a Resveratrol increased the expression of PI3K, p-Akt, Nrf2 and HO-1 protein in PC12 cells with 40 μg/mL Aβ1-42. b The comparative OD values of PI3K, p-Akt, Nrf2 and HO-1 protein expression were analyzed [*P < 0.01, compared with the normal (without Aβ1-42 treatment); #P < 0.05, compared with Aβ1-42 treated group; ##P < 0.001, compared with Aβ1-42 treated group]. c The standalone effects of LY294002 (the PI3K/Akt inhibitor), Nrf2 siRNA and ZnPP (HO-1 inhibitor) on the expression of PI3K, p-Akt, Nrf2 and HO-1 in PC12 cells with 40 μg/mL Aβ1-42. d The comparative OD values of PI3K, p-Akt, Nrf2 and HO-1 protein expression were analyzed (*P < 0.05, compared with Aβ1-42 treated group). e The inducing effects of resveratrol on the PI3K, p-Akt, Nrf2 and HO-1 expression were reversed by LY294002, Nrf2 siRNA and ZnPP. f The comparative OD values of PI3K, p-Akt, Nrf2 and HO-1 after LY294002, Nrf2 siRNA and ZnPP treatment, respectively (*P < 0.01, compared with Aβ1-42 and Res treatment; **P < 0.001, compared with Aβ1-42 and Res treatment)
Discussion
AD, the most prevalent form of dementia in older populations worldwide, is a chronic, progressive neurodegenerative disorder. It is characterized by progressive cognitive dysfunction, memory impairment and the loss of independence. It is well known that many factors contribute to the etiology of AD; the aggregation of Aβ is the most prominent factor and is considered a classic hallmark of AD. Excessive accumulation of the Aβ peptide not only leads to the formation of SPs but also plays a pivotal role in mitochondrial dysfunctions, such as mitochondrial depolarization [18], energy metabolism abnormalities [19] and oxidative stress. The latter is the leading pathophysiological basis of many diseases, including AD [3]. Therefore, reversing Aβ-induced oxidative stress may provide an opportunity to recover AD.
Although the neuroprotective effects of resveratrol have received a great amount attention in the field of AD, the exact molecular mechanisms have not yet been clarified [20, 21]. The major impressive characteristic of the present study is the demonstration of a novel antioxidant effect of resveratrol. In the present study, the results have shown that the exogenous application of resveratrol can protect PC12 cells against oxidative stress induced by Aβ1–42 through the upregulation of HO-1 expression via activation of the PI3K/Akt/Nrf2 signaling pathway.
ROS, including O2−, H2O2, HO2− and –OH, are generated in aerobic cells during the processes of metabolism. Adjusting the concentration of ROS plays a key role in keeping the balance between death and survival and in inducing apoptosis and necrosis. The more extended physiological significance of low concentrations of ROS is that a number of signaling pathways will be activated, triggering transcription factors and promoting cell proliferation, differentiation and regeneration [22]. In fact, previous studies have focused on the high level of damage, such as apoptosis and necrosis, that ROS causes to cells through oxidative stress [23, 24]. In our study, after Aβ1–42 treatment, ROS production was significantly augmented, the proliferation of PC12 cells was inhibited, and more cells were dying. However, resveratrol treatment could counteract the effects of Aβ1–42 on PC12 cells in a concentration- and time-dependent manner. In addition to detecting the changes in ROS as an indicator of oxidative stress, we also examined the concentrations of other important factors: GSH, MDA and SOD. In keeping with previous oxidative stress findings, we found that the concentration of MDA was significantly increased in PC12 cells pretreated with Aβ1–42 and that resveratrol decreased MDA in a concentration-dependent manner. In contrast, the concentrations of GSH and SOD were remarkably decreased in PC12 cells with Aβ1–42 treatment, and resveratrol could increase the concentration of GSH and SOD. It suggested that the concentrations of ROS, MDA, GSH and SOD were implicated in the development of AD and that resveratrol played its neuroprotective roles by either decreasing the expression of both GSH and SOD or by increasing both ROS and MDA to exert a direct free radical scavenging effect.
HO-1 has been proposed to play a cytoprotective role in the regulation of neurons [24]. The molecular basis of the cytoprotective action remains to be elucidated, however, the products of heme degradation, such as carbon monoxide, bilirubin and ferritin, seem to be responsible for the cytoprotective effect attributed to HO-1. Opii et al. reported that HO-1 was low in the brains of AD patients [25], and in the present study, the expression of HO-1 was reduced after Aβ1–42 treatment in PC12 cells compared with PC12 cells without Aβ1–42. Both results were consistent. After resveratrol application, the expression of HO-1 was increased in a concentration-dependent manner, which suggested that the activation of HO-1 may be beneficial to cell proliferation and that resveratrol plays a protective role against oxidative stress induced by Aβ1–42 through the upregulation of HO-1.
