Abstract
Rats with type 2 diabetes exhibit decreased oxidative capacity, such as reduced oxidative enzyme activity, low-intensity staining for oxidative enzymes in fibers, and no high-oxidative type IIA fibers, in the skeletal muscle, especially in the soleus muscle. In contrast, there are no data available concerning the oxidative capacity of spinal motoneurons innervating skeletal muscle of rats with type 2 diabetes. This study examined the oxidative capacity of motoneurons innervating the soleus muscle of non-obese rats with type 2 diabetes. In addition, this study examined the effects of mild hyperbaric oxygen at 1.25 atmospheres absolute with 36 % oxygen for 10 weeks on the oxidative capacity of motoneurons innervating the soleus muscle because mild hyperbaric oxygen improves the decreased oxidative capacity of the soleus muscle in non-obese rats with type 2 diabetes. Spinal motoneurons innervating the soleus muscle were identified using nuclear yellow, a retrograde fluorescent neuronal tracer. Thereafter, the cell body sizes and succinate dehydrogenase activity of identified motoneurons were analyzed. Decreased succinate dehydrogenase activity of small-sized alpha motoneurons innervating the soleus muscle was observed in rats with type 2 diabetes. The decreased succinate dehydrogenase activity of these motoneurons was improved by mild hyperbaric oxygen. Therefore, we concluded that rats with type 2 diabetes have decreased oxidative capacity in motoneurons innervating the soleus muscle and this decreased oxidative capacity is improved by mild hyperbaric oxygen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that non-obese and obese rats with type 2 diabetes exhibit decreased oxidative capacity, such as reduced oxidative enzyme activity, low-intensity staining for oxidative enzymes in fibers, and no high-oxidative type IIA fibers, in the skeletal muscle, especially in the soleus muscle [1, 2]. Skeletal muscle is the primary site of insulin action and glucose metabolism [3]. Reduced oxidative capacity in skeletal muscle impairs glucose metabolism and induces the development and aggravation of type 2 diabetes and its associated complications [4, 5].
The morphological and histochemical properties of fibers in skeletal muscle correspond well with those of motoneurons in the spinal cord [6, 7]. The small-sized and high-oxidative alpha motoneurons innervate high-oxidative fibers, whereas the large-sized and low-oxidative alpha motoneurons innervate low-oxidative fibers in skeletal muscle [7]. We suggest that spinal motoneurons of rats with type 2 diabetes exhibit reduced oxidative capacity because the skeletal muscle, and its fibers innervated by these motoneurons, show decreased oxidative capacity [1, 2]. However, there are no data available for the oxidative capacity of spinal motoneurons in rats with type 2 diabetes. This study determined the oxidative capacity of spinal motoneurons innervating the soleus muscle in non-obese rats with type 2 diabetes.
An elevation in atmospheric pressure accompanied by high oxygen concentration increases blood flow and oxygen, especially dissolved oxygen, in the plasma [8, 9]. An increase in both atmospheric pressure and oxygen concentration enhances oxidative enzyme activity in mitochondria and consequently increases oxidative metabolism in cells and tissues. We have demonstrated that mild hyperbaric oxygen at 1.25 atmospheres absolute (ATA) with 36 % oxygen increased blood flow and dissolved oxygen in the plasma, thereby improving oxidative metabolism [10]. In addition, we found that the increased blood flow and dissolved oxygen induced by mild hyperbaric oxygen were not accompanied by an excessive increase in oxidative stress. We observed that mild hyperbaric oxygen induced a growth-associated increase in neuromuscular oxidative capacity [11] and improved age-associated decrease in muscular oxidative capacity [12]. We observed that mild hyperbaric oxygen inhibited and/or improved lifestyle-related diseases, e.g., type 2 diabetes [13–16], diabetes-induced cataracts [17], and hypertension [18], in rats. In addition, mild hyperbaric oxygen inhibited development of arthritis in rats [19]. A clinical study [20] showed that mild hyperbaric oxygen reversed the increase in melanin pigmentation induced by ultraviolet B irradiation, as well as reduced senile spot size.
We hypothesized that mild hyperbaric oxygen would improve the decreased oxidative capacity of spinal motoneurons as well as skeletal muscles and their fibers [13–16]. This study investigated the effects of mild hyperbaric oxygen on the oxidative capacity of spinal motoneurons innervating the soleus muscle in non-obese rats with type 2 diabetes.
Materials and Methods
Ethical Statement
All experimental procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the Institutional Animal Use Committee at Kyoto University, Kyoto, Japan.
