Abstract
3-Methylglutaric acid (3MGA) is an organic acid that accumulates in various organic acidemias whose patients present neurodegeneration events in children coursing with metabolic acidurias. Limited evidence describes the toxic mechanisms elicited by 3MGA in the brain. Herein, we explored the effects of 3MGA on different toxic endpoints in synaptosomal and mitochondrial-enriched fractions of adult rat brains to provide novel information on early mechanisms evoked by this metabolite. At 1 and 5 mM concentration, 3MGA increased lipid peroxidation, but decreased mitochondrial function only at 5 mM concentration. Despite less intense effects were obtained at 1 mM concentration, its co-administration with the kynurenine pathway (KP) metabolite and N-methyl-d-aspartate receptor (NMDAr) agonist, quinolinic acid (QUIN, 50 and 100 µM), produced toxic synergism on markers of oxidative stress and mitochondrial function. The toxicity of 3MGA per se (5 mM) was prevented by the cannabinoid receptor agonist WIN55,212-2 and the NMDAr antagonist kynurenic acid (KYNA), suggesting cannabinoid and glutamatergic components in the 3MGA pattern of toxicity. The synergic model (3MGA + QUIN) was also sensitive to KYNA and the antioxidant S-allylcysteine, but not to the nitric oxide synthase inhibitor l-nitroarginine methyl ester. These findings suggest various underlying mechanisms involved in the neurotoxicity of 3MGA that may possibly contribute to the neurodegeneration observed in acidemias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inborn errors of amino acid and lipid metabolisms have gained focus in the framework of global research. These recessive hereditary diseases are biochemically characterized by tissue accumulation of carboxylic acids due to deficiency in the activity of specific enzymes during the catabolism of these products. The organic acidemias are the main diseases characterized by elevated levels of these metabolites. In particular, 3-hydroxy-3-methyl glutaric (3HMG) acidemia is caused by a deficiency of the activity of 3-hydroxy-3-methylglutaryl CoA lyase (HL), a key enzyme in the metabolism of leucine and ketogenesis. In this disease there is a large accumulation of organic acids, including 3-hydroxy-3-methylglutaric, 3-metylglutaconic, 3-hydroxy-isovaleric and 3-methylglutaric (3MGA) acids. The accumulation of 3MGA is derived from the hydrolysis of 3-hydroxy-3-methylglutaryl CoA. Since 3MGA is a metabolite derived from leucine catabolism, people affected by 3HMG acidemia do not completely metabolize the carbon chain of leucine, and therefore do not produce ketone bodies in response to a long period of fasting [1].
Among the clinical manifestations, patients affected by 3HMG academia exhibit metabolic acidosis with hypoketotic hypoglycemia, hypotonia, lethargy, convulsions and coma [2, 3]. Hepatomegaly and elevated plasma transaminase levels are also commonly seen in the patients, especially during metabolic crises triggered by situations of excessive catabolism [2]. The magnetic resonance findings highlight lesions in cerebral white matter, in addition to damage in caudate nucleus, dentate gyrus, globus pallidus and corticospinal tract [4, 5]. It is believed that the hypoglycemia associated with reduced production of ketone bodies reduces the energy supply of the brain, thus explaining the neurological changes characteristic of this disease. Furthermore, the hypoglycemia may indirectly provoke the generation of reactive species due to the activation of glutamate receptors (secondary excitotoxicity), leading to increased Ca2+ influx [6, 7].
3MGA concentrations are also increased in tissues from patients affected by the inherited group of disorders known as 3-methylglutaconic aciduria (MGTA). The so-called classical MGTA is a leucine catabolism disorder caused by deficiency of 3-methylglutaconyl-CoA hydratase, which leads to the accumulation of 3-methylglutaconic acid and of its hydrogenated derivative 3MGA. The other five subtypes described so far (Barth syndrome, MEGDEL syndrome, Costeff syndrome, DNAJC19 defect and TMEM70 defect) are considered secondary MGTA once the biochemical deffects in these disorders remain unknown [8, 9]. However, mitochondrial dysfunction is thought to be a common characteristic of all types of MGTA [10]. Clinical features of the primary MGTA are nonspecific and variable, ranging from minimal to severe symptoms and brain abnormalities that include delayed speech, dementia, progressive leukoencephalopathy and ataxia [10, 11]. Psychomotor retardation, microcephaly and progressive neurological impairment with spasticity and seizures have also been reported in several patients [12, 13].
