Abstract
NF-κB is involved in the activation of microglia, which induces secondary spinal cord injury (SCI). This process involves the activation of NF-κB signaling pathway by TRAF6 through its polyubiquitination function. We know that deubiquitination of TRAF6 mediated by deubiquitinating enzyme (DUB) significantly inhibits activation of NF-κB pathway. The ubiquitin-specific protease 4 (USP4) belongs to the deubiquitinase family. Therefore, we hypothesize that USP4 is involved in the microglial activation and subsequent neuronal inflammation after SCI. In this study, we examined the expression and the role of USP4 after SCI. Western blot analysis showed that the expression of USP4 was downregulated and the expression of p-p65 was upregulated in the spinal cord after SCI. Immunohistochemical and immunofluorescence staining showed that USP4 was expressed in microglia but its expression decreased after SCI. In vitro LPS-induced activation of microglia showed decreased expression of USP4 and increased expression of p-p65 and TRAF6. USP4 silencing in LPS-induced activation of microglia promoted the expression of p-p65 and TRAF6 and the secretion of TNF-α and IL-1β. In conclusion, our study provides the first evidence that in microglial cells expression of USP4 decreases after SCI in rats. The downregulation of USP4 expression may promote microglial activation and subsequent neuronal inflammation through NF-κB by attenuating the deubiquitination of TRAF6. This mechanism is of great significance in the pathophysiology of secondary SCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic spinal cord injury is the most common and severe factor in the spinal cord injury (SCI), which leads to neurological dysfunction [1, 2]. It is a serious threat to the patient’s quality of life, and causes financial burden to patients upon prognosis. As to the new WHO report, 500,000 people suffer SCI each year. People with spinal cord injuries are two to five times more likely to die prematurely, with worse survival rates in low- and middle-income countries [3]. SCI causes mechanical damage and secondary biochemical and physiological responses that can induce spinal cord tissue damage and related neurological dysfunction [4]. At present [4, 5], the pathophysiology of SCI include primary injury and secondary injury. Primary injury is the initial mechanical damage caused by direct tissue damage and energy transformation. Secondary injury is a series of biochemical processes occurring in the hours to weeks after SCI that further damage the tissue within and surrounding the initial injury site, which is one of the critical contributing factors of SCI pathology. Inflammation-associated microglial activation is often used to represent neuronal inflammation, this result in second injury in the SCI.
Neuronal inflammation can be induced by activated microglia through the nuclear factor-κB (NF-κB) pathway, which play important roles in inflammation and immune response. Furthermore, it can promote the secretion of IL-1β, IL-6, IL-18, TNF-α and other inflammatory cytokines [6, 7]. NF-κB activation is accompanied by the phosphorylation of p65. Thus, detecting the expression level of p-p65 may reflect the activation of NF-κB. It has been shown that reactive microglia has critical neuroprotective and reparative roles during the initial stages of SCI. At a later time, these can impair axonal regeneration and functional recovery [8, 9]. This means that the appropriate control for the activation of microglia to suppress inflammation and immune response expansion is a key strategy to the treatment of SCI. Therefore, the signaling events which lead to microglial activation following SCI are supposed to be further researched.
As is known to all, TNF (tumor necrosis factor)-receptor-associated factor (TRAF) 2 and 6 are essential adaptor proteins for the NF-κB signaling pathway [10]. In addition, TRAF 6, mainly through their polyubiquitination, realize their activation and functions in TNF-α and IL (interleukin)-1β-induced NF-κB activation [11]. However, the mechanism of how to regulate the deubiquitination of TRAF 6 after SCI remains incompletely understood. It has been reported that the deubiquitination of TRAF 6 by DUBs (deubiquitinating enzymes) markedly inhibits TNF-α and IL-1β-mediated NF-κB activation [12,13,14]. Ubiquitin specific protease 4 (USP4), which is a homolog to murine Unp with two tissue specificity to plasmic isoforms, belongs to the deubiquitin enzyme family [15]. However, it remains unclear whether USP4 is involved in the microglial activation and subsequent neuronal inflammation in SCI through NF-κB.
In the present study, we identified the expression and distribution of USP4 in the spinal cord after traumatic SCI on adult rats, and its correlation with NF-κB in activated microglia. These will help us better understand the function of USP4 and its role in the microglial activation and subsequent neuronal inflammation after SCI.
