Abstract
Astrocytes are multitasking players in brain complexity, possessing several receptors and mechanisms to detect, participate and modulate neuronal communication. The functionality of astrocytes has been mainly unraveled through the study of primary astrocyte cultures, and recently our research group characterized a model of astrocyte cultures derived from adult Wistar rats. We, herein, aim to characterize other basal functions of these cells to explore the potential of this model for studying the adult brain. To characterize the astrocytic phenotype, we determined the presence of GFAP, GLAST and GLT 1 proteins in cells by immunofluorescence. Next, we determined the concentrations of thirteen amino acids, ATP, ADP, adenosine and calcium in astrocyte cultures, as well as the activities of Na+/K+-ATPase and acetylcholine esterase. Furthermore, we assessed the presence of the GABA transporter 1 (GAT 1) and cannabinoid receptor 1 (CB 1) in the astrocytes. Cells demonstrated the presence of glutamine, consistent with their role in the glutamate–glutamine cycle, as well as glutamate and d-serine, amino acids classically known to act as gliotransmitters. ATP was produced and released by the cells and ADP was consumed. Calcium levels were in agreement with those reported in the literature, as were the enzymatic activities measured. The presence of GAT 1 was detected, but the presence of CB 1 was not, suggesting a decreased neuroprotective capacity in adult astrocytes under in vitro conditions. Taken together, our results show cellular functionality regarding the astrocytic role in gliotransmission and neurotransmitter management since they are able to produce and release gliotransmitters and to modulate the cholinergic and GABAergic systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes are multitasking players in brain complexity, acting as secretory cells of the central nervous system (CNS) releasing neurotransmitters, neuromodulators and trophic factors [1–3]. They sense neural communication, as is evident by the expression of numerous neurotransmitter receptors, transporters and enzymes on their membranes [4]. These cells also release gliotransmitters, such as glutamate, d-serine and ATP, which interact with pre- and post-synaptic receptors in the tripartite synapse [5–7]. Astrocytes are also primary homeostatic cells of the brain, and most of their functionality has been unraveled through the study of primary astrocyte cultures, especially those related to glutamate and GABA metabolism, their antioxidant defense and energy capabilities [8–11].
Recent studies have employed astrocyte cultures derived from adult rats, which are unsurprisingly different to those derived from newborn animals, since their connections on the tissue that they are inserted in are far more complex. As such, we previously published a routine astrocyte culture protocol from adult Wistar rats; these cells present classical astrocytic markers, take up glutamate, and actively participate in antioxidant and inflammatory responses [12]. Additionally, these cells presented age-related glial responses, including under neurotoxic and neuroprotective stimuli [13]. However, the presence of gliotransmitters in adult astrocyte cultures remains unclear.
Na+/K+-ATPase (EC 3.6.1.37) is a crucial enzyme that is responsible for the generation of membrane potential, through the active transport of Na+ and K+ ions in the CNS, consuming about 60 % of the ATP generated in this tissue [14]. As astrocytes are involved in the regulation of ionic homeostasis and the glutamate transporter (highly dependent on Na+ ion), there is a close relationship between Na+/K+-ATPase and astrocyte functionality [15, 16]. In addition, as well as the gliotransmitters, acetylcholine serves as an extracellular signaling substance in the neural cells. Acetylcholine (ACh) is specifically hydrolyzed by acetylcholinesterase (AChE, EC 3.1.1.7) and has also been associated with astrocytic activity [17].
The amino acid profile, enzymatic activity and neurotransmitter management in cultivated cells are useful for indicating the culture functionality, which is pivotal for the routine use of a model as a reliable tool when studying neurochemical properties of the adult brain. Thus, the aim of this study was to determine some aspects of our adult astrocyte cultures under basal conditions, such as the concentration of thirteen amino acids, ATP, ADP and adenosine (ADO) in the intra and extracellular medium, as well as the activities of Na+/K+-ATPase and acetylcholinesterase (AChE). Additionally, to study GABAergic and cannabinoid transmission, the presence of GABA transporter 1 (GAT 1) and cannabinoid receptor 1 (CB 1) was assessed in the cells. Morphological analysis of the glial fibrillary acidic protein (GFAP) and of the excitatory amino acid transporters, GLAST (EAAT1) and GLT 1 (EAAT2), were performed to ensure the astrocytic phenotype of the cultured cells.