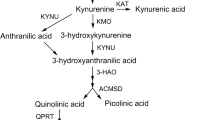
Another neurotoxic metabolite is quinolinic acid (QUIN), a byproduct of tryptophan metabolism produced through the kynurenine pathway (KP) that exerts a role as an endogenous glutamate agonist acting on specific populations of N-methyl-d-aspartate (NMDA) receptors (NMDAr) [14], as well as on other glutamatergic parameters [15]. QUIN could share similar toxic mechanisms as those of 3MGA and other organic acids accumulated in organic acidurias, as described recently by our group [16]. In fact, the combination of this excitotoxin with other toxic metabolites -including glutaric acid (GA), 3-hydroxyglutaric acid (3OHGA), methylmalonic acid (MMA) and propionic acid (PA) at subtoxic concentrations, enhanced the oxidative damage and mitochondrial dysfunction in rat brain synaptic terminals.
On the other hand, we have recently shown that modulation of the endocannabinoid system (ECS) by cannabinoid receptor (CBr) agonists can be beneficial against some harmful actions of these toxic organic acids related to organic acidemias [17]. Thus, the aim of this study was to investigate the effects of 3MGA on different endpoints of toxicity (mitochondrial dysfunction and lipid peroxidation) in isolated synaptic terminals from the rat brain. We also explored a possible synergism between 3MGA and QUIN, as well as the involvement of the ECS and the kynurenine systems in the effects of these metabolites in order to provide further information on the toxic mechanisms elicited by 3MGA in the brain.
Experimental Procedures
Chemicals
HEPES, thiobarbituric acid (TBA), malondialdehyde (MDA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), sucrose, the CBr agonist WIN55,212-2, the NMDAr antagonist and KP metabolite kynurenic acid (KYNA), the well-known antioxidant S-allylcysteine (SAC), the nitric oxide synthase (NOS) inhibitor l-nitroarginine methyl ester (l-NAME), and 3MGA were obtained from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). All other chemicals were obtained from other well-known commercial sources.
Animals
We used adult male Wistar rats (250–300 g) for the collection of synaptosomal and mitochondrial-enriched fractions. Animals (N = 20) were obtained from the vivarium of the Universidad Nacional Autónoma de México, as a donation. Rats were housed in separate groups at a controlled room temperature (25 ± 3 °C) and humidity (60–80 %), and under a 12–12 h light–dark cycle (lights on at 07:00 a.m.). Food and water were available ad libitum in the housing rooms at the vivarium of the Instituto Nacional de Neurología y Neurocirugía. The experimental manipulations were performed according to the “Guidelines for the Use of Animals in Neuroscience Research” from the Society of Neuroscience, the local Ethical Committees, and in compliance of the ARRIVE guidelines.
Isolation of Brain Synaptosomes
Synaptosomal and mitochondrial-enriched P2 fractions were isolated from rat brains according to the method described by Rangel-López et al. [18]. Aliquots were separated and immediately employed for the experiments. The isolated synaptosomal and mitochondrial-enriched fractions were incubated in the presence of the CBr agonist WIN55,212-2 (1 µM) for 30 min and/or 3MGA (1 or 5 mM) for the next 30 min. KYNA (50 µM), SAC (100 µM) and L-NAME (100 µM) were also used in some experiments to test the involvement of NMDAr, the role of oxidative stress and the participation of nitric oxide (NO) species in the effects elicited by the toxic synergism produced by 3MGA (1 mM) + QUIN (50 or 100 µM). KYNA, SAC and L-NAME were also added to synaptosomes 30 min before the addition of the toxic agents. The same incubation conditions were used throughout the study for all markers. The quantification of total protein content in synaptosomal and mitochondrial-enriched fractions was estimated according to the Bradford’s method [19].