Nrf2 is a transcription factor. Once it is activated by oxidative stress, Nrf2 translocates to the nucleus, binds to the ARE and activates the transcription of phase II genes, including HO-1 [26]. Nrf2 is known to induce the expression of a variety of cytoprotective and detoxification genes involved in combating not only oxygen radicals but also products of oxidation, as well as protein and DNA adducts from carbonyls, MDA, or hydroxyl radicals. Thus, activation of Nrf2 has been implicated in protecting against neurodegenerative conditions, and Nrf2 may be a therapeutic target for conditions that are known to involve oxidative stress [27]. It is known that Nrf2 is a key downstream element of the PI3K/Akt signaling pathway, which is involved in the transduction of various signals from the cell surface to the nucleus. When the PI3K/Akt pathway is activated, AKT phosphorylates Nrf2; then the sulfhydryl of the Keap-1 protein physically interacts with Nrf2 and translocates into the nucleus, initiating gene transcription [28]. A few studies have shown that resveratrol could induce HO-1 expression through the PI3K/Akt/Nrf2 pathway in some cells, such as malignant pleural mesothelioma MSTO-211H cells [29], malignant hepatoma H22 cells [30], primary cortical neurons and astrocytes [31], but there has not been report about whether resveratrol plays a role in inducing HO-1 by activating the pathway in AD. Therefore, we assumed that resveratrol upregulated HO-1 expression through activation of the PI3K/Akt signaling pathway, which appears to be responsible for nuclear translocation of Nrf2. In the present study, our results showed that resveratrol significantly increased the expression of PI3K, p-Akt and Nrf2 at the protein level, which was reversed by the PI3K inhibitor LY294002, Nrf2 siRNA and the HO-1 inhibitor ZnPP.
In summary, the study has proven that resveratrol has a neuroprotective effect against oxidative stress induced by Aβ1–42 in PC12 cells by activating the PI3K/Akt/Nrf2 signaling pathway and consequently upregulating HO-1 (Fig. 6). This finding may have therapeutic implications as resveratrol could be an effective therapeutic drug for the treatment of AD, using the mechanism of HO-1 induction through the activation of the PI3K/Akt and Nrf pathway. Further studies should be carried out in vivo to determine whether resveratrol may be beneficial in treating AD.
References
Pan PK, Qiao LY, Wen XN (2016) Safranal prevent rotenone-induced oxidative stress and apoptosis in an in vitro model of Parkinson’s disease through regulating Keap/Nrf2 signaling pathway. Cell Mol Biol (Noisy-le-grand) 62(14):11–17
Machiela E, Dues DJ, Senchuk MM, Van Raamsdonk JM (2016) Oxidative stress is increased in C. elegans models of Huntington’s disease but does not contribute to polyglutamine toxicity phenotypes. Neurobiol Dis 96:1–11
Garcia-Blanco A, Baquero M, Vento M, Gil E, Bataller L, Chafer-Pericas C (2017) Potential oxidative stress biomarkers of mild cognifive impairment due to Alzheimer’s disease. J Neurol Sci 373:295–302
Gupta A, Lacoste B, Pistell PJ, Ingram DK, Hamel E, Alaoui-Jamali MA, Szarek WA, Vlahakis JZ, Jie S, Song W, Schipper HM (2014) Neurotherapeutic effects of novel HO-1 inhibitor in vitro and in a transgenic mouse model of Alzheimer’s disease. J Neurochem 131(6):778–790
Wang ZY, Liu JG, Li H, Yang HM (2016) Pharmacological effects of active components of Chinese Herbal Medicine in the treatment of Alzheimer’s disease: a review. Am J Chin Med 44(8):1525–1541
Huang FC, Kuo HC, Huang YH, Yu HR, Li SC, Kuo HC (2017) Ant-inflammatory effect of resvertrol in human coronary arterial endothelial cells via induction of autophagy: implication for the treatment of Kawasaki disease. BMC Pharmacol Toxicol 18(1):3
Duan GL, Wang CN, Liu YJ, Yu Q, Tang XL, Ni X, Zhu XY (2016) Resvertrol alleviates endotoxemia-associated adrenal insufficiency by suppressing oxidative/nitrative stress. Endocr J 63(6):569–580
Zhai XX, Ding JC, Tang ZM (2015) Resvertrol inhibits proliferation and induces apoptosis of pathological scar fibroblasts through the mechanism involving TGF-beta1/Smads signaling pathway. Cell Biochem Biophys 71(3):1267–1272
Liu Y, Zhou J, Qu Y, Yang X, Shi G, Wang X, Hong Y, Drlica K, Zhao X (2016) Resvertrol antagonizes antimicrobial lethality and stimulates recovery of bacterial mutants. PLoS ONE 11(4):e0153023
Chen P, Wang B, Pan B, Guo W (2016) Resveratrol-4-O-D-(2′-galloyl)-glucopyranoside exerts an anticaner effect on leukemia cells via inducing apoptosis. Mol Med Rep 13(3):2281–2286
Dhooghe B, Bouckaet C, Capron A, Wallemacq P, Leal T, Noel S (2015) Resvertrol increases F508del-CFTR dependent salivary secretion in cystic fibrosis mice. Biol Open 4(7):929–936