Animal Housing
Eight-week-old Wistar (WR, n = 16) and non-obese diabetic Goto-Kakizaki (GK, n = 24) male rats were used in this study. WR (n = 8) and GK (n = 8) rats were sacrificed at 8 weeks of age. The remaining GK rats were assigned randomly to either the normobaric (GK) or mild hyperbaric oxygen (GK-H) group (n = 8/group). The remaining Wistar rats were assigned as the normobaric control (WR) group (n = 8). They were housed in individual cages and housed under normobaric conditions (1 ATA with 20.9 % oxygen). The room was maintained at 22 ± 2 °C, 45–55 % relative humidity, and with a 12 h light/dark cycle (lights on from 08:00 to 20:00). All rats were given standard chow (MF, Oriental East Inc., Tokyo, Japan) and water ad libitum.
Exposure to Mild Hyperbaric Oxygen
Rats in the GK-H group were exposed to 1.25 ATA with 36 % oxygen in a mild hyperbaric oxygen chamber (Japan Patent No. 5076067 dated September 7, 2012; Inventor, Akihiko Ishihara) for 3 h (11:00 to 14:00) daily for 10 weeks from 8 to 18 weeks of age.
Fasting Blood Glucose and Glycosylate Hemoglobin (HbA1c) Analyses
At 8 and 18 weeks of age, blood samples were obtained from the tails of conscious rats. HbA1c levels were measured using a DCA vantage analyzer (Siemens Healthcare Diagnostics, Surrey, UK). Fasting blood glucose levels were measured using a blood glucose meter GT-1650 (Arkray, Kyoto, Japan) after 12 h of fasting.
Injection of Fluorescent Neuronal Tracer
After blood sampling, rats were anesthetized with sodium pentobarbital (5 mg/100 g body weight, i.p.), and the surgical procedure was performed under aseptic conditions. A 2 % fluorescent neuronal tracer, nuclear yellow, which is retrogradely transported from the skeletal muscle to the spinal cord [21, 22], was injected using a microsyringe into multiple sites of the right soleus muscle. Care was taken to inject the neuronal tracer slowly to prevent leakage. After injecting the neuronal tracer, the skin was sutured and the rats were allowed to recover from anesthesia and surgery. The rats were sacrificed by administering an overdose of sodium pentobarbital 2 days after the injection of the neuronal tracer.
Histochemical Analyses
The lumbosacral enlargement of the spinal cord was removed and frozen immediately in isopentane cooled in liquid nitrogen. Serial longitudinal sections (20 μm thick) of the lumbosacral enlargement were cut in a cryostat at −20 °C. The motoneurons innervating the soleus muscle were identified by the golden-yellow fluorescence of the nucleus imparted by nuclear yellow (Fig. 1a). The identified motoneurons in the serial sections were counted. The same sections that were used to identify the motoneurons were subsequently stained for succinate dehydrogenase (SDH) activity, a marker of mitochondrial oxidative capacity [6, 7, 23] (Fig. 1b). The cross-sectional area (CSA) and SDH activity of the identified motoneuron, in which the nucleus was visible, were measured using a computer-assisted image processing system (Neuroimaging System, Kyoto, Japan). Sectional images were digitized as gray-scale images. Each pixel was quantified as 1 of 256 gray levels; a gray level of 0 was equivalent to 100 % light transmission, whereas a gray level of 255 was equivalent to 0 % light transmission. The mean optical density (OD) of all pixels, which were converted to gray-level values, within a motoneuron was determined using a calibration photographic tablet with 21 steps of gradient-density range and corresponding diffused density values. The nucleus was excluded from OD measurements since it does not stain for SDH activity. The cut-off for the distinction among gamma, small-sized alpha, and large-sized alpha motoneurons was based on cell body size. Based on previous studies [24–26], there is a high probability that the very small-sized (<500 μm2) motoneurons are gamma, the small-sized (500–900 μm2) motoneurons are high-oxidative alpha, and the large-sized (>900 μm2) motoneurons are low-oxidative alpha.
Spinal motoneurons innervating the rat soleus muscle were retrogradely identified by the fluorescent neuronal tracer, nuclear yellow (a). After identification of the motoneurons, the section was stained for succinate dehydrogenase activity to determine cell body sizes and oxidative enzyme activity of identified motoneurons (b). WM white matter, GM gray matter. Scale bar 50 μm
Statistics
Values are expressed as mean ± SD. One-way ANOVA was used to determine significant differences among the five groups; WR and GK at 8 weeks and GK, WR, and GK-H at 18 weeks. When significant differences were found, individual group comparisons were performed using Scheffé’s post hoc test. Statistical significance was set at p < 0.05.