Lipid Peroxidation Assay
The lipid peroxidation assay was carried out in synaptosomal and mitochondrial-enriched fractions by the formation of thiobarbituric acid-reactive substances (TBARS), according to a previous report [16]. Two-hundred µL-homogenates were obtained from the synaptosomal and mitochondrial-enriched fractions and further added to 500 µL of the TBA reagent (0.75 g of TBA + 15 g of trichloroacetic acid + 2.54 mL of HCl). Samples were incubated for 30 min at 100 °C. The pink chromophore produced was indicative of the amount of peroxidized lipids. Samples were kept on ice for 5 min to be further centrifuged at 3000×g for 15 min. The optical density of the supernatants was estimated in a CYT3MV Biotek Cytation 3 Imaging Reader at 532 nm. Simultaneous to the formation of TBARS, we analyzed a standard curve with tetramethoxypropane as an index of MDA production that was calculated by interpolation of values. Final results were calculated as nmol of MDA per mg protein and expressed as percent of lipid peroxidation vs. control.
Reactive Oxygen Species (ROS) Formation Assay
Reactive oxygen species (ROS) formation in synaptosomal and mitochondrial-enriched fractions was measured according to a previous report [18]. Briefly, the synaptosomal and mitochondrial-enriched fractions were diluted in nine volumes of 40 mM Tris plus HEPES buffer. Samples were incubated with 5 μM 2′,7′-dichlorofluorescein diacetate (DCFH-DA) for 60 min at 37 °C. The fluorescent signals were recorded at 488 nm of excitation and 525 nm of emission wavelengths in a CYT3MV Biotek Cytation 3 Imaging Reader. Results were expressed as as μmol of 2′,7′-dichlorofluorescein (DCFH) oxidized per g wet tissue.
Mitochondrial Functional Assessment by the MTT Reduction Assay
To assess the functional status of the respiratory chain and mitochondrial function, we performed the MTT reduction assay in rat brain synaptosomes, according to a method previously described by [16]. A CYT3MV Biotek Cytation 3 Imaging Reader was used to estimate the content of formazan at a 570 nm wavelength. The results were expressed as the percentage of MTT reduction vs. control values.
Statistical Analysis
Results are expressed as mean values ± SEM Data were statistically analyzed by one- or two-way analysis of variance (ANOVA) for repeated measures, followed by post hoc Duncan’s test. Values of P < 0.05 were considered of statistical significance. Analytical procedures were performed using the scientific statistic software GraphPad Prism five (GraphPad Scientific, San Diego, CA, USA).
Results
3MGA Increased Lipid Peroxidation in Brain Synaptosomes: Prevention by WIN55,212-2 Pretreatment
Lipid peroxidation was estimated by the TBA reagent as a marker of oxidative damage to lipids (Fig. 1). 3MGA (1 and 5 mM) induced lipid peroxidation in synaptosomal and mitochondrial-enriched fractions (23 and 31 % above the control, respectively; P < 0.05). The pretreatment for 30 min with WIN55,212-2 (1 µM) reduced the 3MGA-induced lipid peroxidation at 1 mM (11 % below 3MGA; P < 0.05), but not at 5 mM concentration. WIN55,212-2 per se produced no changes in oxidative damage to lipids.
Effects of 3-methylglutaric acid (3MGA, 1 and 5 mM) and/or WIN55,212-2 (1 µM) on lipid peroxidation (TBARS levels) in rat brain synaptosomal and mitochondrial-enriched fractions. Data are expressed as mean values ± SEM of n = 6–8 experiments per group. Symbols denote statistical differences vs. control (aP < 0.05) and against 3MGA(1) treatment (bP < 0.05). Two-way ANOVA followed by Duncan’s test
3MGA-Induced Mitochondrial Dysfunction in Brain Synaptosomes: Prevention by WIN55,212-2 Pretreatment
Mitochondrial function was estimated by the MTT assay (Fig. 2). The organic acid 3MGA induced mitochondrial dysfunction evidenced at 5 mM concentration (23 % below the control; P < 0.05), but not at 1 mM. The pre-incubation of synaptosomes with 1 µM WIN55,212-2 for 30 min prevented the 3MGA-induced mitochondrial dysfunction (32 % above 3MGA; P < 0.05). Per se, WIN55,212-2 produced no effect on mitochondrial function.
