Abstract
Intracerebral hemorrhage (ICH) can lead to brain damage and even death, and there is lack of effective therapeutic methods for treating ICH. Although recent studies have focused on the administration of metformin in treating stroke, there is no literature to support whether it can be used to treat ICH. Therefore, the aim of this study was to evaluate the possible effects of metformin on ICH and the underlying mechanisms of those effects. An ICH model was established in adult male Sprague–Dawley rats. Rats were randomly divided into three groups: sham group, ICH group, and ICH+metformin group. The neurobehavioral deficit scoring method was used to examine neurological function in rats. The levels of lipid peroxidation antioxidant enzyme and 8-iso-PGF2α were detected to evaluate oxidative stress. Survival of striatal neurons was examined by TUNEL staining, immunohistochemistry and HE staining. The levels of p-JNK, p-c-Jun and cleaved caspase-3 in the striatum were measured by western blotting. The results demonstrated that metformin protected rats from neurological deficits induced by ICH. Moreover, metformin reduced oxidative stress and preserved the survival of striatal neurons under ICH conditions. Furthermore, metformin downregulated the levels of apoptotic factors (p-JNK3, p-c-Jun and cleaved caspase-3) as well as pro-inflammatory cytokines (IL-1β, IL-4 and IL-6 and TNF-α). Collectively, we speculate that metformin may be a potential clinical treatment for ICH patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) is a subtype of stroke that has a high mortality rate and severe morbidity. Rupturing of a blood vessel within the brain parenchyma leads to brain damage [23, 31]. Multiple factors are involved in brain damage, such as glutamate, thrombin, matrix metalloproteinases, inflammation, oxidative stress, among others [14, 27]. Currently, the only approved interventions to manage ICH are those developed for stroke and blood pressure control; however, the outcome of ICH remains poor [22]. Therefore, effective therapeutic methods are needed to treat ICH patients.
ICH can result in the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which play an important role in pathological processes [17]. ROS can participate in several cellular pathways, including modulating a number of kinases, phosphatases, redox-sensitive transcription factors, and genes, which further contribute to the regulation of cellular growth, differentiation, proliferation, and apoptosis [20]. ICH-induced increases in ROS can result in cellular injury in the form of lipid peroxidation, DNA damage, and protein oxidation [6]. Moreover, activated inflammatory cells, including microglia, neutrophils and macrophages, can secrete various types of pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), which further induce brain damage. ROS can also regulate several signaling pathways, of which the c-Jun N-terminal kinases (C-JNK) signaling pathway is well-studied in ICH.
C-jun-N-terminal kinase is a member of the mitogen-activated protein kinase (MAPK) family and is primarily involved in apoptosis [21]. Several studies have demonstrated that the JNK signaling pathway is involved in cerebral ischemia reperfusion injury and that its activation is crucial in exerting a neuroprotective effect [19, 28]. Furthermore, studies have demonstrated that the JNK signaling pathway plays an important role in ICH. A previous study demonstrated that the JNK signaling pathway is activated following ICH and that iron may contribute to this activation [25]. Michel-Monigadon et al. demonstrated that JNK was activated following ICH in mice and that inhibition of the JNK signaling pathway could attenuate functional impairment [18].
Metformin (Met) is a biguanide drug that has been used since the 1960s in the treatment of type 2 diabetes and metabolic syndrome. It can decrease high blood sugar primarily by suppressing glucose production by the liver [7]. Studies have demonstrated that metformin protects the liver from ischemia reperfusion-induced injury by increasing antioxidant enzyme activity, lowering mitochondrial ROS formation, as well as by reducing post-ischemic inflammation [4]. Metformin also protects rats from ischemia/reperfusion injuries by reducing oxidative stress [1]. Therefore, it is possible to use metformin as a protective drug to treat ICH. However, the mechanism underlying the treatment is still unclear.
In our study, we investigated the effects of metformin in treating ICH as well as its underlying mechanisms. We measured the behavioral score of ICH rats, neuronal loss in the striatum, and levels of modulators in the JNK signaling pathway, and we also examined the activities of superoxide dismutase (SOD), malondialdehyde (MDA) and 8-Iso-Prostaglandin F2α (8-iso-PGF2α) [26] to evaluate lipid peroxidation.