Experimental Procedures
Reagents
Dulbecco’s Modified Eagle’s Medium/F12 (DMEM/F12) and other materials for cell culture were purchased from Gibco/Invitrogen (California, USA). Papain was acquired from Merck (Darmstadt, Germany). DNase, cysteine, albumin and monoclonal anti-GAPDH were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nitrocellulose membrane and an ECL kit were purchased from Amersham (Buckinghamshire, UK). Polyclonal anti-GLAST and anti-GLT 1 were from Alpha Diagnostics (Texas, USA), polyclonal anti-GFAP was from Dako (Glostrup, Denmark), polyclonal anti-GAT 1 was from Merck Millipore (Darmstadt, Germany) and polyclonal anti-CB 1 was from Santa Cruz Biotechnology (California, USA). All other chemicals were from common commercial suppliers.
Animals
Male Wistar rats (90 days old) were obtained from our breeding colony (Department of Biochemistry, UFRGS, Brazil) and maintained under a controlled environment (12 h light/12 h dark cycle; 22 ± 1 °C; ad libitum access to food and water). All animal experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Federal University of Rio Grande do Sul Animal Care and Use Committee (process number 24419).
Cell Culture Preparation and Maintenance
Male Wistar rats (90 days old) were sacrificed by decapitation, had their cerebral cortices aseptically dissected and meninges removed. The tissue was digested using trypsin and papain at 37 °C, as previously described [12]. After mechanical dissociation and centrifugation, the cells were resuspended in DMEM/F12 [10 % fetal bovine serum (FBS), 15 mM HEPES, 14.3 mM NaHCO3, 1 % Fungizone® and 0.04 % gentamicin], plated on 6- or 24-well plates pre-coated with poly-l-lysine and cultured at 37 °C in a 5 % CO2 incubator. The cells were seeded at a density of 3–5 × 105 cells/cm2. Twenty-four hours later, the culture medium was exchanged; during the 1st week, the medium was replaced once every 2 days and from the 2nd week on, once every four days. From the 3rd week on, the astrocytes received medium supplemented with 20 % FBS until they reached confluence (at approximately the 4th week). No dibutyryl cAMP was added to the culture medium in order to observe the naive response of the cells. Specific proteins of neurons and microglia were tested in order to determine the purity of the astrocyte culture, which was found to be around 95 % (data not shown).
Immunofluorescence
Immunocytochemistry was performed as described previously [12]. Briefly, cell cultures were fixed with 4 % paraformaldehyde for 20 min and permeabilized with 0.1 % Triton X-100 in PBS for 5 min at room temperature. After blocking overnight with 4 % albumin, the cells were incubated overnight with anti-GFAP (1:400), anti-GLAST (1:500) or anti-GLT 1 (1:500) at 4 °C, followed by PBS washes and incubation with a specific secondary antibody conjugated with Alexa Fluor® 594 or 488 for 1 h at room temperature. Cell nuclei were stained with 0.2 mg/mL of 4′,6′-diamidino-2-phenylindole (DAPI). The cells were visualized with a Nikon inverted microscope and the images were transferred to a computer with a digital camera (Sound Vision Inc.).
