Abstract
Our previous studies had confirmed that both 3-NP and MCAO induced the behavioral defect as well as striatal neuronal injury and loss in experimental rats. This study aimed to examine different response forms of striatal astrocyte and microglia in 3-NP and MCAO rat models. The present results showed that the immunoreaction for GFAP was extremely weak in the lesioned core of striatum, but in the transition zone of 3-NP model and the penumbra zone of MCAO model, GFAP+ cells showed strong hypertrophic and proliferative changes. Statistical analysis for the number, size and integral optical density (IOD) of GFAP+ cells showed significant differences when compared with their controls and compared between the core and the transition zone or the penumbra zone, respectively, but no differences between the 3-NP and MCAO groups. However, Iba-1+ cells showed obvious hypertrophy and proliferation in the injured striatum in the 3-NP and the MCAO models, especially in the transition zone of 3-NP model and the penumbra zone of MCAO model. These Iba-1+ cells displayed two characteristic forms as branching cells with thick processes and amoeboid cells with thin processes. Statistical analysis showed that the number, size and IOD of Iba-1+ cells were significantly increased in the cores and the transition zone of 3-NP group and the penumbra zone of MCAO group than that of the controls, and the immune response of Iba-1 was stronger in the MCAO group than in the 3-NP group. The present results suggested that characteristic responses of astrocyte and microglia in the 3-NP and the MCAO models display their different effects on the pathological process of brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3-Nitropropionic acid (3-NP) is a suicide inhibitor of succinate dehydrogenase and has been shown to cause a Huntington’s disease (HD)-like syndrome in both primates [3] and rats [7]. I.p. 3-NP induced striatal lesions that resulted in dystonia and other abnormal motor behaviors mimicking the neurochemical and behavioral features of HD in humans. Our previous studies and others had confirmed that 3-NP injection could induce the behavioral defect, striatal lesion and neuronal loss in experimental animals [9, 24]. Middle cerebral artery occlusion (MCAO) in rodents is a common technique used to study mechanisms and potential treatments of cerebral ischemia. Previous studies had showed that MCAO causes motor and cognitive dys-functions as well as histological injuries in the striatum in experimental animals [4, 16]. Our previous studies have confirmed that an obvious zone which could be distinguished from both the lesion core and the peripheral area was present in lesioned striatum induced by both 3-NP (be called the transition zone) and MCAO (be called the penumbra zone) [22, 24]. Both transition and penumbra zone were considered to be the key zone for determining of the neuronal survival or death in pathology, and the important area for the neuronal rescue in clinical treatment [19, 29]. The characteristic reactions of different types of neurons in such zone have been reported in our previous studies, while how the morphological change (number and size) of glia cells respond to different injuries in such zone and if these changes be different from the lesioned core are still unknown.
Astrocyte and microglia are the most abundant cells in the central nervous system and are believed to play a major role in maintaining the normal functions of the brain and spinal cord. They closely interact with neurons to provide structural, metabolic and trophic support and actively participate in modulating neuronal excitability and neurotransmission by controlling the extracellular levels of ions and neurotransmitters [2]. Astrocyte and microglia become activated (reactive) in response to many CNS injuries, including brain infection, inflammation, ischemia, trauma, neurodegenerative diseases, and tumor [10, 15, 32]. Reactive glia cells undergo changes in morphology and in their expression of a wide range of molecules. The hallmarks of astrocyte and microglia activation are enlarged cell body, increased number and length of processes as well as enhanced expression of glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba-1), respectively. However, it is still unknown whether astrocyte and microglia showed different response in the specific zone (such as the transition and penumbra zone) to different injuries of brain. Thus, in the present study both 3-NP and MCAO rat models were used, and the response of astrocyte and microglia was characterized by detecting the number, size and IOD of GFAP+ cells and Iba-1+ cells in the lesioned striatum, especially in the transition zone and the penumbra zone.