Materials and Methods
Animals and Surgery
Experiments were approved by the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Adult male Sprague–Dawley rats (n = 74) with an average body weight of 250 g were obtained from Department of Animal Center, Nantong University. The chosen rats were acclimated for 1–2 weeks. They were placed at 22 ± 3 °C and saturated humidity with a 12 h light/dark cycle. We guarantee plenty of water and food. All the rats were randomly divided into two groups: sham operation (n = 9) and contusion injury (n = 63). In the subgroups, 48 rats (sham and each time point after SCI: n = 6 × 8) were designed as western blot procedures, and 24 rats (sham and each time point after SCI: n = 3 × 8) were designed as frozen cross-sections for immunohistochemistry Dorsal laminectomies at the level of the ninth thoracic vertebra (T9) were carried out under anesthesia with 10% chloral hydrate (3.5 ml/kg, i.p.) [16]. Ketoprofen (5 mg/kg) was administered to minimize postsurgical pain and discomfort. Contusion injury groups were performed using the NYU impactor in the force of 10 g 9.5 cm. Unfortunately, one rat was lost in traumatic SCI group and one rat was lost in sham group during the surgery. The overlying muscles and skin were then sutured in layers with 4.0 silk sutures and staples after contusion. The animals in sham group were anesthetized and surgically prepared without receiving the SCI surgery. The animals were allowed to recover in a new cage with the 30 °C heating pad separated from each other. Postoperative treatments included 2 ml saline (s.c.) for rehydration, ketoprofen (5 mg/kg, i.p.) to minimize postsurgical pain, and discomfortable baytril (0.3 ml, 22.7 mg/ml, s.c., twice daily) to prevent urinary tract infection. Bladders of the rats were manually expressed twice a day until reflex bladder emptying function was recovered. Beddings were changed frequently, and the cage was kept clean and dry. The rats were sacrificed in seven subgroups at 6, 12 h, 1, 3, 5, 7, and 14 days after injury. Ten rats were used as sham controls. All efforts were made to minimize the number of animals used and their suffering.
Western Blot Analysis
In order to get samples for Western blot analysis, the sham or injured spinal cords were taken out and cryopreserved at −80 °C for further use later. The portion of the spinal cord extending from 5 mm caudal to 5 mm rostral in the injury epicenter was immediately removed. To make the lysates, the frozen spinal cord samples were minced with eye scissors on ice. The samples were then well-distributed in lysis buffer (50 mmol/l Tris, 1% NP-40, pH 7.5, 1% SDS, 5 mmol/l EDTA, 1% sodium deoxycholate, 1 mmol/l PMSF, 1% Triton X-100, 1 mg/ml leupeptin, and 10 mg/ml aprotinin) and clarified by centrifugation for 20 min in a microcentrifuge at 4 °C. After the determination of its protein concentration with the Bradford assay (Bio-Rad), the resulting supernatant (50 µg of protein) was accepted to SDS–polyacrylamide gel electrophoresis (PAGE). Then, the separated proteins were transferred to a polyvinylidine difluoride membrane (Millipore) by a transfer apparatus at 350 mA for 2.5 h and blocked with 5% nonfat milk. The membrane was incubated with primary antibodies against USP4 (anti-rabbit, 1:1000; Santa Cruz), p-p65 (antirabbit, 1:1000; Cell Signaling), p65 (antirabbit, 1:1000; Cell Signaling), and GAPDH (antirabbit, 1:1000; Santa Cruz). After incubating with an anti-mouse or anti-rabbit horseradish peroxidase conjugated secondary antibody, the protein was visualized by an enhanced chemiluminescence system (ECL, Pierce Company, USA).
Immunohistochemistry and Immunofluorescent
After the different time decided, the sham and injured rats were terminally anesthetized and perfused through the ascending aorta with saline and next with 4% paraformaldehyde. After perfusion, the sham and injured spinal cords were post-fixed in 4% paraformaldehyde for 12 h and then dehydration in sucrose. After treatment with sucrose solutions, the spinal cords were embedded in O.T.C. compound. Next, they were cut into 4.5 µm frozen cross sections at two spinal cord levels, prepared, and examined. All of the sections were blocked with 10% Donkey serum with 0.3% Triton X-100 and 1% (w/v) bovine serum albumin (BSA) for 2 h at RT and incubated overnight at 4 °C with anti-USP4 antibody (antirabbit, 1:100; Santa Cruz), followed by incubation in biotinylated secondary antibody (Vector Laboratories, Burlingame, CA, USA). Staining was visualized with DAB (Vector Laboratories). Cells with strong or moderate brown staining were counted as positive; cells with no staining were counted as negative; while cells with weak staining were scored separately. For double immunofluorescent staining, sections were removed from the freezer and incubated in an oven at 37 °C for 40 min. The sections were incubated with rabbit polyclonal primary antibodies for USP4 (1:100), NeuN (neuron marker, 1:100), DAPI (nucleus marker 1:1000), GFAP (astrocytes marker, 1:100), and IBa1 (microglia marker, 1:100). Briefly, sections were incubated with both primary antibodies overnight at 4 °C, followed by a mixture of CY2- and CY3-conjugated secondary antibodies for 2 h at 25 °C. The stained sections were examined with a Leica Fluorescence microscope (Germany).