High-Performance Liquid Chromatography (HPLC) Procedure—Amino Acids
The assay was performed with intra and extracellular samples to quantify thirteen amino acids [alanine (Ala), aspartate (Asp), glutamine (Gln), glutamate (Glu), glycine (Gly), isoleucine (Ile), leucine (Leu), lysine (Lys), ornitine (Orn), phenylalanine (Phe), d-serine (Ser), tyrosine (Tyr) and valine (Val), according to [18] ]. Briefly, cells were homogenized in 7 % trifluoroacetic acid (TFA) and centrifuged. The supernatant was collected, neutralized with 1.5 M potassium bicarbonate and filtered (0.22 μm pore). Samples were derivatized with ο-phthalaldehyde and separation was carried out with a reverse phase column (Supelcosil LC-18, 250 mm × 4.6 mm, Supelco) in a Shimadzu Class-VP chromatography system. The mobile phase flowed at a rate of 1.4 mL/min and column temperature was 24 °C. Buffer composition was A: 0.04 mol/L sodium dihydrogen phosphate monohydrate buffer, pH 5.5, containing 20 % of methanol; B: 0.01 mol/L sodium dihydrogen phosphate monohydrate buffer, pH 5.5, containing 80 % of methanol. The gradient profile was modified according to the content of buffer B in the mobile phase: 0 % at 0.00 min, 100 % at 55 min, 0 % at 55–60.00 min. Absorbance was read at 360 nm and 455 nm, excitation and emission respectively, with a Shimadzu fluorescence detector. For glutamine determination, samples were diluted 10×. Samples of 100 µL were used and concentration was expressed as mean ± SEM, in nmol/mg protein for intracellular content and µmol/dL for extracellular medium. The amino acids appear in the chromatogram in the following order: Asp; Glu; Ser; Gln; Gly; Ala; Tyr; Val; Phe; Ile; Leu; Orn and Lys.
HPLC Procedure—Purines
The assay was performed in culture supernatant in order to measure the ATP, ADP and ADO concentration, according to Domanski and colleagues [19]. Analyses were performed with a Shimadzu LC-20A series chromatography system, consisting of a quaternary gradient pump with vacuum degassing and piston desalting modules, auto injector and UV detector. Separations were achieved on a reverse-phase column (Supelcosil LC-18, 250 mm × 4.6 mm, Supelco). The mobile phase flowed at a rate of 1.2 mL/min and the column temperature was 24 °C. Buffer composition was: (a) 150 mmol/L phosphate buffer, pH 6.0, containing 150 mmol/L potassium chloride and (b) 15 % acetonitrile in buffer A. The gradient profile underwent the following changes of buffer B in the mobile phase: 0 % at 0.00 min, 2 % at 0.05 min, 7 % at 2.45 min, 50 % at 10.00 min, 100 % at 11.00 min, and 0 % at 12.40 min. Samples of 20 µL were injected. Absorbance was read at 254 nm. Concentrations of purines are expressed as mean ± SEM in µmol/dL.
AChE Activity Assay
For the AChE assay, the cells were homogenized in ten volumes of 0.1 mM potassium phosphate buffer, pH 7.4, and centrifuged for 10 min at 1000×g. The supernatants were used for the enzymatic AChE analyses. AChE activity was determined according to the method of Ellman and colleagues [20], with some modifications [21]. Hydrolysis rates were measured at ACh concentration of 0.8 mM in 300 μL assay solution with 30 mM phosphate buffer, pH 7.5, and 1.0 mM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) at 25 °C. About 15 μL of cell homogenate supernatant was added to the reaction mixture and preincubated for 3 min. The hydrolysis was monitored by formation of the thiolate dianion of DTNB at 412 nm for 2–3 min (intervals of 30 s). Samples were run in triplicate, data are expressed as mean ± SEM, in μmol/h/mg protein.
Na+/K+-ATPase Activity Assay
The reaction mixture for the Na+/K+-ATPase activity assay contained 5.0 mM MgCl2, 80.0 mM NaCl, 20.0 mM KCl and 40.0 mM Tris–HCl, pH 7.4, in a final volume of 170 μL. The reaction was initiated by the addition of ATP. Controls were carried out under the same conditions with the addition of 1.0 mM ouabain. The activity was calculated by the difference between the two assays, as previously described [22]. Released inorganic phosphate (Pi) was measured by the method of Chan and colleagues [23]. Samples were run in triplicate. Specific activity of the enzyme was expressed as mean ± SEM, in nmol Pi/min/mg protein.