Materials and Methods
Experimental Design and Animal Groups
Sprague–Dawley rats were obtained from the Center for Experimental Animals of Sun Yat-sen University and housed with a 12-h light/dark cycle and ad libitum access to food and water. 25 adult Sprague–Dawley male rats (body weight, 250–280 g) were randomly assigned to one of the following five experimental groups (five per group): the 3-NP group, the saline group, the MACO group, the sham-operated group and the control group (the normal rats).
All animal procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Guangdong Medical Laboratory Animal Center Institutional Animal Care and Use Committee.
Animal Model of Systemic 3-NP Treatment and Middle Cerebral Artery Occlusion
The 3-NP group received 3-NP (dissolved in saline to a concentration of 15 mg/ml, pH7.4; Sigma, St. Louis, MO) administered intraperitoneally twice daily for 5 days at a dose of 25 mg/kg/day. The saline group was matched by weight and received only vehicle i.p. The animals were sacrificed 1 day after the last injection.
The rats in the MCAO group underwent permanent MCAO procedures, as described previously [25]. The animals were anesthetized with a solution of ketamine (75 mg/kg) and xylazine (12.5 mg/kg) and incised medially in the neck using blunt dissection techniques. The right common, external, and internal carotid arteries were exposed and isolated carefully from the vagus nerve. After clamping the common and internal carotid artery, the external carotid artery was tied and a small hole was made in its branch. A monofilament (0.34 mm in diameter, GUANGZHOU BOYA BIOTECH CO, LTD, CHINA) was inserted through the hole and advanced to a length of 18–20 mm via the internal carotid, finally reaching the middle cerebral artery (MCA). Then the incision was sutured. The rats in the sham-operated group received the same procedures, except that the MCA was not occluded. The animals were sacrificed 5 day after the operation.
Immunohistochemistry
Animals were anesthetized with a solution of ketamine (75 mg/kg) and xylazine (12.5 mg/kg) and perfused first with 400 ml of saline and then 400 ml of 4 % paraformaldehyde (in 0.1 M PBS, pH 7.4). Brains were then removed and postfixed in the same fixative, and then coronal Sects. (30 μm) were cut on a vibratome (VIBRATOME, #053746). Sections were pretreated with 0.3 % H2O2 in 0.01 M PBS at 37 °C for 30 min and then incubated overnight at 4 °C in mouse anti-glial fibrillary acidic protein (GFAP) (1:1000, Santa Cruz Biotechnology), and rabbit anti-ionized calcium binding adaptor molecule 1 (Iba-1) (1:1000, Millipore). Sections were then rinsed and incubated in anti-mouse IgG or anti-rabbit IgG (1:200, Sigma), followed by incubating in the appropriate mouse or rabbit PAP complex (1:200, Sigma) at room temperature for 2 h. The DAB-peroxidase reaction (0.05 % in 0.01 M PBS, pH 7.4, Sigma) was carried out for 2–8 min and mounted onto gelatin-coated slides, dried, dehydrated, cleared with xylene, and covered with neutral balsam.
Data Collection and Statistical Analysis
In each group, ten sections for each rat were analyzed per labeling type. The sections were taken from levels corresponding approximately to the interaural plane from 10.70 to 8.74 mm (according to the atlas of Paxinos and Watson) [30]. Sections were viewed and images captured using an Olympus BHS microscope (Tokyo, Japan), using a ×100 objective. The integral optical density (IOD) of positive cells in five randomly selected areas (0.01 mm2 for each) in both core and zone (transition and penumbra) was measured with an image analysis program (Image-Pro Plus 6.0). The number of labeled perikarya was counted in five randomly selected areas (0.01 mm2 for each) in both core and zone (transition and penumbra) for each section. The size of ten randomly selected cells in both core and zone (transition and penumbra) of each section was measured by Image-Pro Plus.
All data in this study are presented as mean ± SEM. The statistical significance of the results was evaluated by One-Way ANOVA with multiple comparison t-tests with SPSS analytical software and P < 0.05 was considered as significant.
Results
There was no significant difference in weight among groups (data not shown). Statistical analyses were made between the saline and control groups as well as between the sham-operated and control groups, respectively, and there also were no significant differences in number, size and IOD of labeled cell (data not shown). To make the following report concise and easier to understand, we provide data and statistical analyses only for the control, 3-NP, and MCAO groups in the following text.