Cell Culture and Treatments
Glial cultures were prepared from the spinal cords of postnatal day-1 Sprague–Dawley rats [17]. The meninges and blood vessels were removed from the spinal cords, and then tissue was finely minced and dissociated enzymatically by 0.25% Trypsin–EDTA for 20 min at 37 C. Spinal cord tissues were triturated mechanically in Dulbecco Modified Eagle Medium/Ham’s F12 (DMEM/F12; Gibco) containing 10% fetal bovine serum (FBS; Gibco) and 2% Penicillin/Streptomycin (P/S; Gibco) and then plated on poly-l-lysine coated plates. After 21 days of culture, microglia was isolated from glial cultures by mild trypsinization (0.25% Trypsin–EDTA was diluted in 1:3) for 20–50 min. Then DMEM/F12 containing 2% P/S was added to the isolated spinal cord microglia (SCM) [17]. SCM were further tagged with a PE-conjugated anti-CD11b+ antibody (BD Biosciences, San Jose, CA, USA) followed by an anti-PE antibody conjugated to a magnetic bead. Magnetically tagged CD11b+ cells were isolated using MS columns according to the Miltenyi MACS protocol. As previously reported, this method results in a 97% pure population of CD11b+ cells [18]. Isolated CD11b+ cells will subsequently be referred to as “microglia.” Isolate microglia cells were cultured again in DMEM/F12 containing 2% P/S at 37 °C in 5% CO2. Cells of passages 3–4 were used for all experiments. For hypoxia treatment, SCM were set in an airtight experimental hypoxia chamber (Billups-Rothenberg, San Diego, CA, USA) containing a gas mixture composed of 95% N2/5% CO2.
LPS Treatment
Cell culture medium was switched to serum-free DMEM/F12 culture medium. One group of the six-well plates of microglia was synchronized for 24 h in the absence of serum, then incubated in the presence of serum, and in the presence or absence of LPS with an incubation density of 0, 0.01, 0.1, 1, 10, 20 μg/ml. LPS-induced microglia were then harvested for Western blot analysis. Then we choose the incubation density of LPS which made p-p65 expression most obviously changed to incubate microglia for 1, 3, 6, 12, or 24 h. LPS-treated microglia were then harvested for Western blot analysis.
RNA Interference of USP4
HA-USP4 plasmid was purchased from Public Protein/Plasmid Library (PPL) and siRNA transfection was purchased from GenePharma. Transient transfection of plasmid and siRNA was done using a protocol recommended by the manufacturer. The three siRNA sequences, which were obtained online from the GenePharma siRNA library, were directed against USP4. The USP4 siRNA sequence was sense (5′–3′): Si-Usp4a: GAGCAAGCUAGACAACACUTT; Si-Usp4b: CCAAAUGGAUGAAGGUUUATT; Si-Usp4c: GGUGGUUCAUCUCAAACGUTT. Negative control, UUCUCCGAACGUGUCACGUTT. Cells were transfected with 100 nmol/l of siRNA duplexes using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol.
Enzyme-Linked Immunosorbent Assay (ELISA)
TNF-α and IL-1β in the SCM media were quantified by solid-phase sandwich ELISA (R&D systems, Mannheim, Germany), according to the manufacturer’s instructions, and all samples were run in duplicate. Spinal cords were isolated and homogenized in 300 μ PBS. The homogenates were frozen at −20 °C overnight and were centrifuged at 12,000×g at 4 °C. Protein concentration was measured in the supernatants using the BCA method (Pierce, Rockford, IL, USA) and 1 mg proteins of each group were used for ELISA assay. Samples and standards were incubated on plates coated with anti-TNF-α and anti-IL-1β antibodies (n = 5 per group). Biotinylated antibody was added to mixtures of bound cytokines. To quantify the binding of the secondary antibody, streptavidin-peroxidase conjugate and substrate (tetramethylbenzidine) were added. After stopping the reaction by the addition of citric acid, the absorbance was measured at 450 nm. Concentrations were determined from a standard curve.