Results
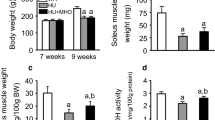
Body Weight
Body weight was lower in the GK group at 8 weeks of age than in the age-matched WR group (Fig. 2a). Body weight was higher in the WR, GK, and GK-H groups at 18 weeks of age than in the WR and GK groups at 8 weeks of age. Body weight was lower in the GK and GK-H groups at the 18 weeks of age than in the age-matched WR group.
Body weight (a) and fasting blood glucose (b) and HbA1c (c) levels of the WR and GK groups at 8 weeks of age and WR, GK, and GK-H groups at 18 weeks of age. WR, normal rats; GK, type 2 diabetic rats; GK-H, type 2 diabetic rats exposed to mild hyperbaric oxygen for 3 h per day; HbA1c, glycosylated hemoglobin. Values are means ± SD for 8 animals. a p < 0.05 compared with WR at 8 weeks; b p < 0.05 compared with GK at 8 weeks; c p < 0.05 compared with WR at 18 weeks; d p < 0.05 compared with GK at 18 weeks
Fasting Blood Glucose Levels
Fasting blood glucose levels were higher in the GK group at 8 weeks of age than in the WR group at 8 and 18 weeks of age (Fig. 2b). Fasting blood glucose levels were higher in the GK group at 18 weeks of age than in the WR group at 8 and 18 weeks of age and the GK group at 8 weeks of age. Fasting blood glucose levels in the GK-H group at 18 weeks of age were higher than those of the WR group at 8 and 18 weeks of age and lower than those of GK group at 18 weeks of age.
HbA1c Levels
HbA1c levels were higher in the GK group at 8 weeks of age than in the WR group at 8 and 18 weeks of age (Fig. 2c). HbA1c levels were higher in the GK group at 18 weeks of age than in the WR group at 8 and 18 weeks of age and the GK group at 8 weeks of age. HbA1c levels in the GK-H group at 18 weeks of age were higher than those of the WR group at 8 and 18 weeks of age and lower than those of GK group at 8 and 18 weeks of age.
Number of Motoneurons Innervating the Soleus Muscle
There was no significant difference in the number of alpha (Fig. 3a) or gamma + alpha (Fig. 3b) motoneurons, as identified by the fluorescent neuronal tracer, innervating the soleus muscle.
Number of alpha (a) and gamma + alpha (b) motoneurons innervating the soleus muscle of the WR and GK groups at 8 weeks of age and WR, GK, and GK-H groups at 18 weeks of age. WR, normal rats; GK, type 2 diabetic rats; GK-H, type 2 diabetic rats exposed to mild hyperbaric oxygen for 3 h per day. Values are means ± SD for 8 animals
SDH Activity of Motoneurons Innervating the Soleus Muscle
The SDH activity of small-sized alpha motoneurons innervating the soleus muscle was lower in the GK group at 8 weeks than in the WR group at 8 and 18 weeks (Fig. 4b). The SDH activity of small-sized alpha motoneurons was lower in the GK group at 18 weeks of age than in the WR group at 8 and 18 weeks. The SDH activity of small-sized alpha motoneurons was higher in the GK-H group at 18 weeks of age than in the GK group than 8 and 18 weeks.
SDH activity of spinal motoneurons innervating the soleus muscle of the WR and GK groups at 8 weeks of age and WR, GK, and GK-H groups at 18 weeks of age. SDH, succinate dehydrogenase; OD, optical density. Spinal motoneurons were divided into three types according to their cell body sizes; gamma (<500 μm2, a), small-sized alpha (500–900 μm2, b), and large-sized alpha (>900 μm2, c) motoneurons. Values are means ± SD for 8 animals. a p < 0.05 compared with WR at 8 weeks; b p < 0.05 compared with GK at 8 weeks; c p < 0.05 compared with WR at 18 weeks; d p < 0.05 compared with GK at 18 weeks
Discussion
Fasting Blood Glucose and HbA1c Levels
At 8 and 18 weeks of age, GK rats with type 2 diabetes showed higher blood glucose and HbA1c levels compared with age-matched normal WR rats (Fig. 2b, c). The increased blood glucose and HbA1c levels of GK rats in this study correspond well with those observed in GK rats at 7 [14], 9 [2, 14],15 [27, 28], and 20 [2] weeks of age. In this study, the GK rats had developed diabetes by 8 weeks of age.