WIN55,212-2 (1 µM) prevented the 3-methylglutaric acid (3MGA, 1 and 5 mM)-induced changes in mitochondrial function (MTT reduction) in brain synaptosomal and mitochondrial-enriched fractions. Data are expressed as mean values ± SEM of n = 6–8 experiments per group. Symbols denote statistical differences vs. control (aP < 0.05) and against 3MGA (bP < 0.05). Two-way ANOVA followed by Duncan’s test
3MGA Exhibited a Synergism with QUIN in Mitochondrial Dysfunction in Brain Synaptosomes
In order to test whether 3MGA and QUIN may exert synergic toxic actions when added together to incubated synaptosomal and mitochondrial-enriched fraction, we first challenged this preparation with a toxic concentration of QUIN (100 µM) plus a non-toxic concentration of 3MGA (1 mM). As expected, when testing the effect of QUIN at a toxic concentration on mitochondrial function (Fig. 3), this metabolite caused per se some level of mitochondrial dysfunction (18 % below the control; P < 0.05). In contrast, 3MGA per se at 1 mM concentration did not significantly change mitochondrial function. However, when synaptosomes were co-incubated with QUIN and 3MGA, the degree of mitochondrial dysfunction was synergistically enhanced (39 and 47 % below QUIN and 3MGA per se, respectively; P < 0.05). In further experiments (Fig. 5), we investigated whether an apparently subtoxic concentration of QUIN (50 µM) was able to evoke synergism with 3MGA.
Toxic synergism between 3MGA (1 mM) and quinolinic acid (QUIN, 100 µM) in mitochondrial function (MTT reduction) in rat brain synaptosomes. Data are expressed as mean values ± SEM of n = 6–8 experiments per group. Symbols denote statistical differences vs. control (aP < 0.05) and against the QUIN (bP < 0.05) or 3MGA (cP < 0.05) treatments. Two-way ANOVA followed by Duncan’s test
3MGA-Induced Mitochondrial Dysfunction was Partially Prevented by Kynurenic Acid (KYNA) in Brain Synaptosomes
When 3MGA was administered at a toxic concentration to synaptosomes and mitochondrial-enriched (5 mM, Fig. 4), it significantly decreased the mitochondrial function (23 % below the control; P < 0.05). In contrast, pretreatment of synaptosomal fractions with the NMDAr antagonist KYNA (50 µM) for 30 min prevented the 3MGA-induced mitochondrial dysfunction (22 % above 3MGA and 5 % below the control; P < 0.05). KYNA per se did not modify mitochondrial function (2 % above the control).
Kynurenic acid (KYNA, 50 µM) partially prevented the changes in mitochondrial function (MTT reduction) caused by 3MGA in rat brain synaptosomes. Data are expressed as mean values ± SEM of n = 6–8 experiments per group. Symbols denote statistical differences vs. control (aP < 0.05) and against the 3MGA treatment (bP < 0.05). Two-way ANOVA followed by Duncan’s test
The Toxic Synergism Induced by 3MGA and QUIN was Prevented at Variable Degrees by KYNA, the Antioxidant SAC and the NOS Inhibitor L-NAME
Lipid peroxidation (Fig. 5a), ROS formation (Fig. 5b) and MTT reduction (Fig. 5c) were estimated as endpoints of the toxicity exerted by the synergic action of 3MGA and QUIN (50 µM) in synaptosomal and mitochondrial-enriched fractions. The effects of the NMDAr antagonist KYNA, the antioxidant and radical scavenger SAC, and the NOS inhibitor L-NAME were explored throughout these indicators.
The toxic synergism between 3MGA (1 mM) and quinolinic acid (QUIN, 50 uM) is prevented at different levels by KYNA kynurenic acid, SAC S-allylcysteine and L-NAME L-nitroarginine methyl ester. a–c Lipid peroxidation, ROS reactive oxygen species formation and mitochondrial function, respectively. Data are expressed as mean values ± SEM of n = 6–8 experiments per group. Symbols denote statistical differences vs. control (aP < 0.05) and against the 3MGA + QUIN treatment (bP < 0.05). One-way ANOVA followed by Duncan’s test
Figure 5a shows that lipid peroxidation was increased by 3MGA (16 % above the control) and the combination of 3MGA and QUIN (26 % above the control; P < 0.05), but not by QUIN per se. KYNA and SAC attenuated the oxidative damage to lipids induced by 3-MGA plus QUIN by 11 % (P < 0.05) and 20 % (P < 0.05), respectively. L-NAME had no effect on the 3MGA + QUIN-induced oxidative damage (3 % below). None of the tested drugs with potential protective actions (KYNA, SAC or L-NAME) had effect per se on lipid peroxidation.