Statistical Analysis
All dates are analyzed in Stata 7.0 software. The values were expressed as means ± SEM. One-way ANOVA followed by Tukey’s post hoc multiple comparisons tests were used for statistical analysis. P < 0.05 was considered statistically significant. Each experiment consisted of at least three replicates per condition.
Results
Changes in Protein Expression for USP4 Following Spinal Cord Injury
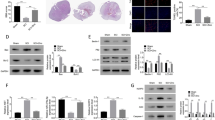
In order to investigate the temporal patterns of USP4 expression in the spinal cord, rats were euthanized in different time points after SCI or sham operation. The expression of USP4 was examined by western blot analysis. As shown in Fig. 1a, b, USP4 began to decrease at 6 h after SCI, and was the lowest in day three (P < 0.05). Then, This gradually increased to the level of sham operation group. These above data meant that the USP4 protein level could possibly be downregulated after SCI.
USP4 expression in rat spinal cords. a Spinal cord tissues from rats in different time points after SCI and the sham operation were homogenized and examined by western blot analysis. The expression of USP4 at 1, 3 and 5 days after SCI were significantly lower than that in the sham group. b Quantification of USP4 protein levels: the relative levels of protein expression were normalized to GAPDH expression. The data are means ± SD. (n = 6, *P < 0.05, significantly different from the sham group)
The Expression and Distribution of USP4 in Normal or Injured Spinal Cord
To further research the alteration of USP4 protein expression and distribution in the spinal cord after SCI, we performed immunohistochemical staining on the transverse cryo-sections of the spinal cord tissues with anti-USP4 antibody. As shown in Fig. 1a, the expression level of USP4 in day three was the lowest. Hence, day three was selected as the time point of injury immunofluorescent staining. As shown in Fig. 2a, b, USP4 was expressed in the white matter and gray matter. However, USP4 expression apparently decreased in the white matter in the injury group compared to the sham group (Fig. 2e, f), while the positively stained intensity in gray matter did not change obviously (Fig. 2c, d). The number of USP4 staining positive cells was relatively high in the sham operated controls, and markedly decreased at day three after SCI (Fig. 2g) (P < 0.05). These above data argued that SCI-induced downregulated USP4 expression in the white matter of rat spinal cord.
Immunohistochemical staining of USP4 in rat spinal cords in sham operation and SCI. Low-power views of immunohistochemical staining on transverse cryo-sections of the spinal cord with antibody to USP4 in the sham (a) and 3 days after SCI (b). High-power views in the gray matter (c, d) and the white matter (e, f) of the spinal cord are shown. Quantitative analysis of the number of USP4 positive cells/mm2 in the sham group and in 3 days after SCI (g) is shown. The data are means ± SD. (n = 3, *P < 0.05, significantly different from the sham group). Scale bar 200 μm (a, b) and 20 μm (c–f)
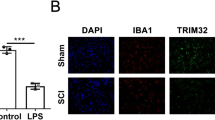
Colocalization of USP4 with Cell-Specific Markers by Double Immunofluorescent Staining After SCI
To further address the exact cellular localization of USP4 in the rat SCI model, we double labeled USP4 and the cell-specific markers for neurons (NeuN), astrocytes (GFAP), or microglias (Iba-1) on the sham and day three injured transverse cryosections of rat spinal cords (Fig. 3a–r). We found USP4 was expressed in microglia. Significant downregulation of USP4 expression was observed in microglia (Iba-1 positive) at day three after SCI, in comparison with the sham-operated control (Fig. 3s) (P < 0.05). Based on the results above, compared to the sham groups, USP4 expression significantly decreased in microglia in the injury group, which indicate that USP4 might be related to microglia activation after SCI.