The blood glucose and HbA1c levels were lower in GK rats exposed to mild hyperbaric oxygen than in age-matched GK rats not exposed to mild hyperbaric oxygen (Fig. 2b, c), indicating that mild hyperbaric oxygen improved the blood glucose and HbA1c levels of GK rats. This improvement corresponds well with previous findings [13–16] although the blood glucose and HbA1c levels of GK rats exposed to mild hyperbaric oxygen did not attain the normal level of these parameters as observed in WR rats (Fig. 2b, c). We concluded that mild hyperbaric oxygen is effective in improving the blood glucose and HbA1c levels of rats with type 2 diabetes.
Skeletal Muscle Properties of Humans and Animal Models with Type 2 Diabetes
Skeletal muscles in humans and animal models of type 2 diabetes have decreased oxidative capacity. In clinical studies [29–32], a low percentage of high-oxidative type I fibers and a high percentage of low-oxidative type II fibers, especially type IIB fibers, are observed in the vastus lateralis muscle of patients with type 2 diabetes. The altered fiber-type composition is correlated closely with insulin concentrations, which regulate myosin synthesis in skeletal muscle.
The soleus muscles have high oxidative capacity and are required to function against gravity, e.g., maintaining posture and walking [33], indicating that the soleus muscles function most effectively at relatively low intensity for long durations. The rat soleus muscle contains type I, type IIA, and type IIC fibers; type IIA and type IIC fibers have high oxidative enzyme activity, whereas type I fibers have low oxidative enzyme activity [34, 35]. In previous studies using animal models of type 2 diabetes, Yasuda et al. [1, 2] found that both obese Otsuka Long-Evans Tokushima fatty (OLETF) and non-obese GK rats exhibited a decreased percentage of high-oxidative type IIA and type IIC fibers and an increased percentage of low-oxidative type I fibers in the soleus muscle during postnatal development. In addition, there are no high-oxidative type IIA and type IIC fibers in the soleus muscle of adult OLETF and GK rats.
Skeletal muscle plays a major role in insulin-stimulated glucose uptake. Type 2 diabetes is associated with impaired insulin-stimulated glucose uptake and disposal capacity, which is attributed to insulin resistance in skeletal muscle. The altered pattern of fiber types in skeletal muscles of humans and animal models with type 2 diabetes is tightly linked to impaired glucose tolerance and insulin sensitivity.
Effect of Mild Hyperbaric Oxygen on Skeletal Muscle Properties
A decrease in the percentage of low-oxidative type I fibers and an appearance in high-oxidative type IIA and type IIC fibers, which are not detected in the soleus muscle of type 2 diabetic rats [1, 2], are observed following exposure to mild hyperbaric oxygen [14]. A high percentage of low-oxidative type I fibers with no high-oxidative type IIA fibers is observed in the soleus muscle of obese OLETF rats compared with that in normal Long-Evans Tokushima Otsuka (LETO) rats [1]. In addition, a growth-associated increase in the percentage of low-oxidative type IIB fibers and a decrease in the percentage of high-oxidative type I and type IIA fibers are observed in the plantaris muscle of OLETF rats compared with that in LETO rats [15]. In contrast, there were no differences in the fiber-type composition of the soleus and plantaris muscles between LETO and OLETF rats after OLETF rats were exposed to mild hyperbaric oxygen. The increased blood flow and availability of oxygen induced by mild hyperbaric oxygen has a beneficial impact on the type shift of fibers in skeletal muscle of diabetic rats to a normal pattern.
Endurance exercise restores decreased oxidative capacity in skeletal muscle of rats with type 2 diabetes [36]. The percentage of type I fibers in the soleus muscle is related to running distance in exercised OLETF rats. Endurance exercise inhibits a diabetes-induced type shift of fibers from high-oxidative type II to low-oxidative type I in the soleus muscle of OLETF rats that run distances of more than 7000 m/day. In contrast, changes in the fiber-type composition in the plantaris muscle of OLETF rats are inhibited by endurance exercise, irrespective of the distance they run. Thus, endurance exercise can inhibit the diabetes-induced type shift of fibers from high-oxidative to low-oxidative in skeletal muscle of rats with type 2 diabetes because of increased muscle oxidative capacity. However, the increase in the blood flow following an endurance exercise is induced mostly in active skeletal muscles, but not in internal organs. In addition, because of the increased atmospheric pressure and oxygen concentration, mild hyperbaric oxygen has an advantage in that it can increase the amount of dissolved oxygen in the plasma, which does not occur with endurance exercise.