In Fig. 5b, ROS formation was increased by the combination of 3MGA and QUIN (26 % above the control), but not by 3MGA or QUIN per se. KYNA and L-NAME did not decreased ROS formation induced by 3-MGA + QUIN in a significant manner, in contrast to SAC that reduced this endpoint not only as compared with the 3-MGA + QUIN treatment (53 % below; P < 0.05), but also as compared with the control (29 % below; P < 0.05). Finally, neither KYNA, nor L-NAME, had any effect per se on ROS formation.
Figure 5c demonstrates that MTT reduction was decreased by the combination of 3MGA and QUIN (41 % below the control), but not by 3MGA or QUIN per se. KYNA, SAC and L-NAME prevented in a similar manner the 3MGA + QUIN-induced mitochondrial dysfunction (36, 42 and 39 % above the 3MGA + QUIN treatment, respectively; P < 0.05). Finally, none of the tested drugs with potential protective actions (KYNA, SAC or L-NAME) had effect per se on mitochondrial function.
Discussion
In this study we have collected evidence supporting the concept that different and synergic noxious mechanisms could be part of the toxic pattern elicited by 3MGA in the brain. Through simple experimental approaches applied to synaptosomal and mitochondrial-enriched fractions, it was demonstrated that 3MGA exerts toxicity through the early activation of mechanism like oxidative stress (ROS formation and lipid peroxidation) and mitochondrial dysfunction (MTT reduction). Toward these endpoints, a cannabinoid-sensitive component was revealed by the synthetic CBr agonist WIN55,212-2, since this compound prevented some of the effects exerted by 3MGA. In addition, a kynurenine component further indicating a glutamatergic component was revealed by the synergic action exerted by 3MGA and the excitotoxin QUIN on the toxic markers, as well as the preventive actions of the NMDAr antagonist KYNA on the 3MGA-induced toxicity. The active participation of the oxidative component in the synergic model produced by the concerted actions of 3MGA plus QUIN was confirmed through the use of the potent antioxidant SAC. This evidence allows us to suggest that the mechanisms involved in the toxic effects of 3MGA are mediated at least in part by NMDAr-mediated excitotoxicity, which leads secondarily to high intracellular calcium influx and a potential increase in reactive oxygen and nitrogen species generation. Altogether, the occurrence of these mechanisms as part of the early toxic events elicited by 3MGA suggest their possible occurrence during the progression of the 3HMG and the 3-methylglutaconic acidemias in children as potential triggering factors for the neurodegeneration. Nonetheless, this hypothesis requires further demonstration at both the experimental and clinical levels. Of note, the protective effect of the NOS inhibitor L-NAME exerted only on 3MGA + QUIN-induced mitochondrial dysfunction suggests that a nitrosative component is only partially involved in this toxic model. Sensing nitrosative stress is important in toxic paradigms to evidence a role of NO in the toxic mechanisms elicited by different insults.
The toxic model induced by 3MGA and used in the present work was previously validated in synaptosomes by Ribeiro et al. [20]. In this study, using synaptosomes of cerebral cortex from young rats, the authors found reduced mitochondrial function, increased ROS formation and inhibition of the Na+,K+-ATPase enzyme activity, but no changes in lipid peroxidation were observed. Thus, our present study represents a logic extension of the one from Ribeiro et al. [20] and confirms part of the effects previously described for the toxic pattern of 3MGA. Interestingly, in contrast to that study we found changes in the levels of TBARS at concentrations of 1 and 5 mM. The observed differences could obey to a differential susceptibility of the synaptosomal and mitochondrial-enriched fractions employed in these two reports: once again, Ribeiro et al. [20] used cortical synaptosomes from young rats whereas we tested synaptosomes obtained from the whole brain of adult rats, which in turn and by unknown reasons, could be more vulnerable to oxidative damage of lipids. In any case, reproducibility of the toxic model was achieved in our present report, thus validating it for further studies. In addition, we found that 3MGA-induced oxidative damage and mitochondrial dysfunction were correlated only at the highest concentration tested of the metabolite (5 mM). Interestingly, there was a clear correlation between oxidative damage and mitochondrial dysfunction induced by 3MGA at 1 mM concentration when this metabolite was co-incubated with QUIN, suggesting that endogenous agents could act synergically to potentiate cell damage if pathological conditions prevail.
Evidence in the literature suggests that the inhibition of Na+,K+-ATPase is involved in deleterious effects leading to neuronal dysfunction and neurodegeneration in many pathological conditions [20–22]. Although in this study we did not test the activity of this enzyme, we can assume that the same effect is occurring in synaptosomes of adult rats.