Morphological Observation of Striatal Injury Induced by 3-NP and MCAO
Our previous studies had confirmed that both 3-NP and MCAO model rats yielded movement, motor coordination and cognitive dysfunction, and histochemical and immunohistochemical stainings showed that a clear lesion was located in the dorsolateral striatum with almost all neuronal loss in the lesion core and lesser damage around the area, where was called the transition zone in the 3-NP model and the penumbra zone in the MCAO model [18, 21–24], and were considered to be the key zone for determining of the neuronal survival or death in pathology, and were the important area for the neuronal rescue in clinical treatment [19, 29]. In accord with our previous studies, immunohistochemical staining for both GFAP and Iba-1 confirmed the presence of a lesioned core surrounding by the transition or penumbra zone in the striatum of this experiment (dots in Figs. 1b, c and 2b, c). In the 3-NP and the MCAO models, GFAP immunohistochemistry showed nearly no positive cells in the central area of lesions, whereas numerous hyperplastic GFAP+ cells were observed in the transition and in the penumbra zone, respectively. However, immunoreactivity for Iba-1 showed an identical increase in the lesion core, especially a strong immunoreactivity in the transition of 3-NP model and the penumbra zone of MCAO model, respectively.
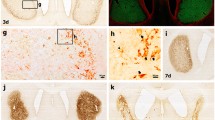
Reaction of striatal GFAP+ astrocytes to 3-NP and MCAO. GFAP+ cell reactions were showed in a–a″ (control), b–b″ (3-NP group) and c–c″ (MCAO group). b′ and c′ showed the strong immunoreactions of GFAP+ cells in the transition zone of 3-NP (TZ, b′) and the penumbra zone of MCAO (PZ, c′), respectively. b″ and c″ showed the absence of GFAP+ cell reactions in the core of 3-NP (b″) and MCAO groups (c″), respectively. The lesion core (filled star) was surrounded by the transition zone (dots in b) and penumbra zone (dots in c). Arrows showed positive fibers or fibrotic cells, and arrowheads for somas. a′–c′ and a″–c″ were the higher magnifications of the area outlined in a–c, respectively. a–c were the same magnification; a′–c′ and a″–c″ were the same magnification. The 3 groups were presented as Ctrl (control group), 3-NP (3-NP group) and MCAO (MCAO group)
Activation of striatal microglia cells induced by 3-NP and MCAO. Iba-1+ cell reactions were showed in a–a″ (control), b–b″ (3-NP group) and c–c″ (MCAO group). b′ and c′ showed the immunoreactions of Iba-1+ cells in the transition zone of 3-NP (TZ, b′) and the penumbra zone of MCAO (PZ, c′), respectively. b″ and c″ showed the strong Iba-1+ cell reactions in the core of 3-NP (b″) and MCAO groups (c″), respectively. The lesion core (filled star) was surrounded by the transition zone (dots in b) and penumbra zone (dots in c). Arrows showed amoeboid cells, and arrowheads for branching cells. a′–c′ and a″–c″ were the higher magnifications of the area outlined in a–c, respectively. a–c were the same magnification; a′–c′ and a″–c″ were the same magnification. The 3 groups were presented as Ctrl (control group), 3-NP (3-NP group) and MCAO (MCAO group)
Characteristic Response of Striatal Astrocyte to the Injury Induced by 3-NP and MCAO