Western Blot Analyses
Cells were solubilized in lyses solution containing 4 % SDS, 2 mM EDTA, 50 mM Tris–HCl (pH 6.8). Protein content was measured, the samples were standardized in sample buffer [62.5 mM Tris–HCl (pH 6.8), 4 % (v/v) glycerol, 0.002 % (w/v) bromophenol blue] and boiled at 95 °C for 5 min. Samples were separated by SDS/PAGE (45 mg protein per sample), and transferred to nitrocellulose membranes, as previously described [12]. Adequate loading of each sample was confirmed using Ponceau S staining. Membranes were incubated overnight (4 °C) with anti-GAT 1 (1:100) or anti-CB 1 (1:100). The membranes were then washed and incubated with a peroxidase-conjugated anti-rabbit immunoglobulin in a dilution of 1:3000 for 2 h. Chemiluminescence signals were detected in an Image Quant LAS4000 system (GE Healthcare) using ECL kit.
Calcium Measurement
Calcium intracellular levels were evaluated in 40 μL of cells homogenate, using the Calcium colorimetric assay kit from Sigma Aldrich; results are expressed as mean ± SEM for six experimental determinations performed in duplicate.
Protein Assay
Protein content was measured using Bicinchoninic Acid method with bovine serum albumin as a standard [24].
Results
Astrocytic Characterization
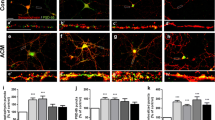
The morphology of the cultured astrocytes was verified by immunofluorescence for GFAP, an intermediary filament of cytoskeleton that is one of the principal markers of astrocytes in in vivo and in vitro conditions (Fig. 1a). Additionally, to insure that cells expressed an astrocytic phenotype, we determined the presence of the two major glutamate transporters, GLAST (Fig. 1b) and GLT 1 (Fig. 1c).
Astrocyte characterization. Under normal conditions, primary adult astrocytes present a polygonal-to-fusiform and flat morphology. a Astrocytes present intense immunolabeling for GFAP. The cells express (b) GLAST and (c) GLT 1. Nuclei were counterstained with DAPI (blue). Scale bar 50 μm (Color figure online)
Amino Acids and Purine Profile
With regard to gliotransmission, we determined the concentration of amino acids in the intra and extracellular medium of the astrocyte cultures. Figure 2a shows the chromatogram for the thirteen amino acids measured in the intracellular content. Figure 2b shows their quantification in nmol/mg protein: Ala 206.5 ± 22.0; Asp 15.7 ± 1.2; Gln 12016.2 ± 1114.3; Glu 157.3 ± 17.1; Gly 484.0 ± 18.1; Ile 143.7 ± 9.4; Leu 197.5 ± 11.4; Lys 292.0 ± 14.5; Orn 68.7 ± 4.3; Phe 112.9 ± 7.2; Ser 122.8 ± 3.7; Tyr 97.3 ± 6.7 and Val 202.4 ± 11.0. Figure 3a shows the chromatogram for the thirteen amino acids measured in the extracellular medium. Figure 3b shows their quantification in μmol/dL: Ala 34.3 ± 3.5; Asp 1.07 ± 0.28; Gln 4795.7 ± 427.5; Glu 9.3 ± 2.1; Gly 70.2 ± 12.1; Ile 23.1 ± 5.1; Leu 29.6 ± 6.8; Lys 42.4 ± 8.5; Orn 10.3 ± 2.1; Phe 15.8 ± 2.7; Ser 19.9 ± 3.7; Tyr 14.6 ± 2.4 and Val 29.9 ± 5.5.
Amino acid separation of intracellular medium of adult astrocyte cultures by HPLC. a Representative chromatogram showing the amino acids separation (inset shows glutamine dosage, which was performed by dilution). b Concentration of each amino acid in the intracellular medium (nmol/mg protein). Data are presented as the means + SEM, n = 5
HPLC procedure for amino acid separation in the extracellular medium of adult astrocyte cultures. a Representative chromatogram showing the amino acid separation (inset shows glutamine dosage, which was performed by dilution). b Concentration of each amino acid in the extracellular medium (μmol/dL). Data are presented as the means + SEM, n = 5
Figure 4 depicts the extracellular concentrations of ATP, ADP and ADO. The values obtained for ATP, ADP and ADO in extracellular medium were, respectively, 646.5 ± 22.7; 126.28 ± 32.1 and 56.2 ± 5.68 nmol/dL.