Materials and Methods
Animals
Adult male Sprague–Dawley rats weighing 200–250 g were obtained from Shanghai Experimental Animal Center (Chinese Academy of Science, Shanghai, China). All rats were maintained in standard cages at a temperature of 25 ± 1 °C and were given free access to water and food. The animals were maintained in a 12 h light/12 h dark cycle. The number of animals studied was the minimum to obtain significant results. All experiments and animal care were approved by the Science and Technology Department of Jiangsu Province.
Drugs and Antibodies
The following drugs were used: metformin (M0605000), anti-NeuN primary antibody (Rabbit, SAB4300883-100UL) and Alexa Fluor 488 goat-anti-rabbit secondary antiserum (SAB4600234) obtained from Sigma (USA). The following antibodies were employed: Anti-p-c-jun (sc-16312), anti-p-JNK3 (sc-6254) and anti-actin (sc-10731), obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-cleaved caspase-3 (No. 9664) obtained from Cell Signaling Biotechnology.
Experimental Groups and ICH model
All rats were randomly divided into three groups: Sham group, ICH group, ICH+ metformin group (14 rats in each group). For the ICH group, the procedure to induce ICH was performed as described in previous studies [29]. Rats were anesthetized intraperitoneally with 10% pentobarbital sodium and then positioned in a stereotaxic frame (Shanghai Ruanlong Science and Technology Development Co., Ltd.). A 1-mm-diameter burr hole was drilled in the skull (0.7 mm anterior and 3 mm lateral to the bregma), and a 26-gauge needle was inserted into the skull (5.5 mm ventral to the bregma) to infuse 80 μl of whole blood at a rate of 10 μl/min into the right brain striatum. Ten minutes after infusion, the needle was removed slowly. The ICH+ metformin group received metformin (100 mg/kg) intragastrically once a day following ICH surgery (metformin diluted in water containing 0.1% Tween-80) [Asghari et al. 2]. Rats from the sham group were infused with 80 μl of PBS. In the ICH+ metformin group, rats were treated with metformin 30 min before surgery. Next, the burr hole was fixed with bone wax and the skin incision was sutured.
Neurobehavioral Deficit Scoring
Neurobehavioral deficit scoring was performed as previously described by Julio H and colleagues [12]. Testing and scoring were carried out on a daily basis, starting the day before ICH surgery. The measurement details are described as follows.
-
1.
Spontaneous activity: animals were observed for 5 min in a normal environment (cage). 3 = moved and approached at least three sides of the cage; 2 = moved slightly but did not approach at least three sides of the cage; 1 = barely moved; 0 = did not move at all.
-
2.
Symmetry in movement of the four limbs: rats were held in the air by the tail to observe symmetry in the movement of the four limbs. 3 = moved symmetrically on both sides; 2 = moved relatively slowly on the left side; 1 = minimal movement of left side; 0 = did not move on the left side at all.
-
3.
Forepaw outstretching: rats were made to walk on their forelimbs while being held by the tail. 3 = stretched and walked symmetrically on both forelimbs; 2 = left forelimb outstretched less than the right; 1 = left forelimb moved minimally; 0 = left forelimb did not move at all.
-
4.
Climbing: rats were placed on the wall of a wire cage. 3 = climbed easily and gripped tightly; 2 = left side was weak; 1 = failed to climb.
-
5.
Body proprioception: rats were touched with a blunt stick on each side of the body. 3 = turned head and were startled on both sides; 2 = slow response to the left side stimulus; 1 = no response to the left side stimulus.
-
6.
Response to vibrissae touch: A blunt stick was brushed against the vibrissae on each side. 3 = turned head and startled on both sides; 2 = slow response to the left side stimulus; 1 = no response to the left side stimulus.
Tissue Preparation
Rats were anesthetized and sacrificed following the behavior scoring test on day 7. Next, rats were perfused with 500 ml of 0.9% normal saline, followed by infusion of 500 ml of 4% paraformaldehyde. The brains were isolated and stored in 4% paraformaldehyde for post-fixation for 24 h. The brains were then transferred to a 10% sucrose solution and stored until they sank, followed by 20% sucrose until they sank, and were finally transferred to 30% sucrose until they sank. The tissues were then embedded into an O.C.T. compound and sectioned at a thickness of 16 μm for TUNEL staining and immunostaining and at 6 μm for HE staining.