The co-localization of USP4 with various phenotype specific markers by double-immunofluorescent staining in the spinal cord. The tissue sections of the sham group and 3 days after SCI were immunolabeled with USP4 (red) and phenotype-specific markers (green), namely, GFAP, IBa-1 and NeuN (a–r). The co-localization of USP4 and the phenotype-specific markers (yellow) is shown in the microglia (i, l). Quantitative analysis of cells co-expressing is shown in the sham group and 3 days after SCI. The data are means ± SD. (n = 3, *P < 0.05, significantly different from the sham groups, Scale bar 20 μm). (Color figure online)
USP4 was Associated with the p-p65 After SCI
As shown in Fig. 4a, b, the protein level of p-p65 increased at 6 h after SCI, and reached a peak at day three (P < 0.05); and these were in contrast with the temporal patterns of the expression of USP4. Besides, the protein level of p65 was not change. These results attracted our attention to examine whether USP4 is involved in inflammation after SCI. Next, double-labeled immunofluorescent staining was performed to probe the colocalization of USP4 with p-p65 in the injured spinal cord. The colocalization of USP4 and p-p65 was observed in day three after SCI (data not shown). These results indicate that USP4 was involved in the inflammation in the spinal cord after SCI.
p-p65 increased in the spinal cord. a Spinal cord tissue from rats in different time points after SCI and sham operation were homogenized and examined by western blot analysis. The expression of p-p65 was increased at 6 h after SCI. b Quantification of p-p65 protein levels: the relative levels of protein expression were normalized to GAPDH expression. The data are means ± SD. (n = 6,*P < 0. 05, significantly different from the sham groups)
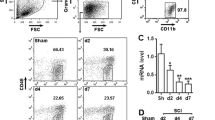
The Expression Changes of USP4, p-p65 and TRAF6 in Activated Microglia In Vitro
We used LPS simulation to induce the activation of primary microglia. As shown in Fig. 5a, b, the level of USP4 expression was markedly reduced after 6 h of LPS induction, compared with naïve cells (P < 0.05). It has been known that TRAF 6 plays a critical role in NF-κB activation. Therefore, western blot was performed to further measure the expression profiles of p-p65 and TRAF 6. It was found that p-p65 and TRAF 6 protein levels were low in naïve cells, but both remarkably increased after LPS treatment for 6 h (Fig. 5c–e) (P < 0.05).
Protein expression of USP4, p-p65 and TRAF 6 in activated microglia. a Western blot analysis for USP4 in microglia after being induced by LPS for 1, 3, 6, 12 or 24 h. b Quantification of USP4 protein levels: the relative levels of protein expression were normalized to GAPDH expression. c Western blot analysis for p-p65 and TRAF6 in microglia after being induced by LPS for 1, 3, 6, 12 or 24 h. d, e Quantification of p-p65 and TRAF6 protein levels: the relative levels of protein expression were normalized to GAPDH expression. The data are means ± SD (*P < 0.05, significantly different from the naïve groups)
The Silencing of USP4 Promotes the Inflammation in Activated Microglia Cells
In order to further characterize the function of USP4 in microglial activation and subsequent neuronal inflammation, siRNA was synthesized specifically against USP4 (si-USP4a, si-USP4b, si-USP4c). Microgliais were transfected with si-USP4 for 24 h prior to treatment with LPS for 12 h. It was found that si-USP4c effectively decreased the protein level of USP4 in microglia. (Fig. 6a, b) (P < 0.05). Thus, si-USP4c was employed in the following experiments. As expected, USP4 silencing promotes the expression of p-p65 and TRAF 6. In addition, the p-p65 and TRAF 6 protein levels of microglia triggered by LPS were significantly higher than that in controls (Fig. 6c, d) (P < 0.05). To judge the effect of USP4, we examine TNF-α and IL-1β secretion after overexpressing or silencing USP4 (HA-USP4 or si-USP4c) in microglia cells with or without LPS stimulation by ELISA. Similar to the expression patterns of p-p65 and TRAF6, the secretion levels of TNF-α and IL-1β are significantly increased after treating USP4 knock down, however, reduced after transfecting HA-USP4 plasmid primary cultured microglia (Fig. 7) (P < 0.05). In brief, these results indicated that decreased expression of USP4 may contribute to microglial activation and subsequent neuronal inflammation of SCI by affecting the NF-κB signaling pathway through TRAF 6.