Motoneuron Property of Animal Models
The neuromuscular unit is comprised of an alpha motoneuron in the dorsolateral region of the ventral horn in the spinal cord and the fibers in the skeletal muscle that the motoneuron innervates. The metabolic properties of alpha motoneurons are tightly related to those of their target muscle fibers, indicating that there is matching between the cell size and metabolic properties of the alpha motoneurons and the muscle fibers that they innervate. There is an inverse relationship between motoneuron cell body size and oxidative capacity [6, 7]. In general, alpha motoneurons are classified into low-oxidative and high-oxidative types. The high-oxidative alpha motoneurons have small cell body sizes, whereas the low-oxidative alpha motoneurons have large cell body sizes. There were no data available concerning effects of mild hyperbaric oxygen on motoneuron properties of rats with type 2 diabetes. Therefore, this study examined the effects of mild hyperbaric oxygen on motoneuron properties of rats with type 2 diabetes.
Effect of Mild Hyperbaric Oxygen on Motoneuron Oxidative Capacity
Heavy ion radiation [37] and exposure to microgravity [38–42] induce decreased oxidative capacity of spinal motoneurons in rats. The oxidative enzyme activity of all motoneurons was decreased following administration of heavy ion radiation to the spinal cord at doses of 40, 50, and 70 Gy compared with that of non-irradiated controls [37]. The decreased oxidative capacity in the irradiated motoneurons returned to the oxidative capacity of non-irradiated controls after a 6-month recovery period.
Previous studies [43, 44] observed atrophy of fibers, a type shift of fibers from high-oxidative to low-oxidative, decreased oxidative enzyme activity, and down-regulation of the mRNA expression of heat shock proteins in skeletal muscles, especially in the slow muscle, of rats following exposure to microgravity. In addition, previous studies [38–40] observed that the oxidative capacity of small-sized and high-oxidative alpha motoneurons, but not gamma or large-sized and low-oxidative alpha motoneurons, in the lumbar segment of the rat spinal cord decreased after exposure to microgravity. In contrast, there were no changes in the oxidative capacity of alpha motoneurons in the ventromedial region of the ventral horn in the lumbar segment [41] or in the dorsolateral region of the ventral horn in the cervical segment [42] of the rat spinal cord after exposure to microgravity. These responses in the oxidative capacity of alpha motoneurons correspond well with those of fibers in the skeletal muscles that the motoneurons innervate. The findings collectively indicate that the oxidative capacity of the small-sized alpha motoneurons innervating high-oxidative fibers in skeletal muscle with gravity-dependent functions is decreased by exposure to microgravity with atrophy and decreased oxidative capacity in the slow muscle and its fibers that were innervated by the small-sized alpha motoneurons.
It is expected that mild hyperbaric oxygen would improve decreased oxidative capacity in spinal motoneurons of rats with type 2 diabetes, because the metabolic properties of alpha motoneurons are tightly related to those of their target muscle and its fibers [7] and mild hyperbaric oxygen has been shown to improve the decreased oxidative capacity of muscles in rats with type 2 diabetes [14–16]. The present study found that mild hyperbaric oxygen improved the decreased oxidative capacity of small-sized and high-oxidative alpha motoneurons innervating the slow muscle of rats with type 2 diabetes (Fig. 4b). The improved oxidative capacity in motoneurons corresponds well with those observed in fibers in the soleus muscle that the motoneurons innervate [11, 45], indicating that the properties and responses of motoneurons and their innervating fibers are related closely under diabetic conditions, as well as under normal conditions (Fig. 5). However, which changes in the oxidative capacity of motoneurons and their innervating fibers occur earlier and/or are triggered by mild hyperbaric oxygen was not clear in this study.