Whitehouse S, Chen PL, Greenshields AL, Nightingale M, Hoskin DW, Bedard K (2016) Resveratrol, piperine and apigenin differ in their NADPH-oxidase inhibitory and reactive oxygen species-scavenging properties. Phytomedicine 23(12):1494–1503
Shen C, Cheng W, Yu P, Wang L, Zhou L, Zeng L, Yang Q (2016) Resveratrol pretreatment attenuates injury and promotes proliferation aof neural stem cells following oxygen-glucose deprivation/reoxygenation by upregulating the expression of Nrf2, HO-1 and NQO1 in vitro. Mol Med Rep 14(4):3646–3654
Liu XQ, Wu BJ, Pan WH, Zhang XM, Liu JH, Chen MM, Chao FP, Chao HM (2013) Resveratrol mitigates rat retinal ischemic injury: the roles of matrix metalloproteinase-9, inducible nitric oxide, and heme oxygenase-1. J Ocul Pharmacol Ther 29(1):33–40
Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL (2011) Resveratrol protects rats from Abeta-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE 6(12):e29102
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages.. J Biol Chem 275:16023–16029
Lei Y, Yang L, Ye CY, Qin MY, Yang HY, Jiang HL, Tang XC, Zhang HY (2015) Involvement of intracellular and mitochondrial Abeta in the ameliorative effects of Huperzine A against Oligomeric Abeta42-induced injury in primary rat neurons. PLoS ONE 10(5):e0128266
Fong S, Teo E, Ng LF, Chen CB, Lakshmanan LN, Tsoi SY, Moore PK, Inoue T, Halliwell B, Gruber J (2016) Energy crisis precedes global metabolic failure in a novel Caenorhabditis elegans Alzheimer disease model. Sci Rep 6:33781
Braidy N, Jugder BE, Poljak A, Jayasena T, Mansour H, Nabavi SM, Sachdev P, Grant R (2016) Resveratrol as a potential therapeutic candidate for the treatment and management of Alzheimer’s disease. Curr Top Med Chem 16(17):1951–1960
He X, Li Z, Rizak JD, Wu S, Wang Z, He R, Su M, Qin D, Wang J, Hu X (2017) Resveratrol attenuates formaldyhyde induced hyperphosphorylation of Tau protein and cytotoxicity in N2a cells. Front Neurosci 10:598
Luo L, Sun Z, Zhang L, Li X, Dong Y, Liu TC (2013) Effects of low-level laser therapy on ROS homeostasis and expression of IGF-1 and TGF-beta1 in skeletal muscle during the repair process. Lasers Med Sci 28(3):725–734
Wu N, Shen H, Liu H, Wang Y, Bai Y, Han P (2016) Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovas Diabetol 15(1):109
Xipell E, Gonzalez-Huarriz M, Martinez de Irujo JJ, Garcia-Garzon A, Lang FF, Jiang H, Fueyo J, Gomez-Manzano C, Alonso MM (2016) Salinomycin induced ROS results in abortive autophagy and leads to regulated necrosis in glioblastoma. Oncotarget 7(21):30626–30641
Opii WO, Joshi G, Head E, Milgram NW, Muggenburg BA, Klein JB (2008) Proteomic identification of brain proteins in the canine model of human aging following a long-term treatment with antioxidants and a program of behavioral enrichment: relevance to Alzheimer’s disease. Neurobiol Aging 29:51–70
Nguyen T, Yang CS, Pickett CB (2004) The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress. Free Radic Biol Med 37:433–441
Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP (2009) The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxi Redox Signal 11:497–509
Li MH, Jang JH, Na HK, Cha YN, Surh YJ (2007) Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamatecysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J Biol Chem 282:28577–28586
Lee YJ, Im JH, Lee DM, Park JS, Won SY, Cho MK, Nam HS, Lee YJ, Lee SH (2012) Synergistic inhibition of mesothelioma cell growth by the combination of clofarabine and resveratrol involves Nrf2 downregualtion. BMB Rep 45(11):647–652
Huang WY, Chao XJ, Ouyang Y, Liu AM, He XX, Chen MH, Wang LH, Liu J, Yu SW, Rapposelli S, Pi RB (2012) Tacrine-6-ferulic acid, a novel multifunctional dimer agianst Alzheiemr’s disease, prevents oxidative stress-induced neuronal death through activating Nrf/ARE/HO-1 pathway in HT22 cells. CNS Neurosci Ther 18(11):950–951
Kwon KJ, Kim JN, Kim MK, Lee J, Ignarro LJ, Kim HJ, Shin CY, Han SH (2011) Melatonin sygergistically increases resveratrol-induced heme oxygenase-1 experssion through the inhibition of ubiquitin-dependent proteasome pathway: a possible role in neuroprotection. J Pineal Res 50(2):110–123
Acknowledgements
This work was supported by the research projects of the Chongqing Municipal Health Bureau (CSTC: 2008-2-381).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hui, Y., Chengyong, T., Cheng, L. et al. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-β1–42 in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem Res 43, 297–305 (2018). https://doi.org/10.1007/s11064-017-2421-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2421-7