Immunohistochemistry for GFAP showed that astrocytes with small bodies and slender processes were detected in the striatum of controls (Fig. 1a, a′, a″). However, in both 3-NP and MCAO groups, the enlargement and increase of GFAP+ soma, as well as the thickening of processes were observed in the transition zone and the penumbra zone, respectively, and a lots of fibrotic GFAP+ fibers were present simultaneously (arrows in Fig. 1b′, c′). In contrast, in the lesioned core in both 3-NP and MCAO groups, few positive GFAP cells could be detected (Fig. 1b″, c″). Data for the count of GFAP + cells showed that the number of GFAP+ cells was 13.13 ± 0.57 in the control, however, a significant increase was observed in both the transition zone in the 3-NP group (18.87 ± 0.49, P < 0.05, Table 1) and the penumbra zone in the MCAO group (16.93 ± 0.30, P < 0.05, Table 1), but there was no significant difference when compared between the 3-NP group and the MCAO group (P > 0.05, Table 1). In the lesioned core, the GFAP+ number was 1.8 ± 0.01 in the 3-NP and 3.5 ± 0.01 in the MCAO groups, which were significant decreased than their controls, respectively (P < 0.05, P < 0.05, Table 1). In addition, statistical analysis also showed that the size of GFAP+ soma in the control was 4.68 ± 0.21, but 2.97 ± 0.13 for 3-NP group and 3.12 ± 0.22 for MCAO group in their cores, which were significantly smaller than their controls (P < 0.05, P < 0.05, Table 1). However, the soma size in the transition zone of 3-NP group and in the penumbra zone of MCAO group, respectively, were 9.47 ± 0.56 and 9.29 ± 0.48, which were significantly larger than their controls, (P < 0.05, P < 0.05, Table 1), but the statistical analysis showed no significant differences between the 3-NP and the MCAO groups (P > 0.05, Table 1). Furthermore, analysis for the IOD showed that obvious increase in the transition zone of the 3-NP group and in the penumbra zone of the MCAO group were observed when compared with their controls (P < 0.05, P < 0.05, Table 1), but decrease in the core than their controls (P < 0.05, P < 0.05, Table 1). However, no statistical difference was found between the 3-NP and the MCAO groups (P > 0.05, Table 1).
Morphological Changes of Striatal Microglia Induced by 3-NP and MCAO
Morphological changes of striatal microglia induced by 3-NP and MCAO were examined by Iba-1 immunohistochemistry. In the control, Iba-1+ cells with small soma, slender and rare processes distributed uniformly in all the striatum (Fig. 2a, a′). In the lesion areas of the 3-NP and the MCAO groups, there was a significant increase in Iba-1 immunoreactivity with hypertrophic soma, thicken processes, and an increased number of processes, especially in the transition zone and the penumbra zone (arrowheads in Fig. 2b′, c′, b″, c″). In addition, some Iba-1+ amoeboid cells characterized as round and small soma with thin processes were found scattered among the branching cells (arrows in Fig. 2b′, c′), and their sizes were obviously smaller than the Iba-1+ branching cells. To detect morphological changes of Iba-1+ cells induced by the 3-NP and the MCAO, a count of Iba-1+ cells was performed in both the core and zone of lesioned striatum, as well as in the striatal corresponding area of the control group. The statistical analysis showed significant increases on Iba-1 + cell number both in the 3-NP group (17.57 ± 0.54 for the core and 19.00 ± 0.71 for the transition zone, P < 0.05) and in the MCAO group (16.45 ± 0.78 for the core and 21.53 ± 0.63 for the penumbra zone, P < 0.05) compared to their control groups (10.60 ± 0.51, Table 2). The size of Iba-1+ cells in the 3-NP group (6.98 ± 0.34 for the core and 7.88 ± 0.25 for the transition zone,P < 0.05) and the MCAO group (7.10 ± 0.44 for the core and 9.25 ± 0.31 for the penumbra zone,P < 0.05) were significantly larger than their control groups (5.75 ± 0.19, Table 2). Statistical results for IOD detection also showed that the Iba-1+ cells in the 3-NP group and the MCAO group were significantly higher than their controls (P < 0.05, P < 0.05, Table 2). Furthermore, the number, size and IOD of Iba-1+ cells in the MCAO group were significantly increased than that in the 3-NP group, (P < 0.05, P < 0.05, P < 0.05, Table 2).