Measurement of purines in the extracellular medium of adult astrocyte cultures. After cells reached confluence, the extracellular ATP, ADP and ADO concentrations in extracellular medium were determined: 646.5 ± 22.7; 126.28 ± 32.1 and 56.2 ± 5.7 nmol/dL, respectively, whereas basal values for these purines in DMEM/F12 20 % FBS are, 543.5 ± 52.3; 217.3 ± 41.5 and 52.3 ± 0.4 nmol/dL, respectively. Data are presented as the mean + SEM, n = 5
Calcium and Enzymatic Measurement
Enzymatic activity was measured in the adult astrocytes as depicted in Table 1. AchE can end acetylcholine signaling; therefore, the findings that adult astrocytes presented an AchE enzymatic activity of 2.50 ± 0.15 μmol/h/mg protein indicates that these cells may modulate the cholinergic system. Na+/K+-ATPase maintains the sodium and potassium transmembrane gradients needed for the functionality of the nervous tissue, and its basal value in astrocyte cultures was found to be 42.33 ± 4.33 nmol PI/min/mg protein. The concentration of intracellular calcium, fundamental for glial functionality as well as gliotransmission in adult astrocytes, was 125.54 ± 1.14 nM (Table 1).
Neurotransmitter Receptor Expression
To investigate GABAergic transmission in adult astrocytes functionality, we determined the expressions of the GAT 1 transporter and of the CB 1 receptor by immunoblotting. Figure 5a shows that adult astrocytes expressed the GAT 1 transporter, the classical GABA transporter described for astrocytes. However, adult astrocyte cultures did not express the CB 1 receptor, which controls cannabinoid actions in astrocytes (Fig. 5b).
Neurotransmitter transporters in adult astrocyte cultures. Representative immunoblot band for a GAT 1 and b CB 1. The left band represents rat cortex homogenate and the right band represents adult astrocyte culture. Adult astrocytes express the GABA transporter 1 (GAT 1), however they do not express the cannabinoid receptor 1 (CB 1); n = 5
Discussion
Numerous studies, conducted in primary cultures, have facilitated the progress of astrocytic biology comprehension, both under physiological and pathological conditions. We, herein, investigated some parameters related to cellular functionality in astrocyte cultures prepared from adult rats to determine their amino acid and purine profiles, neurotransmitter management and enzymatic activity. We firstly determined the presence of important astrocytic markers in the cells (Fig. 1a). GFAP staining shows that the adult cultures present typical polygonal-to-fusiform and flat morphology. The presence of the glutamatergic transporters also confirmed the astrocytic character of the cultures (Fig. 1b, c).
In addition to its pivotal role in neurotransmitter management, astrocytes also manage gliotransmitters. Several molecules are known to act as gliotransmitters, and these may be exchanged through GAP junctions for communication between astrocytes and/or for communication between astrocytes and other types of cells. Our data indicate the presence intra and extracellular aspartate, glutamate, d-serine and glycine in the cell cultures (Fig. 2, 3). Furthermore, it has been reported that astrocytes discharge gliotransmitters to modulate or respond to neuronal activity; these gliotransmitters activate receptors located at several sites of surrounding cells, leading to alterations in synaptic transmission and plasticity, therefore, turning astrocytes into active elements in brain functionality [2]. Glutamine is the major amino acid present in the intracellular and extracellular medium of the cultures, consistent with its role in glutamate–glutamine cycle (closely associated to glutamine synthetase activity [26]) and its ability to act as ammonia carrier. Glycine, the second most abundant amino acid, besides being a gliotransmitter, is a glutathione precursor and, therefore, essential for astrocytic function, as glutathione is an important antioxidant in the brain. Glutamate, aspartate and d-serine, some of the principal gliotransmitters, are present in low concentrations, as are the branched-chain amino acids isoleucine, leucine and valine.