During the progression of acidemias, persistent accumulation of toxic organic acids takes place, and these metabolites—especially dicarboxylic acids, such as 3MGA- accumulate more in the brain than in serum due to the trapping of these metabolites by neural cells, because of their limited flux across the blood–brain barrier [23, 24]. Therefore, since 3MGA and QUIN are dicarboxylic acids, the toxic synergism created by these two metabolites could be partially explained through their tendency to accumulate in neuronal cells with the subsequent intensification of cell damage.
Noteworthy, in recent reports, and in further support to this study, we have characterized a cannabinoid component involved in the in vitro toxic events elicited by other toxic organic acids, including glutaric acid, 3-hydroxyglutaric acid, MMA and PA, that are found at higher concentrations in the corresponding organic acidurias [17]. In such report, we collected evidence demonstrating that the synthetic cannabinoid WIN can attenuate the toxic effects caused by the accumulation of these carboxylic acids. In addition, in another study of our group [16], we have demonstrated a toxic synergism occurring between some of these organic acids and QUIN, supporting a glutamatergic component in these novel toxic models. The cannabinoid component has also been characterized in the toxic model provoked by QUIN per se [18]. Altogether, these studies clearly suggest that excitotoxicity, oxidative damage and mitochondrial dysfunction could underlie the toxicity of 3MGA and its related carboxylic acids during the progression of organic acidurias in children leading to neurodegeneration. Furthermore, it was here demonstrated that these events could be attenuated though the pharmacological manipulation of the KP and the ECS.
Conclusion
The findings described herein support an active role of excitotoxicity, oxidative damage and mitochondrial dysfunction as part of the toxic pattern elicited by 3MGA and prompt more detailed investigation on the precise role of the ECS on the NMDA-mediated 3MGA-induced neurotoxicity.
References
Wysocki SJ, Hähnel R (1986) 3-Hydroxy-3-methylglutaryl-coenzyme a lyase deficiency: a review. J Inherit Metab Dis 9:225–233
Gibson KM, Breuer J, Nyhan WL (1988) 3-Hydroxy-3-methylglutarylcoenzyme a lyase deficiency: review of 18 reported patients. Eur J Pediatr 148:180–186
Leipnitz G, Vargas CR, Wajner M (2015) Disturbance of redox homeostasis as a contributing underlying pathomechanism of brain and liver alterations in 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. J Inherit Metab Dis 38:1021–1028
Yylmaz Y, Ozdemir N, Ekinci G, Baykal T, Kocaman C (2006) Corticoespinal tract involvement in a patient with 3-HMG coenzyme a lyase deficiency. Pediatr Neurol 35:139–141
Zaifeiriou DI, Vargiami E, Mayapetek E, Augoustidou-Savvopoulou P, Mitchell GA (2007) 3-Hydroxy-3-methylglutaryl coenzyme a lyase deficiency with reversible white matter changes after treatment. Pediatr Neurol 37:47–50
Singh P, Jain A, Kaur G (2004) Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 260:153–159
Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA (2007) Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADH oxidase. J Clin Invest 117:910–918
Wortmann SB, Kluijtmans LA, Engelke UFH, Wevers RA, Morava E (2012) The 3-methylglutaconic acidurias: what’s new? J Inherit Metab Dis 35:13–22
Wortmann SB, Kluijtmans LAJ, Sequeira S, Wevers RA, Morava E (2014) Leucine loading test is only discriminative for 3-methylglutaconic aciduria due to AUH defect. JIMD Rep 16:1–6
Wortmann SB, Duran M, Anikster Y, Barth PG, Sperl W, Zschocke J, Morava E, Wevers RA (2013) Inborn errors of metabolism with 3-methylglutaconic aciduria as discriminative feature: proper classification and nomenclature. J Inherit Metab Dis 36:923–928