Silencing of USP4 promotes the expression of p-p65 and TRAF 6 in activated microglia. The protein levels of USP4 in microglia transfected with USP4 si-RNA were detected by western blot analysis (a), and the quantification of USP4 levels (b) in the indicated groups. Protein levels of p-p65 and TRAF 6 were determined by western blot analysis (c) and quantification of p-p65 and TRAF 6 levels in the indicated groups (d) (*P < 0.05, significantly different from the sham groups)
Discussion
Spinal cord injury mostly occurs in young adults and has a relatively high disability rate. It causes great harm to the psychology and physiology of the patient [19]. Thus, further research of its pathophysiology after SCI is necessary. Secondary SCI refers to the progressive and self-destructive cascade damage of spinal cord tissues caused by multiple factors on the basis of the primary injury [20]. Neuronal inflammation induced by activated microglia through the NF-κB pathway is the critical contributing factors of secondary injury [21, 22]. The parenchymal damage of secondary injury following SCI is far more than the primary SCI. Primary injury is considered irreversible. However, the subsequent secondary injury is reversible [20, 23]. These findings provide the possibility for researchers to study regeneration and repair of SCI. If intervention could be performed early during the secondary injury, this might be able to improve the living conditions of spinal cord tissues, and save the necessary anatomical structure for functional recovery. The study conducted by Cartier et al. [24] revealed that when SCI occurs, microglia in the SCI area and its surroundings react to the activation. Excessive activation of microglia can produce a number of neurotoxic inflammatory cytokines and mediate immune inflammatory responses, which would exacerbate damage to surrounding tissues. This is involved in the pathological process of secondary injury. Therefore, an in-depth understanding of the molecular mechanism of microglial activation and neuronal inflammation response after SCI would help in exploring the early intervention method of secondary injury.
Our results revealed that the protein level of USP4 was downregulated after traumatic SCI by western blot analysis. And USP4 expression and distribution decreased in the white matter by immunohistochemical staining. Furthermore, we found the percentage of USP4 positive cells in microglias was significantly decreased. The protein level of p-p65 was in contrast the temporal patterns of USP4 expression. We known that microglias are in a resting or sleep state under normal conditions in the central nervous system [25]. When trauma stimulates the nervous system, microglias are excessively activated, which induce changes in form, function and immune phenotype [26]. The excessive activation of microglias can generate immune response, exert the phagocytic effects of macrophages, and release a large number of inflammatory cytokines such as IL-1β, IL-6, IL-18 and TNF-α. These inflammatory cytokines can contribute to inflammatory response, and increase neuronal injury and apoptosis; thus, causing or aggravating secondary injury [27]. In addition, USP4 is a member of the Ub-specific peptidases (USPs), which represent the largest subclass of DUBs (deubiquitinating enzymes). Ubiquitination is a reversible process and plays an important role in the activation of the NF-κB signaling pathway, which is an important signaling pathway for microglia activation and secretion of inflammatory factors [28, 29]. Therefore, we consider that USP4 may participate in microglia activation-mediated inflammation through NF-κB after SCI.
To confirm the veracity of the results in vivo research, we performed the model of activation of microglia cultured in vitro. LPS was used to induce the activation of microglias in vitro. Consistent with the inverse relationship between USP4 and p-p65 levels in the spinal cord, USP4 was markedly reduced and p-p65 increased in LPS-induced microglias. In addition, we found that the protein levels of TRAF6, a critical E3 ubiquitin ligase to regulate the NF-κB signaling pathway, were increased, and was consistent with the change in p-p65. These results indicate that low levels of USP4 promote the expression of p-p65 and TRAF 6 after SCI. This are consistent with a previous literature, which reported that USP4 is potentially a negative regulator of TRAF 6 activity [13]. Our further research found that USP4 silenced effectively promotes the up-regulation of p-p65 and TRAF 6 in LPS-induced activated microglias. Moreover, the secretion levels of TNF-α and IL-1β are significantly increased after treating USP4 knock down, however, reduced after transfecting HA-USP4 plasmid primary cultured microglia. This shows that down-regulation of USP4 facilitates the neuroinflammatory response in secondary injury by activation of NF-κB after SCI. Under the normal state, NF-κB dimmers (p65/p50) exist as an inactive state by combining with one of the inhibitory factors (IκBα, IκBβ, IκBε). When IκB ubiquitination, it will be separated from p65/p50, leading to activation of NF-κB [28, 30]. In the present study, USP4 expression was downregulated in the SCI rat model. Therefore, we believe that USP4 downregulated in microglia promote IκB ubiquitination by attenuating the deubiquitination of TRAF 6 after SCI. This activates the NF-κB pathway and promotes inflammatory response.