In addition to its classical energetic role, ATP has also been demonstrated to act as a gliotransmitter, and when released from astrocytes may act in a bidirectional fashion, regulating neuronal excitability depending on concentration. In the astrocyte cytosol, ATP is produced via glycolysis and oxidative phosphorylation and reaches high cytosolic concentrations, thus establishing a concentration gradient favoring its exit from the cell [25], as also observed in the present study, under in vitro conditions. Some studies have shown that, once released, ATP is rapidly converted into ADO; however, the quantity of ATP measured was higher than that of ADO. As such, this signaling might be lost in astrocytes when in vitro (Fig. 4). Moreover, all synapses in the CNS are highly dependent on ATP and approximately 60 % of ATP produced in the CNS is consumed by Na+/K+-ATPase. For this reason, we measured Na+/K+-ATPase activity, under basal conditions, due to its vital role in cellular physiology, especially considering that astrocytes interact with neurons to promote the clearance of synaptically-released glutamate, in association with the co-transport of Na+ ions. Together with Ca2+, Na+ regulates the communication between astrocytes and neurons, since the intracellular levels of these ions rise when stimulation occurs at the tripartite synapse. After the entry of Na+ ions into the cytosol, the basal concentration of Na+ must be restored; the Na+/K+-ATPase acts as the major transporter of Na+ ions in astrocytes, and the activity of this enzyme is essential for the function of glutamate transporters. Since the clearance of glutamate from the synaptic cleft is a function of astrocytes, Na+/K+-ATPase might be impaired in aging and in neuropathological conditions, where excitotoxicity is an ordinary feature.
Previous studies have shown that astrocytes take up glutamate and GABA in physiological conditions; moreover, astrocytes play a pivotal role in the synthesis of these neurotransmitters, since they express glutamine synthetase, which provides the precursor for both glutamate and GABA [26]. Once taken up by astrocytes, GABA is completely metabolized to yield energy, whereas glutamate may, besides being metabolized via the tricarboxilic acids cycle, also replenish neurotransmitter pools via the glutamate–glutamine cycle or be used as a substrate for glutathione synthesis. We, herein, showed that astrocyte cultures express the GAT 1 protein, the major astrocytic GABA transporter (Fig. 5).
In addition to controlling the glutamatergic and GABAergic systems, astrocytes may also control the cholinergic system, since they present AchE activity, as shown in Table 1. The brain cholinergic system may modulate several important functions such as learning, memory, control over cerebral blood flow and inflammatory response; thus, our findings indicate that, even after weeks in culture, astrocyte cultures from adult animals continue to play roles that are important for neurotransmitter management. As such, our data indicate that these astrocytes are functional and suitable to better comprehend adult and aged brain.
Astroglial cannabinoid signaling plays a role in the modulation of synaptic plasticity, Ca2+ signaling and in the communication between astrocytes and neurons, through the release of gliotransmitters, via the cannabinoid receptors CB 1 and CB 2. Previous studies have shown that astrocytes from different brain regions may present either CB 1 or CB 2, where the CB 1 receptor is involved in the exocytosis of gliotransmitters, and, hence, in synaptic plasticity [27]. Herein, we have not found the presence of CB 1 in adult astrocyte cultures under basal conditions; this lack of receptor expression may compromise some astrocytic functions, particularly those related to neuroprotection. In fact, adult astrocytes culture and astrocytes aged in vitro have already demonstrated less capacity of fulfilling neuroprotective roles and perhaps the loss of CB 1 through the process of adaptation to the in vitro condition may be related to that constraint [13, 28].