Wortmann SB, Kremer BH, Graham A, Willemsen MA, Loupatty FJ, Hogg SL, Engelke UF, Kluijtmans LA, Wanders RJ, Illsinger S, Wilcken B, Cruysberg JR, Das AM, Morava E, Wevers RA (2010) 3-Methylglutaconic aciduria type I redefined: a syndrome with late-onset leukoencephalopathy. Neurology 75:1079–1083
Ijlst L, Loupaty FJ, Ruiter JPN, Duran M, Lehnert W, Wanders RJA (2002) 3-Methylglutaconic aciduria type I is caused by mutations in AUH. Am J Hum Genet 71:1463–1466
Arn P, Funanage VL (2006) 3-methylglutaconic aciduria disorders: the clinical spectrum increases. J Pediatr Hematol Oncol 28:62–63
Stone TW, Mackay GM, Forrest CM, Clark CJ, Darlington LG (2003) Tryptophan metabolites and brain disorders. Clin Chem Lab Med 41:852–859
Tavares RG, Tasca CI, Santos CE, Alves LB, Porciúncula LO, Emanuelli T, Souza DO (2002) Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int 40:621–627
Colín-González AL, Paz-Loyola AL, Serratos IN, Seminotti B, Ribeiro CAJ, Leipnitz G, Souza DO, Wajner M, Santamaría A (2015) Toxic synergism between quinolinic acid and organic acids accumulating in glutaric academia type I and in disorders of propionate metabolism in rat brain synaptosomes: relevance for metabolic acidemias. Neuroscience 308:64–74
Colín-González AL, Paz-Loyola AL, Serratos IN, Seminotti B, Ribeiro CAJ, Leipnitz G, Souza DO, Wajner M, Santamaría A (2015) The effect of WIN 55,212-2 suggests a cannabinoid-sensitive component in the early toxicity induced by organic acids accumulating in glutaric acidemia type I and in related disorders of propionate metabolism in rat brain synaptosomes. Neuroscience 310:578–588
Rangel-López E, Colín-González AL, Paz-Loyola AL, Pinzón E, Torres I, Serratos IN, Castellanos P, Wajner M, Souza DO, Santamaría A (2015) Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience 285:97–106
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Ribeiro CAJ, Hickmann FH, Wajner M (2011) Neurochemical evidence that 3-methylglutaric acid inhibits synaptic Na+,K+-ATPase activity probably through oxidative damage in brain cortex of young rats. Int J Dev Neurosci 29:1–7
de Freitas RM (2010) Lipoic acid alters delta-aminolevulinic dehydratase, glutathione peroxidase and Na+,K+-ATPase activities and glutathione-reduced levels in rat hippocampus after pilocarpine-induced seizures. Cell Mol Neurobiol 30:381–387
Santos IM, Tome Ada R, Feitosa CM, de Souza GF, Feng D, de Freitas RM, Jordan J (2010) Lipoic acid blocks seizures induced by pilocarpine via increases in delta-aminolevulinic dehydratase and Na+,K+-ATPase activity in rat brain. Pharmacol Biochem Behav 95:88–91
Sauer SW, Okun JG, Fricker G, Mahringer A, Muller I, Crnic LR, Muhlhausen C, Hoffmann GF, Horster F, Goodman SI, Harding CO, Koeller DM, Kolker S (2006) Intracerebral accumulation of glutaric and 3-hydroxyglutaric acids secondary to limited flux across the blood–brain barrier constitute a biochemical risk factor for neurodegeneration in glutaryl-CoA dehydrogenase deficiency. J Neurochem 97:899–910
Stellmer F, Keyser B, Burckhardt BC, Koepsell H, Streichert T, Glatzel M, Jabs S, Thiem J, Herdering W, Koeller DM, Goodman SI, Lukacs Z, Ullrich K, Burckhardt G, Braulke T, Muhlhausen C (2007) 3-Hydroxyglutaric acid is transported via the sodium-dependent dicarboxylate transporter NaDC3. J Mol Med 85:763–770
Acknowledgments
This work was supported by CNPq Grant 404883/2013-3 (M.W., Brazil) and CONACyT Grant 205648 (A.S., Mexico). Ana Laura Colín-González is scholarship holder of CONACyT-Mexico (239954).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Ana Laura Colín-González, Ariana Lizbeth Paz-Loyola, María Eduarda de Lima, Bianca Seminotti and César Augusto João Ribeiro have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Colín-González, A.L., Paz-Loyola, A.L., de Lima, M.E. et al. Experimental Evidence that 3-Methylglutaric Acid Disturbs Mitochondrial Function and Induced Oxidative Stress in Rat Brain Synaptosomes: New Converging Mechanisms. Neurochem Res 41, 2619–2626 (2016). https://doi.org/10.1007/s11064-016-1973-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1973-2