TUNEL Assay
The number of apoptotic cells was determined using an in situ Cell Death Detection Kit (TMR Green; Roche Applied Sciences, Indianapolis, IN) that allows visualization of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). TUNEL + nuclei were visualized on a confocal microscope and were quantified using Image-J.
Immunohistochemistry
We performed an immunohistochemistry assay to stain for NeuN. Brain sections were blocked with 5% goat serum for 1 h. After washing with PBS, monoclonal rabbit anti-NeuN primary antiserum (1:1000) was used, followed by Alexa Fluor 488 goat-anti-rabbit secondary antiserum (1:1000). NeuN-ir nuclei were visualized using the same confocal microscope as described for TUNEL staining. Five microscopic fields per sample were examined. The neuron quantity was measured by NeuN positive neurons minus TUNEL positive neurons.
HE Staining
This method was applied to assess neuronal damage in the striatum, as has been described in a previous study [5]. Brain sections from the damaged area were stained with hematoxylin and eosin. Next, histological evaluations were performed using HE staining. the cell numbers were assessed using unbiased stereological techniques. In brief, a cell count was performed under 400× magnification using an Olympus BH-2 (Madison, WI) microscope connected to a Sony charge-coupled video device camera, motorized stage system and commercial stereology software. The mean values were calculated from data obtained from three sections of each rat.
Western Blot Analysis
Rats were anesthetized on day 7 after the behavioral scoring test. Next, in a frozen environment, the striatum was removed quickly and stored in liquid nitrogen. The striatum was homogenized in ice-cold tissue lysis buffer (W:V 1:5). The lysed tissue was then centrifuged, followed by 30 min pyrolysis at 12,000 r for 20 min at 4 °C. A bicinchoninic acid (BCA) protein assay kit (Beyotime, Haimen, China) was used to determine the total protein concentration in the supernatant. Thereafter, the samples were subjected to SDS–PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes. Next, the membranes were blocked with 5% skimmed milk at room temperature for 2 h and incubated overnight at 4 °C with anti-p-JNK3, anti-c-Jun and anti-cleaved caspase-3 primary antibodies. After washing in phosphate-buffered saline (PBS), the membranes were incubated with secondary antibodies and then washed with PBS. Finally, immunoreactivity was detected using an enhanced chemiluminescence detection system (Beyotime, Haimen, China) and visualized on an X-ray film (Kodak, Shanghai, China). β-actin was used as the control.
ELISA
ELISA experiments were performed following the manufacturer’s instructions. The expression levels of IL-1β, IL-4, IL-6 and TNF-α in the striatum were measured using ELISA kits from R&D system (Minneapolis, MN, USA). The absorbance values were analyzed at 450 nm on a Microplate Reader (Tecan Group AG, Mannedorf, Switzerland) according to the color reaction.
Determination of Brain Content
The dry-wet weight method was used to measure the brain water content. Rats were sacrificed on day 7 after the behavioral scoring test. Following decapitation, brains were isolated, and the right caudate nucleus were obtained. Tissues were weighed immediately to obtain the wet weight. Next, tissues were dried at 100 °C for 24 h and reweighed to obtain the dry weight. The brain water content was calculated as (wet weight − dry weight)/wet weight × 100%.
Lipid Peroxidation and Antioxidant Enzyme Assays
We measured the SOD activity and MDA levels in the striatum. Sample preparation was the same as that for western blotting. Assay kits were purchased from Nanjing Jiancheng Company (A001, Nanjing, China). The assays were conducted according to the manufacturer’s instructions. The SOD activity and MDA levels were detected using a Beckman DU-640 UV/visual spectrometer (St. George, Utah).
Determination of 8-iso-PGF2α in Plasma
Blood samples were obtained on day 7, after the behavioral scoring test and before other experiments and then immediately placed into sterile ethylenediaminetetraacetic acid test tubes coated with ice and centrifuged at 1500×g for 20 min at 4 °C to collect plasma. The concentration of 8-iso-PGF2α in plasma was analyzed by enzyme-linked immunosorbent assay (ELISA) using commercial kits (Cayman Chemicals) according to the manufacturer’s instructions.
Data Analysis
Each experiment included seven rats to obtain the results. One-way ANOVA followed by the Newman–Keuls test was employed to analyze all of the results. GraphPad Prism 6.0 software was used for all statistical analyses. Values of P < 0.05 were considered significant.