Discussion
Our previous studies and the others had confirmed the serious injury of striatum induced by 3-NP and MCAO, respectively, especially the numerous neuronal losses in striatum. Many factors are involved in this pathological process, whereas striatal astrocyte and microglia may also participate in the injury and repair of striatum. The present study designed to detect the characteristic response of astrocyte and microglia to striatal injury induced by 3-NP and MCAO morphologically. Our present results confirmed that both 3-NP administration and MCAO for 5 days to rats strongly induced reactive gliosis in lesioned striatum. However, astrocyte and microglia showed different responses to different injuries, especially the Iba-1+ microglia showed stronger hypertrophy and proliferation in the penumbra zone of MCAO than in the transition zone of 3-NP rats.
Experimental results over the last decade have led to consideration of glial cells as major active players in the function of the CNS in health and disease [28]. It has been reported that both an injury to the central nervous system and neurodegenerative diseases activate resting microglial cells within the nervous system, a process that sustains the astrocytic activation through the production and release of inflammatory mediators that in turn act on other glial cells and neurons, thus sensitizing neural cells and facilitating neurodegenerative processes [11]. This leads to an imbalance in neurotransmitter homeostasis, disruption of synaptic connectivity and neuronal death, probably through increased excitotoxicity [27]. However, it is different to answer the question whether reactive glial cells are harmful or beneficial. Several studies have been conducted to fully characterize the complex molecular and structural changes that occur during reactive glial cells. Recently, team of Giovannini characterized the interplay among neurons and glia in several animal models. Their results demonstrated that astrocytes and microglia in the hippocampus of aged and adult LPS-treated rats participate in the clearance of neuronal debris associated with programmed cell death and phagocytosis of apoptotic neurons [5, 13]. These efforts only produced unequivocal evidence of the role played by reactive glial cells in different experimental models suggesting that glial cells are engaged in neurological diseases determining the onset, progression and outcome of the neuropathological process [17]. In this study, we only characterized the different response forms of microglia and astrocytes in ischemic and neurodegenerative brain injury, however, our result has potential to provide a morphological data to further study the interactions between neurons, microglia and astrocytes within the brain injury.
The changes of astrocytes induced by 3-NP was reported in vivo [7, 33] and in vitro [8]. Mainly, it was already described that GFAP increased after 3-NP high dosage chronic treatment and this finding is well correlated with behavioral impairments [33]. Increases of GFAP immunoreaction had also been observed previously in the MCAO rat model and the other brain ischemia models [12, 14, 26], which demonstrated that ischemia resulted in increased number of astrocytes in ischemic penumbra area as early as 30 min after reperfusion in the ipsilateral hemisphere of the animals. The GFAP-positive astrocytes remained increased thereafter, reached to peak level after 1–3 days of reperfusion, and then gradually decreased to control level after 28 days of reperfusion [12]. Here, we also detected the characterized response of astrocytes by measuring the number and size of GFAP+ cells after 5 days of 3-NP injection and MCAO in rats. Our results showed that GFAP-positive astrocytes can hardly be detected in the lesioned core but showed hypertrophy and proliferation in both the transition zone and the penumbra zone, which is consistent with the previous studies [35]. Obviously, although astrocytes increase could be caused by the subchronic treatment around the lesion area, this may develop into loss of astrocytes next to extensive neuronal death in the core of a severe striatal lesion eventually. Astrocytes have been found to play a fundamental activity in the developing central nervous system and exert a neuroprotective in response to many CNS injuries [32, 34]. This can let us hypothesize that the activated astrocytes could only work effectively in the chronic injury or at the early stage of brain injury.