Three types of soleus neuromuscular units in normal WR rats. Type 2 diabetes induces decreased oxidative capacity in small-sized and high-oxidative alpha motoneurons innervating the soleus muscle, which shifts fiber types from type IIA and type IIC to type I [1, 2]. In contrast, mild hyperbaric oxygen restores these changes to normal [14, 16]
It is possible that the response to mild hyperbaric conditions involved changes in physiological properties as well as metabolic properties, i.e., oxidative capacity, of motoneurons innervating the soleus muscle of rats with type 2 diabetes, although we did not measure physiological properties including conduction velocity, input resistance, and duration of the after hyperpolarization period of motoneurons. Previous studies [46, 47] observed that there is a relationship between the physiological and morphological (i.e., cell body size) properties of motoneurons innervating the skeletal muscle. In addition, there is a relationship between the morphological and metabolic properties, e.g., smaller-sized motoneurons have higher oxidative enzyme activity in the extensor digitorum longus [6], tibialis anterior [7], and soleus [21, 23, 25, 26] neuron pools in rats. In this study, with our previous findings observed in the soleus muscle of rats with type 2 diabetes [1, 2] and exposed to mild hyperbaric oxygen [14, 16], it is suggested that physiological properties of motoneuron shift from slow to fast type with type 2 diabetes, whereas mild hyperbaric oxygen inhibit such changes in the motoneuron’s physiological properties.
Conclusions
This study determined the oxidative capacity of motoneurons innervating the soleus muscle of rats with type 2 diabetes. In addition, this study examined the effects of exposure to mild hyperbaric oxygen at 1.25 ATA with 36 % oxygen on the oxidative capacity of motoneurons innervating the soleus muscle of rats with type 2 diabetes. Spinal motoneurons innervating the soleus muscle were retrogradely identified using a fluorescent neuronal tracer. Thereafter, cell body sizes and SDH activity of identified motoneurons were analyzed. We concluded that rats with type 2 diabetes have decreased oxidative capacity in the motoneurons innervating the soleus muscle and that the decreased oxidative capacity of the motoneurons induced by type 2 diabetes was improved by mild hyperbaric oxygen.
Abbreviations
- ATA:
-
Atmosphere absolute
- CSA:
-
Cross-sectional area
- GK:
-
Goto-Kakizaki
- HbA1c:
-
Glycosylate hemoglobin
- LETO:
-
Long-Evans Tokushima Otsuka
- OD:
-
Optical density
- OLETF:
-
Otsuka Long-Evans Tokushima fatty
- SDH:
-
Succinate dehydrogenase
- WR:
-
Wistar
References
Yasuda K, Ishihara A, Adachi T, Shihara N, Seino Y, Tsuda K (2001) Growth-related changes in skeletal muscle fiber type and insulin resistance in diabetic Otsuka Long-Evans Tokushima fatty rats. Acta Histochem Cytochem 34:371–382
Yasuda K, Nishikawa W, Iwanaka N, Nakamura E, Seino Y, Tsuda K, Ishihara A (2002) Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol 29:1001–1008
Ballantyne CM, Hoogeveen RC, McNeill AM, Heiss G, Schmidt MI, Duncan BB, Pankow JS (2008) Metabolic syndrome risk for cardiovascular disease and diabetes in the ARIC study. Int J Obes 32:S21–S24
Kelley DE, Goodpaster BH, Wing RR, Simoneau JA (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277:1130–1141
Abdul-Ghani MA, DeFronzo RA (2010) Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010:476279. doi:10.1155/2010/476279
Ishihara A, Taguchi S, Araki H, Nishihira Y (1991) Retrograde neuronal labeling of motoneurons in the rat by fluorescent tracers, and quantitative analysis of oxidative enzyme activity in labeled neurons. Neurosci Lett 124:141–143
Ishihara A, Roy RR, Edgerton VR (1995) Succinate dehydrogenase activity and soma size of motoneurons innervating different portions of the rat tibialis anterior. Neuroscience 68:813–822
Tibbles PM, Edelsberg JS (1996) Hyperbaric-oxygen therapy. N Engl J Med 334:1642–1648
Leach RM, Rees PJ, Wilmshurst P (1998) ABC of oxygen: hyperbaric oxygen therapy. BMJ 317:1140–1143