Results
Effects of Metformin on Neurological Function in Rats Following ICH
We used a neurobehavioral deficit scoring method to investigate the functional changes in rats following ICH and to evaluate the effects of metformin. Following ICH, rats exhibited a remarkable decrease in behavioral scores. Both the ICH and ICH +metformin groups demonstrated recovery after operation; however, compared to the sham group, the neurological deficit was significantly greater in the ICH group (p < 0.05). The metformin treatment group demonstrated significantly higher behavioral scores compared to those observed in the ICH group (p < 0.05, Fig. 1). The results suggested that metformin exerted neuroprotective effects following ICH.
Effects of metformin on the locomotor function in rats following ICH analyzed using the behavioral scoring method. The diagram shows the behavioral score of each group. Values are expressed as the mean ± SD (n = 7). Asterisk P < 0.05 relative to the sham group. Hash P < 0.05 relative to the ICH group
Effects of Metformin on Lipid Peroxidation Following ICH
ICH can increase ROS, which results in cellular injury in the form of lipid peroxidation [9]. To investigate whether the neuroprotective mechanism of metformin is associated with its antioxidant ability, we examined the activity of total SOD and the levels of MDA in all groups. Since the 8-iso-PGF2α concentration in plasma is related to disease severity and the clinical outcome of ICH, it can be used as a tool to evaluate lipid peroxidation following ICH [10]. In the ICH group, the SOD levels were significantly decreased compared to the sham group, while MDA and 8-iso-PGF2α levels were significantly increased compared to those of the sham group (p < 0.05). Metformin treatment remarkably increased SOD activity and reduced the levels of MDA and 8-iso-PGF2α induced by ICH (p < 0.05, Table 1). Our results suggested that metformin exerted a neuroprotective effect by inhibiting lipid peroxidation induced by ICH.
Metformin Protects Neuron Loss in the Striatum Following ICH
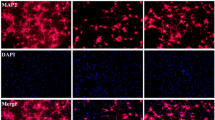
To examine whether metformin exhibits a neuroprotective function against ICH, we employed the TUNEL assay, immunohistochemistry methods as well as HE staining. In the striatum, compared to the sham group, the ICH group showed a large number of apoptotic neurons, while treatment with metformin demonstrated that it had a protective function against neuronal loss due to ICH (Fig. 2a–g). The neuron quantity in the striatum of ICH rats was significantly decreased compared to that in the striatum of sham-operated rats (p < 0.05), while the neuron quantity in the metformin treatment group was significantly increased compared to that in the ICH group (p < 0.05, Fig. 2h). These results suggest that there was neuronal apoptosis in the striatum following ICH and that metformin partly reversed this neuronal loss.
TUNEL assay, immunohistochemistry and HE staining results in the striatum. a Total NeuN staining and TUNEL assay in the striatum of rats from the sham group; b partially enlarged image from the sham group; c partially enlarged image from the ICH group; d partially enlarged image from the ICH +metformin group; e HE staining in the striatum from the sham group; f HE staining in the striatum from the ICH group; g HE staining in the striatum from the ICH+ metformin group; h neuron quantity in the striatum of all groups detected using the TUNEL assay and HE staining. Values are expressed as the mean ± SD (n = 7). Asterisk P < 0.05 relative to the sham group. Hash P < 0.05 relative to the ICH group
Effects of Metformin on p-JNK3, p-c-Jun Levels Following ICH
Previous studies have indicated that ICH activated the JNK signaling pathway and eventually increased JNK phosphorylation [11]. To elucidate the possible mechanism of the protective function of metformin, we examined the levels of phosphorylated JNK3 and phosphorylated c-Jun using western blotting. In our study, we observed that ICH caused a significant increase in p-JNK levels compared to those observed in the sham group (P < 0.05). Metformin reduced phosphorylation of JNK3 induced by ICH (P < 0.05). There was no significant difference in the expression of JNK3 in the striatum between any of the groups (Fig. 3a, b). Similarly, we observed that p-c-Jun levels were also increased following ICH, while metformin treatment reduced the p-c-Jun levels (p < 0.05). The expression of c-Jun in striatum showed no significant difference in any of the groups (p > 0.05, Fig. 3c, d).