Calcium signaling constitutes a primitive pathway of cellular communication that is conserved in most living organisms [29]. Several events are able to transiently elevate cytosolic Ca2+ in the astrocyte intracellular medium, including synaptically released neurotransmitters and neuromodulators and retrograde messengers such as endocannabinoids [1]. In order maintain a low Ca2+ threshold to permit [Ca2+] changes in the cytosol, Ca2+ concentrations must be kept low, with the extracellular concentration being ~10,000–20,000 times higher than the intracellular concentration. Accordingly, herein we showed that adult astrocytes cultures maintain their intracellular levels of Ca2+ at around 125 nm, in line with previous reports [29]. This large difference between intra and extracellular medium [Ca2+] allows the rapid pro-gradient diffusion of Ca2+ ions into the cells, to induce intracellular signaling.
Figure 6 summarizes the findings of our research group concerning the main properties of cultures of astrocytes from adult rats. Since we showed here that adult astrocytes possess and release gliotransmitters and are able to affect synaptic communication, not only at glutamatergic sites, we provide some evidence that this methodology is suitable for the routine study of the physiology and pathology of astrocytes. These cultures are an innovative and functional tool to study the cellular, biochemical and molecular properties of adult and aged brains; and further studies may employ these cultures for investigations of astrocytic biology, to characterize the roles of these cells in aging and senescence.
Functionality of adult astrocyte cultures. Figure 6 summarizes some of our findings regarding adult astrocyte cultures. These cells are able to take up glutamate through high-affinity transporters, in association with the co-transport of sodium ions (Na+). The Na+ ions can be released from the cell by Na+/K+ ATPase, while glutamate may have several fates within the cell. Herein we show that glutamate may enter the mitochondria for energy yielding or be released from astrocytes as a gliotransmitter, similarly to d-serine. GABA may enter the astrocyte through GAT 1 and generate ATP in the mitochondria, via the TCA cycle and ETC. ATP may either be used for cellular energetic requirements or be released as a gliotransmitter. EAAT excitatory amino acid transporter, TCA tricarboxylic acids cycle, ETC electron transport chain
References
De Pitta M, Brunel N, Volterra A (2015) Astrocytes: orchestrating synaptic plasticity? Neuroscience. doi:10.1016/j.neuroscience.2015.04.001
Perea G, Sur M, Araque A (2014) Neuron-glia networks: integral gear of brain function. Front Cell Neurosci 8:378. doi:10.3389/fncel.2014.00378
Allen NJ, Barres BA (2009) Neuroscience: glia—more than just brain glue. Nature 457(7230):675–677. doi:10.1038/457675a
Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121(1):4–27. doi:10.1111/j.1471-4159.2012.07664.x
Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD (2008) What is the role of astrocyte calcium in neurophysiology? Neuron 59(6):932–946. doi:10.1016/j.neuron.2008.09.004
Vedam-Mai V, van Battum EY, Kamphuis W, Feenstra MG, Denys D, Reynolds BA, Okun MS, Hol EM (2012) Deep brain stimulation and the role of astrocytes. Mol Psychiatry 17(2):124–131, 115. doi:10.1038/mp.2011.61
Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18(7):942–952. doi:10.1038/nn.4043
Booher J, Sensenbrenner M (1972) Growth and cultivation of dissociated neurons and glial cells from embryonic chick, rat and human brain in flask cultures. Neurobiology 2(3):97–105
Skytt DM, Madsen KK, Pajecka K, Schousboe A, Waagepetersen HS (2010) Characterization of primary and secondary cultures of astrocytes prepared from mouse cerebral cortex. Neurochem Res 35(12):2043–2052. doi:10.1007/s11064-010-0329-6
Lindsay RM, Barber PC, Sherwood MR, Zimmer J, Raisman G (1982) Astrocyte cultures from adult rat brain. Derivation, characterization and neurotrophic properties of pure astroglial cells from corpus callosum. Brain Res 243(2):329–343. doi:10.1016/0006-8993(82)90257-8