In summary, we provide the first evidence that USP4 decreases in microglial cells after acute SCI in rats. In addition, our results provide an important clue to understand the downregulation of USP4 expression may promote microglial activation and subsequent neuronal inflammation after SCI. However, several mechanisms to further understand the role of USP4 in LPS-induced microglial activation model still remain unclear. Thus, further investigations are required to delineate the precise molecular mechanism of USP4 in NF-κB mediated inflammatory response after SCI.
Abbreviations
- USP4:
-
Ubiquitin specific protease 4
- SCI:
-
Spinal cord injury
- CNS:
-
Central nervous system
- SD:
-
Sprague–Dawley
- NeuN:
-
Neuronal nuclear antigen
- GFAP:
-
Glial fibrillary acidic protein
- Iba-1:
-
Ionized calcium-binding adapter molecule-1
- LPS:
-
Lipopolysaccharide
- siRNA:
-
Short interferingRNA
References
Huang S, Liu X, Zhang J, Bao G, Xu G, Sun Y, Shen Q, Lian M, Huang Y, Cui Z (2015) Expression of peroxiredoxin 1 after traumatic spinal cord injury in rats. Cell Mol Neurobiol 35(8):1217–1226. doi:10.1007/s10571-015-0214-6
Ren J, Mao X, Chen M, Zhang W, Liu Y, Duan C, Zhang H, Sun C, Wu W, Zhu X, Ge J, Tao W, Wang Y, Lu H (2015) TCTP expression after rat spinal cord injury: implications for astrocyte proliferation and migration. J Mol Neurosci 57(3):366–375. doi:10.1007/s12031-015-0628-0
Sminkey L (2013) Spinal cord injury: as many as 500 000 people suffer each year. http://www.who.int/mediacentre/news/releases/2013/spinal-cord-injury-20131202/en/.
Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, Isa T, Nishimura Y (2015) Function of the nucleus accumbens in motor control during recovery after spinal cord injury. Science 350(6256):98–101. doi:10.1126/science.aab3825
Ambrozaitis KV, Kontautas E, Spakauskas B, Vaitkaitis D (2006) Pathophysiology of acute spinal cord injury. Medicina 42(3):255–261
Liu G, Fan G, Guo G, Kang W, Wang D, Xu B, Zhao J (2016) FK506 attenuates the inflammation in rat spinal cord injury by inhibiting the activation of NF-kappaB in microglia cells. Cell Mol Neurobiol. doi:10.1007/s10571-016-0422-8
Raha S, Lee HJ, Yumnam S, Hong GE, Saralamma VVG, Ha YL, Kim JO, Kim YS, Heo JD, Lee SJ, Kim EH, Kim GS (2016) Vitamin D2 suppresses amyloid-beta 25–35 induced microglial activation in BV2 cells by blocking the NF-kappaB inflammatory signaling pathway. Life Sci 161:37–44. doi:10.1016/j.lfs.2016.07.017
Yaguchi M, Ohta S, Toyama Y, Kawakami Y, Toda M (2008) Functional recovery after spinal cord injury in mice through activation of microglia and dendritic cells after IL-12 administration. J Neurosci Res 86(9):1972–1980. doi:10.1002/jnr.21658
Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P (2014) Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain 137(Pt 3):707–723. doi:10.1093/brain/awt341
Xiao N, Li H, Luo J, Wang R, Chen H, Chen J, Wang P (2012) Ubiquitin-specific protease 4 (USP4) targets TRAF2 and TRAF6 for deubiquitination and inhibits TNFalpha-induced cancer cell migration. Biochem J 441(3):979–986. doi:10.1042/bj20111358
Hadweh P, Habelhah H, Kieff E, Mosialos G, Hatzivassiliou E (2014) The PP4R1 subunit of protein phosphatase PP4 targets TRAF2 and TRAF6 to mediate inhibition of NF-kappaB activation. Cell Signal 26(12):2730–2737. doi:10.1016/j.cellsig.2014.08.001
Martinez-Forero I, Rouzaut A, Palazon A, Dubrot J, Melero I (2009) Lysine 63 polyubiquitination in immunotherapy and in cancer-promoting inflammation. Clin Cancer Res 15(22):6751–6757. doi:10.1158/1078-0432.ccr-09-1225
Yasunaga J, Lin FC, Lu X, Jeang KT (2011) Ubiquitin-specific peptidase 20 targets TRAF6 and human T cell leukemia virus type 1 tax to negatively regulate NF-kappaB signaling. J Virol 85(13):6212–6219. doi:10.1128/jvi.00079-11
Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G (2003) CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature 424(6950):793–796. doi:10.1038/nature01803
Wijnhoven P, Konietzny R, Blackford AN, Travers J, Kessler BM, Nishi R, Jackson SP (2015) USP4 auto-deubiquitylation promotes homologous recombination. Mol Cell 60(3):362–373. doi:10.1016/j.molcel.2015.09.019
Zhang J, Cui Z, Feng G, Bao G, Xu G, Sun Y, Wang L, Chen J, Jin H, Liu J, Yang L, Li W (2015) RBM5 and p53 expression after rat spinal cord injury: implications for neuronal apoptosis. Int J Biochem Cell Biol 60:43–52. doi:10.1016/j.biocel.2014.12.020
Baskar Jesudasan SJ, Todd KG, Winship IR (2014) Reduced inflammatory phenotype in microglia derived from neonatal rat spinal cord versus brain. PLoS ONE 9(6):e99443. doi:10.1371/journal.pone.0099443
Nikodemova M, Watters JJ (2012) Efficient isolation of live microglia with preserved phenotypes from adult mouse brain. J Neuroinflamm 9:147. doi:10.1186/1742-2094-9-147
Stein DM, Pineda JA, Roddy V, Knight WAt (2015) Emergency neurological life support: traumatic spine injury. Neurocrit Care 23(Suppl 2):S155–S164. doi:10.1007/s12028-015-0169-y
Kingwell K (2011) A new approach to respiratory recovery after spinal cord injury? Nat Rev Neurol 7(9):473. doi:10.1038/nrneurol.2011.124
Jin X, Yamashita T (2016) Microglia in central nervous system repair after injury. J Biochem 159(5):491–496. doi:10.1093/jb/mvw009
Liu N, Zang KK, Zhang YQ (2015) Activation of microglia and astrocytes in different spinal segments after peripheral nerve injury in mice. Sheng Li Xue Bao 67(6):571–582
Antar V, Baran O, Yuceli S, Erdogan H, Altintas O, Eryigit Baran G, Tasdemiroglu E (2015) Assessment of the neuroprotective effects of the acetylcholinesterase inhibitor Huperzine A in an experimental spinal cord trauma model. J Neurosurg Sci
Cartier N, Lewis CA, Zhang R, Rossi FM (2014) The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol 128(3):363–380. doi:10.1007/s00401-014-1330-y
Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M (2006) Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci USA 103(35):13174–13179. doi:10.1073/pnas.0603747103
Nakajima K, Kohsaka S (2004) Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord 4(1):65–84
Magni P, Ruscica M, Dozio E, Rizzi E, Beretta G, Facino RM (2012) Parthenolide inhibits the LPS-induced secretion of IL-6 and TNF-alpha and NF-kappaB nuclear translocation in BV-2 microglia. Phytother Res 26(9):1405–1409. doi:10.1002/ptr.3732
Li Z, Hao Q, Luo J, Xiong J, Zhang S, Wang T, Bai L, Wang W, Chen M, Wang W, Gu L, Lv K, Chen J (2016) USP4 inhibits p53 and NF-kappaB through deubiquitinating and stabilizing HDAC2. Oncogene 35(22):2902–2912. doi:10.1038/onc.2015.349
Fan YH, Yu Y, Mao RF, Tan XJ, Xu GF, Zhang H, Lu XB, Fu SB, Yang J (2011) USP4 targets TAK1 to downregulate TNFalpha-induced NF-kappaB activation. Cell Death Differ 18(10):1547–1560. doi:10.1038/cdd.2011.11
Tao Z, Fusco A, Huang DB, Gupta K, Young Kim D, Ware CF, Van Duyne GD, Ghosh G (2014) p100/IkappaBdelta sequesters and inhibits NF-kappaB through kappaBsome formation. Proc Natl Acad Sci USA 111(45):15946–15951. doi:10.1073/pnas.1408552111
Funding
Funding was provided by The Biomechanical and Clinical Research of Lumbar Cortical Bone Tract Screw System (Grant No. MS22015074).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xingjie Jiang and Mingchen Yu have contributed equally.
Rights and permissions
About this article
Cite this article
Jiang, X., Yu, M., Ou, Y. et al. Downregulation of USP4 Promotes Activation of Microglia and Subsequent Neuronal Inflammation in Rat Spinal Cord After Injury. Neurochem Res 42, 3245–3253 (2017). https://doi.org/10.1007/s11064-017-2361-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2361-2