Effects of metformin on apoptotic factors following ICH. a Protein expression of p-JNK3, JNK3 and β-actin. b Intensity of the bands is presented and normalized to β-actin corresponding to p-JNK3, JNK3 in the sham, ICH and ICH+metformin groups. c Protein expression of p-c-Jun, c-Jun and β-actin. d Intensity of the bands is presented and normalized to β-actin corresponding to p-c-Jun, c-Jun in the sham, ICH and ICH+metformin groups. e Protein expression of cleaved caspase-3, caspase-3 and β-actin. f Intensity of the bands is presented and normalized to β-actin corresponding to cleaved caspase-3, caspase-3 in the sham, ICH and ICH+metformin groups. Data are presented as the mean ± SD (n = 7). Asterisk P < 0.05 relative to the sham group. Hash P < 0.05 relative to the ICH group
Effects of Metformin on Cleaved Caspase-3 Levels Following ICH
Cleaved caspase-3, which acts as a lethal protease during the final phase of the apoptotic pathway, is an active form of caspase-3 and is also a downstream protein of the JNK signaling pathway [15]. To evaluate the effects of metformin on cell apoptosis, we examined the cleaved caspase-3 levels. Our data suggested that ICH caused a significant increase in the cleaved caspase-3 levels compared to those observed in the sham group (P < 0.05). Metformin reduced the cleaved caspase-3 levels induced by ICH (P < 0.05). The expression of caspase-3 in the striatum showed no significant difference in any of the groups (P > 0.05, Fig. 3e, f).
Effects of Metformin on Pro-Inflammatory Cytokines Following ICH
Finally, we measured the anti-inflammatory effect of metformin to further investigate its effects following ICH. Compared to the sham group, the ICH group demonstrated remarkably increased expression levels of IL-1β, IL-4 and IL-6 and TNF-α, as analyzed using ELISA and western blotting. In addition, treatment with metformin significantly inhibited increases in the protein levels of IL-1β, IL-4 and IL-6 and TNF-α induced by ICH (P > 0.05, Fig. 4).
Anti-inflammatory effects of metformin following ICH. a ELISA for IL-Iβ. b ELISA for IL-4. c ELISA for IL-6. d ELISA for TNF-α. e Protein levels of IL-Iβ and β-actin and the intensity of the bands normalized to β-actin in the sham, ICH and ICH+metformin groups. f Protein levels of IL-4 and β-actin and the intensity of the bands normalized to β-actin in the sham, ICH and ICH+metformin groups. g Protein levels of IL-6 and β-actin and the intensity of the bands normalized to β-actin in the sham, ICH and ICH+metformin groups. h Protein levels of TNF-α and β-actin and the intensity of the bands normalized to β-actin in the sham, ICH and ICH+metformin groups. Data are presented as the mean ± SD (n = 7). Asterisk P < 0.05 relative to the sham group. Hash P < 0.05 relative to the ICH group
Discussion
Intracerebral hemorrhage can result in an extensive range of neurological impairments through oxidative stress, neuronal apoptosis, and so on, which can further cause behavioral deficits [16, 30]. In our study, we observed that metformin exerted protective effects by reversing neuronal apoptosis in the striatum and reducing the oxidative stress induced by ICH.
In the present study, we investigated a clinically used drug, metformin, which is known to exert neuroprotective effects in treating ischemia. A series of studies suggested that metformin had antioxidant activities as well as an anti-inflammatory effect. It has been reported that metformin has a protective effect against ischemia/reperfusion injury in the testis by reducing oxidative stress (Asghari et al. [2, 3]). Other studies have provided evidence that metformin administration following stroke exerts beneficial effects on long-term post-stroke recovery through AMPK activation [13]. Pretreatment with metformin demonstrated its neuroprotective function in cerebral ischemia/reperfusion injury, which involves AMPK reduction [8]. However, there is no relevant literature to suggest whether metformin can be used to treat ICH. In our study, we observed that administration of metformin for 7 consecutive days following ICH had a neuroprotective effect.