Lange SC, Bak LK, Waagepetersen HS, Schousboe A, Norenberg MD (2012) Primary cultures of astrocytes: their value in understanding astrocytes in health and disease. Neurochem Res 37(11):2569–2588. doi:10.1007/s11064-012-0868-0
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS ONE 8(3):e60282. doi:10.1371/journal.pone.0060282
Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2014) Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol In Vitro 28(4):479–484. doi:10.1016/j.tiv.2014.01.006
de Lores Arnaiz GR, Ordieres MG (2014) Brain Na(+), K(+)-ATPase activity in aging and disease. Int J Biomed Sci 10(2):85–102
Benarroch EE (2010) Glutamate transporters: diversity, function, and involvement in neurologic disease. Neurology 74(3):259–264. doi:10.1212/WNL.0b013e3181cc89e3
Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32(1):1–14. doi:10.1002/1098-1136(200010)32:1<1:AID-GLIA10>3.0.CO;2-W
Thullbery MD, Cox HD, Schule T, Thompson CM, George KM (2005) Differential localization of acetylcholinesterase in neuronal and non-neuronal cells. J Cell Biochem 96(3):599–610. doi:10.1002/jcb.20530
Schmidt AP, Tort AB, Silveira PP, Bohmer AE, Hansel G, Knorr L, Schallenberger C, Dalmaz C, Elisabetsky E, Crestana RH, Lara DR, Souza DO (2009) The NMDA antagonist MK-801 induces hyperalgesia and increases CSF excitatory amino acids in rats: reversal by guanosine. Pharmacol Biochem Behav 91(4):549–553. doi:10.1016/j.pbb.2008.09.009
Domanski L, Sulikowski T, Safranow K, Pawlik A, Olszewska M, Chlubek D, Urasinska E, Ciechanowski K (2006) Effect of trimetazidine on the nucleotide profile in rat kidney with ischemia-reperfusion injury. Eur J Pharm Sci 27(4):320–327. doi:10.1016/j.ejps.2005.10.012
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Scherer EB, da Cunha MJ, Matte C, Schmitz F, Netto CA, Wyse AT (2010) Methylphenidate affects memory, brain-derived neurotrophic factor immunocontent and brain acetylcholinesterase activity in the rat. Neurobiol Learn Mem 94(2):247–253. doi:10.1016/j.nlm.2010.06.002
Wyse AT, Streck EL, Barros SV, Brusque AM, Zugno AI, Wajner M (2000) Methylmalonate administration decreases Na + , K + -ATPase activity in cerebral cortex of rats. NeuroReport 11(10):2331–2334
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157(2):375–380
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):76–85
Zorec R, Verkhratsky A, Rodriguez JJ, Parpura V (2015) Astrocytic vesicles and gliotransmitters: slowness of vesicular release and synaptobrevin2-laden vesicle nanoarchitecture. Neuroscience. doi:10.1016/j.neuroscience.2015.02.033
Schousboe A, Bak LK, Waagepetersen HS (2013) Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol (Lausanne) 4:102. doi:10.3389/fendo.2013.00102
Rasooli-Nejad S, Palygin O, Lalo U, Pankratov Y (2014) Cannabinoid receptors contribute to astroglial Ca(2)(+)-signalling and control of synaptic plasticity in the neocortex. Philos Trans R Soc Lond B Biol Sci 369(1654):20140077. doi:10.1098/rstb.2014.0077
Souza DG, Bellaver B, Raupp GS, Souza DO, Quincozes-Santos A (2015) Astrocytes from adult Wistar rats aged in vitro show changes in glial functions. Neurochem Int. doi:10.1016/j.neuint.2015.07.016
Verkhratsky A, Rodriguez JJ, Parpura V (2012) Calcium signalling in astroglia. Mol Cell Endocrinol 353(1–2):45–56. doi:10.1016/j.mce.2011.08.039
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Financiadora de Estudos e Projetos (FINEP)—IBN Net (Instituto Brasileiro de Neurociências) 01.06.0842-00, Universidade Federal do Rio Grande do Sul (UFRGS), and Instituto Nacional de Ciência e Tecnologia para Excitotoxicidade e Neuroproteção (INCTEN/CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Souza, D.G., Bellaver, B., Hansel, G. et al. Characterization of Amino Acid Profile and Enzymatic Activity in Adult Rat Astrocyte Cultures. Neurochem Res 41, 1578–1586 (2016). https://doi.org/10.1007/s11064-016-1871-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1871-7