Ishihara A, Nagatomo F, Fujino H, Kondo H (2014) Exposure to mild hyperbaric oxygen increases blood flow and resting energy expenditure but not oxidative stress. J Sci Res Rep 3:1886–1896
Matsumoto A, Okiura T, Morimatsu F, Ohira Y, Ishihara A (2007) Effects of hyperbaric exposure with high oxygen concentration on the physical activity of developing rats. Dev Neurosci 29:452–459
Nishizaka T, Nagatomo F, Fujino H, Nomura T, Sano T, Higuchi K, Takeda I, Ishihara A (2010) Hyperbaric oxygen exposure reduces age-related decrease in oxidative capacity of the tibialis anterior muscle in mice. Enzyme Res 2010:824763. doi:10.4061/2010/824763
Yasuda K, Aoki N, Adachi T, Tsujimoto G, Gu N, Matsunaga T, Kikuchi N, Tsuda K, Ishihara A (2006) Hyperbaric exposure with high oxygen concentration inhibits growth-associated increase in the glucose level of diabetic Goto-Kakizaki rats. Diabetes Obes Metab 8:714–715
Yasuda K, Adachi T, Gu N, Matsumoto A, Matsunaga T, Tsujimoto G, Tsuda K, Ishihara A (2007) Effects of hyperbaric exposure with high oxygen concentration on glucose and insulin levels and skeletal muscle-fiber properties in diabetic rats. Muscle Nerve 35:337–343
Matsumoto A, Nagatomo F, Yasuda K, Tsuda K, Ishihara A (2007) Hyperbaric exposure with high oxygen concentration improves altered fiber types in the plantaris muscle of diabetic Goto-Kakizaki rats. J Physiol Sci 57:133–136
Gu N, Nagatomo F, Fujino F, Takeda I, Tsuda K, Ishihara A (2010) Hyperbaric oxygen exposure improves blood glucose level and muscle oxidative capacity in rats with type 2 diabetes. Diabetes Technol Ther 12:125–133
Nagatomo F, Roy RR, Takahashi H, Edgerton VR, Ishihara A (2011) Effect of exposure to hyperbaric oxygen on diabetes-induced cataracts in mice. J Diabetes 3:301–308
Nagatomo F, Fujino H, Takeda I, Ishihara A (2010) Effects of hyperbaric oxygenation on blood pressure levels of spontaneously hypertensive rats. Clin Exp Hypertens 32:193–197
Nagatomo F, Gu N, Fujino H, Okiura T, Morimatsu F, Takeda I, Ishihara A (2010) Effects of exposure to hyperbaric oxygen on oxidative stress in rats with type II collagen-induced arthritis. Clin Exp Med 10:7–13
Nishizaka T, Nomura T, Sano T, Higuchi K, Nagatomo F, Ishihara A (2011) Hyperbaric oxygen improves UVB irradiation-induced melanin pigmentation and diminishes senile spot size. Skin Res Technol 17:332–338
Ishihara A, Kawano F, Ishioka N, Oishi H, Higashibata A, Shimazu T, Ohira Y (2003) Growth-related changes in cell body size and succinate dehydrogenase activity of spinal motoneurons innervating the rat soleus muscle. Int J Dev Neurosci 21:461–469
Ishihara A, Kawano F, Ishioka N, Oishi H, Higashibata A, Shimazu T, Ohira Y (2004) Effects of running exercise during recovery from hindlimb unloading on soleus muscle fibers and their spinal motoneurons in rats. Neurosci Res 48:119–127
Nagatomo F, Ishihara A, Ohira Y (2009) Effects of hindlimb unloading at early postnatal growth on cell body size in spinal motoneurons innervating soleus muscle of rats. Int J Dev Neurosci 27:21–26
Ishihara A, Hayashi S, Roy RR, Tamada Y, Yokoyama C, Ohira Y, Edgerton VR, Ibata Y (1997) Mitochondrial density of ventral horn neurons in the rat spinal cord. Acta Anat 160:248–253
Ishihara A, Hori A, Roy RR, Oishi Y, Talmadge RJ, Ohira Y, Kobayashi S, Edgerton VR (1997) Perineal muscles and their innervation: metabolic and functional significance of the motor unit. Acta Anat 159:156–166
Ishihara A, Ohira Y, Tanaka M, Nishikawa W, Ishioka N, Higashibata A, Izumi R, Shimazu T, Ibata Y (2001) Cell body size and succinate dehydrogenase activity of spinal motoneurons innervating the soleus muscle in mice, rats, and cats. Neurochem Res 26:1301–1304
Nagatomo F, Gu N, Fujino H, Takeda I, Tsuda K, Ishihara A (2009) Skeletal muscle characteristics of rats with obesity, diabetes, hypertension, and hyperlipidemia. J Atheroscler Thromb 16:576–585
Nagatomo F, Fujino H, Kondo H, Gu N, Takeda I, Ishioka N, Tsuda K, Ishihara A (2011) PGC-1α mRNA level and oxidative capacity of the plantaris muscle in rats with metabolic syndrome, hypertension, and type 2 diabetes. Acta Histochem Cytochem 44:73–80