Previous studies have reported that breakdown of ROS and the antioxidant system balance is a contributor to ICH damage. Excessive ROS derived from metabolic products causes oxidative stress injury [14, 24], which further lead to aggravated brain injury. In addition, proinflammatory cytokines were secreted to interact with peripheral cells, which triggered neuron damage in brain. To elucidate the underlying mechanisms of the neuroprotective effects of metformin, we first examined the effect of metformin as an antioxidant. In our study, we observed that metformin remarkably increased the SOD activities and reduced the levels of MDA and 8-iso-PGF2α induced by ICH. Therefore, our results suggested that metformin demonstrated an antioxidant effect and decreased the brain water content. Next, the levels of IL-4, IL-6, IL-1β and TNF-α were examined. ICH dramatically increased the proinfammatory cytokines levels and metformin treatment significantly alleviated the production of those cytokines. Thus, metformin could exert neuroprotective effect through anti-oxidative effect and anti-inflammatory effect.
As hypertensive ICH often occurs in the human striatum [27], leading to apoptosis, we further investigated the neuron quantity in the striatum. We performed TUNEL staining and immunohistochemistry to check whether metformin protected neurons against apoptosis in the striatum. Our results suggested that metformin reversed apoptosis induced by ICH in the striatum. Because ROS contributes to stroke-induced apoptosis through multiple pathways, especially the JNK signaling pathway, and the JNK signaling pathway has been demonstrated to be involved in ICH-induced brain damage, we proposed that metformin prevented activation of the JNK signaling pathway, which further resulted in neuronal protection in the striatum. Interestingly, our results demonstrated that metformin reduced the levels of p-JNK, p-c-Jun and cleaved caspase-3 in the striatum. Therefore, we concluded that metformin remarkably inhibited activation of the JNK pathway following ICH.
In summary, metformin alleviates neurological impairments in ICH rats, and this protection is accomplished by a reduction of oxidative stress in the brain and protection of striatal neurons against apoptosis by the JNK signaling pathway. Therefore, metformin has great future potential in treating intracerebral hemorrhage.
References
Abd-Elsameea AA, Moustaf AA, Mohamed AM (2014) Modulation of the oxidative stress by metformin in the cerebrum of rats exposed to global cerebral ischemia and ischemia/reperfusion. Eur Rev Med Pharmacol Sci 18(16):2387–2392
Asghari A, Akbari G, Meghdadi A, Mortazavi P (2016) Effects of melatonin and metformin co-administration on testicular ischemia/reperfusion injury in rats. J Pediatr Urol 12:410
Asghari A, Akbari G, Meghdadi A, Mortazavi P (2016) Protective effect of metformin on testicular ischemia/reperfusion injury in rats. Acta Cir Bras 31(6):411–416
Cahova M, Palenickova E, Dankova H, Sticova E, Burian M, Drahota Z, Cervinkova Z, Kucera O, Gladkova C, Stopka P, Krizova J, Papackova Z, Oliyarnyk O, Kazdova L (2015) Metformin prevents ischemia reperfusion-induced oxidative stress in the fatty liver by attenuation of reactive oxygen species formation. Am J Physiol Gastrointest Liver Physiol 309(2):G100–G111
Cui GY, Gao XM, Qi SH, Gillani A, Gao L, Shen X, Zhang YD (2011) The action of thrombin in intracerebral hemorrhage induced brain damage is mediated via PKCalpha/PKCdelta signaling. Brain Res 1398:86–93
Davies KJ (1995) Oxidative stress: the paradox of aerobic life. Biochem Soc Symp 61:1–31
de Oliveira Santana KN, Lelis DF, Mendes KL, Lula JF, Paraiso AF, Andrade JM, Feltenberger JD, Cota J, da Costa DV, de Paula AM, Guimaraes AL, Santos SH (2016) Metformin reduces lipogenesis markers in obese mice fed a low-carbohydrate and high-fat diet. Lipids 51(12):1375–1384
Deng T, Zheng YR, Hou WW, Yuan Y, Shen Z, Wu XL, Chen Y, Zhang LS, Hu WW, Chen Z, Zhang XN (2016) Pre-stroke Metformin Treatment is Neuroprotective Involving AMPK Reduction. Neurochem Res 41(10):2719–2727
Dong YS, Wang JL, Feng DY, Qin HZ, Wen H, Yin ZM, Gao GD, Li C (2014) Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int J Med Sci 11(3):282–290
Du Q, Yu WH, Dong XQ, Yang DB, Shen YF, Wang H, Jiang L, Du YF, Zhang ZY, Zhu Q, Che ZH, Liu QJ (2014) Plasma 8-iso-Prostaglandin F2alpha concentrations and outcomes after acute intracerebral hemorrhage. Clin Chim Acta 437:141–146
Duan ZZ, Zhou XL, Li YH, Zhang F, Li FY, Su-Hua Q (2015) Protection of Momordica charantia polysaccharide against intracerebral hemorrhage-induced brain injury through JNK3 signaling pathway. J Recept Signal Transduct Res 35(6):523–529
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634 (discussion 635)
Jia J, Cheng J, Ni J, Zhen X (2015) Neuropharmacological actions of metformin in stroke. Curr Neuropharmacol 13(3):389–394
Ke K, Li L, Rui Y, Zheng H, Tan X, Xu W, Cao J, Xu J, Cui G, Xu G, Cao M (2013) Increased expression of small heat shock protein alphaB-crystallin after intracerebral hemorrhage in adult rats. J Mol Neurosci 51(1):159–169
Kobayashi T, Masumoto J, Tada T, Nomiyama T, Hongo K, Nakayama J (2007) Prognostic significance of the immunohistochemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin Cancer Res 13(13):3868–3874
Lu H, Shen J, Song X, Ge J, Cai R, Dai A, Jiang Z (2015) Protective effect of pyrroloquinoline quinone (PQQ) in rat model of intracerebral hemorrhage. Cell Mol Neurobiol 35(7):921–930
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75(2):209–219
Michel-Monigadon D, Bonny C, Hirt L (2010) c-Jun N-terminal kinase pathway inhibition in intracerebral hemorrhage. Cerebrovasc Dis 29(6):564–570
Pei B, Yang M, Qi X, Shen X, Chen X, Zhang F (2016) Quercetin ameliorates ischemia/reperfusion-induced cognitive deficits by inhibiting ASK1/JNK3/caspase-3 by enhancing the Akt signaling pathway. Biochem Biophys Res Commun 478(1):199–205
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24(5):981–990
Shah SA, Lee HY, Bressan RA, Yun DJ, Kim MO (2014) Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis 5:e1026
Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M, European Stroke Organisation (2014) European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 9(7):840–855
Taylor RA, Chang CF, Goods BA, Hammond MD, Grory BM, Ai Y, Steinschneider AF, Renfroe SC, Askenase MH, McCullough LD, Kasner SE, Mullen MT, Hafler DA, Love JC, Sansing LH (2016) TGF-beta1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J Clin Invest 127:280
Taylor RA, Sansing LH (2013) Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol 2013:746068
Wan S, Zhan R, Zheng S, Hua Y, Xi G (2009) Activation of c-Jun-N-terminal kinase in a rat model of intracerebral hemorrhage: the role of iron. Neurosci Res 63(2):100–105
Wiswedel I (2009) F(2)-isoprostanes: sensitive biomarkers of oxidative stress in vitro and in vivo: a gas chromatography-mass spectrometric approach. Methods Mol Biol 580:3–16
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5(1):53–63
Xu L, Li Y, Fu Q, Ma S (2014) Perillaldehyde attenuates cerebral ischemia-reperfusion injury-triggered overexpression of inflammatory cytokines via modulating Akt/JNK pathway in the rat brain cortex. Biochem Biophys Res Commun 454(1):65–70
Xu R, Wang S, Li W, Liu Z, Tang J, Tang X (2016) Activation of peroxisome proliferator-activated receptor-gamma by a 12/15-lipoxygenase product of arachidonic acid: a possible neuroprotective effect in the brain after experimental intracerebral hemorrhage. J Neurosurg 14:1–10
Yang Y, Chen S, Zhang J (2016) The updated role of oxidative stress in subarachnoid hemorrhage. Curr Drug Deliv [Epub ahead of print]
Yang Z, Yu A, Liu Y, Shen H, Lin C, Lin L, Wang S, Yuan B (2014) Regulatory T cells inhibit microglia activation and protect against inflammatory injury in intracerebral hemorrhage. Int Immunopharmacol 22(2):522–525
Acknowledgements
This work was supported by the bureau of Xuzhou city science and technology (KC15SH037).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Qi, B., Hu, L., Zhu, L. et al. Metformin Attenuates Neurological Deficit after Intracerebral Hemorrhage by Inhibiting Apoptosis, Oxidative Stress and Neuroinflammation in Rats. Neurochem Res 42, 2912–2920 (2017). https://doi.org/10.1007/s11064-017-2322-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2322-9