Mårin P, Andersson B, Krotkiewski M, Björntorp P (1994) Muscle fiber composition and capillary density in women and men with NIDDM. Diabetes Care 17:382–386
Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL (1995) Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol Endocrinol Metab 268:453–457
Nyholm B, Qu Z, Kaal A, Pedersen SB, Gravholt CH, Andersen JL, Saltin B, Schmitz O (1997) Evidence of an increased number of type IIb muscle fibers in insulin-resistant first-degree relatives of patients with NIDDM. Diabetes 46:1822–1828
Gaster M, Staehr P, Beck-Nielsen H, Schrøder HD, Handberg A (2001) GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes 50:1324–1329
Hennig R, Lømo T (1985) Firing patterns of motor units in normal rats. Nature 314:164–166
Nakatani T, Nakashima T, Kita T, Hirofuji C, Itoh K, Itoh M, Ishihara A (1999) Succinate dehydrogenase activities of fibers in the rat extensor digitorum longus, soleus, and cardiac muscles. Arch Histol Cytol 62:393–399
Nakatani T, Nakashima T, Kita T, Ishihara A (2003) Cell size and oxidative enzyme activity of type-identified fibers in rat hindlimb muscles: a review. Acta Histochem Cytochem 36:105–114
Yasuda K, Adachi T, Kikuchi N, Tsujimoto G, Aoki N, Tsuda K, Ishihara A (2006) Effects of running exercise on fibre-type distribution of soleus and plantaris muscles in diabetic Otsuka Long-Evans Tokushima fatty rats. Diabetes Obes Metab 8:311–321
Ishihara A, Nagatomo F, Fujino H, Kondo H, Nojima K (2012) A threshold dose of heavy ion radiation that decreases the oxidative enzyme activity of spinal motoneurons in rats. Neurochem Res 37:387–393
Ishihara A, Ohira Y, Roy RR, Nagaoka S, Sekiguchi C, Hinds WE, Edgerton VR (1996) Influence of spaceflight on succinate dehydrogenase activity and soma size of rat ventral horn neurons. Acta Anat 157:303–308
Ishihara A, Ohira Y, Roy RR, Nagaoka S, Sekiguchi C, Hinds WE, Edgerton VR (2002) Succinate dehydrogenase activity in rat dorsolateral ventral horn motoneurons at L6 after spaceflight and recovery. J Gravit Physiol 9:39–48
Ishihara A, Nagatomo F, Fujino H, Kondo H, Ohira Y (2013) Decreased succinate dehydrogenase activity of gamma and alpha motoneurons in mouse spinal cords following 13 weeks of exposure to microgravity. Neurochem Res 38:2160–2167
Ishihara A, Ohira Y, Roy RR, Nagaoka S, Sekiguchi C, Hinds WE, Edgerton VR (2000) Comparison of the response of motoneurons innervating perineal and hind limb muscles to spaceflight and recovery. Muscle Nerve 23:753–762
Ishihara A, Yamashiro J, Matsumoto A, Higashibata A, Ishioka N, Shimazu T, Ohira Y (2006) Comparison of cell body size and oxidative enzyme activity in motoneurons between the cervical and lumbar segments in the rat spinal cord after spaceflight and recovery. Neurochem Res 31:411–415
Edgerton VR, Roy RR (1996) Neuromuscular adaptations to actual and simulated spaceflight. In: Fregly MJ, Blatteis CM (eds) Handbook of physiology. Section 4. Environmental physiology. III. The gravitational environment. Oxford University Press, New York, pp 721–763
Ishihara A, Fujino H, Nagatomo F, Takeda I, Ohira Y (2008) Gene expression levels of heat shock proteins in the soleus and plantaris muscles of rats after hindlimb suspension or spaceflight. J Physiol Sci 58:413–417
Ishihara A, Kawano F, Okiura T, Morimatsu F, Ohira Y (2005) Hyperbaric exposure with high oxygen concentration enhances oxidative capacity of neuromuscular units. Neurosci Res 52:146–152
Burke RE, Strick PL, Kanda K, Kim CC, Walmsley B (1977) Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol 40:667–680
Burke RE, Dum RP, Fleshman JW, Glenn LL, Lev-Tov A, O’Donovan MJ, Pinter MJ (1982) An HRP study of the relation between cell size and motor unit type in cat ankle extensor motoneurons. J Comp Neurol 209:17–28
Acknowledgments
This study was supported by a Grand-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Takemura, A., Ishihara, A. Mild Hyperbaric Oxygen Improves Decreased Oxidative Capacity of Spinal Motoneurons Innervating the Soleus Muscle of Rats with Type 2 Diabetes. Neurochem Res 41, 2336–2344 (2016). https://doi.org/10.1007/s11064-016-1947-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1947-4