Besides astrocytes, microglia also played a crucial role in cellular proliferation and glial scar formation post brain injury. As a major source of proinflammatory factors in brain, microglia is significantly activated after 3-NP treatment [6] and ischemic stroke [1]. Compared with astrocytes, Iba1+ microglia recruits to ischemic regions more rapidly in response to ischemia. Another difference between astrocytes and microglia is that the percentage of proliferating microglia from total proliferating cells is much higher than the percentage of proliferating reactive astrocytes after stroke [14]. Our present study showed that Iba-1+ microglia, different from astrocytes, showed obvious hypertrophy and proliferation in the damaged striatum in the 3-NP and the MCAO models, especially in the transition zone and the penumbra zone. This is consistent with a recent study showing that microglial response was local, confined to the core and neighboring lesion [31]. First activated microglia were observed in the infarct core within 24 h after photothrombotic stroke, on day 4 the microglial reaction had intensified, and isolectin IB4 binding delineated numerous ameboid and round cells accumulating in the infarct core [26]. Such ameboid cells were also observed scattered among the ramified glia cells in our present study. Ameboid cell is also called ameboid microglia, is the undifferentiated microglia, have the ability to proliferate, phagocytose apoptotic cells and migrate long distances toward their final destinations throughout all CNS regions, where they acquire a mature ramified morphological phenotype. Recent studies indicate that ameboid microglial cells not only have a scavenger role during development but can also promote the death of some neuronal populations [20]. Thus, the appearance of ameboid cells in the injured striatum indicates that it may assist the mature glia cells participating in the neuronal injury and repair of striatum. Besides, our results showed a stronger response of microglia in the MCAO group than in the 3-NP model, which might be due to more serious damage in striatum induced by MCAO than 3-NP. Together, we can infer that, in comparison with astrocyte, mitroglia might show more quick and persistent activation in response to the brain injury.
In conclusion, our results suggest that astrocyte and microglia showed different spatiotemporal dynamics in response to 3-NP- and MCAO-induced striatal injury, which is important for understanding the mechanism of astrocyte and microglia action in the pathological process of brain injury.
References
Annunziato L, Boscia F, Pignataro G (2013) Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia. J Cereb Blood Flow Metab 33:969–982
Bezzi P, Volterra A (2001) A neuron-glia signalling network in the active brain. Curr Opin Neurobiol 11:387–394
Brouillet E, Jacquard C, Bizat N, Blum D (2005) 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J Neurochem 95:1521–1540
Carmichael ST (2005) Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx 2:396–409
Cerbai F, Lana D, Nosi D, Petkova-Kirova P, Zecchi S, Brothers HM, Wenk GL, Giovannini MG (2012) The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS ONE 7:e45250
Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP (2014) Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s Disease. CNS Neurosci Ther 20:10–19
Cirillo G, Maggio N, Bianco MR, Vollono C, Sellitti S, Papa M (2010) Discriminative behavioral assessment unveils remarkable reactive astrocytosis and early molecular correlates in basal ganglia of 3-nitropropionic acid subchronic treated rats. Neurochem Int 56:152–160
Deshpande SB, Nishino H (1998) In vitro protection of 3-nitropropionic acid-induced toxicity of astrocytes by basic fibroblast growth factor and thrombin. Brain Res 783:28–36
Fernagut PO, Diguet E, Stefanova N, Biran M, Wenning GK, Canioni P, Bioulac B, Tison F (2002) Subacute systemic 3-nitropropionic acid intoxication induces a distinct motor disorder in adult C57Bl/6 mice: behavioural and histopathological characterisation. Neuroscience 114:1005–1017
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11(1):56–64.
Ji RR, Strichartz G (2004) Cell signaling and the genesis of neuropathic pain. Science 2004:reE14
Jing L, He Q, Zhang JZ, Li PA (2013) Temporal profile of astrocytes and changes of oligodendrocyte-based myelin following middle cerebral artery occlusion in diabetic and non-diabetic rats. Int J Biol Sci 9:190–199
Lana D, Melani A, Pugliese AM, Cipriani S, Nosi D, Pedata F, Giovannini MG (2014) The neuron-astrocyte-microglia triad in a rat model of chronic cerebral hypoperfusion: protective effect of dipyridamole. Front Aging Neurosci 6:322
Li H, Zhang N, Lin HY, Yu Y, Cai QY, Ma L, Ding S (2014) Histological, cellular and behavioral assessments of stroke outcomes after photothrombosis-induced ischemia in adult mice. BMC Neurosci 15:58
Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481
Liu F, Schafer DP, McCullough LD (2009) TTC, fluoro-Jade B and NeuN staining confirm evolving phases of infarction induced by middle cerebral artery occlusion. J Neurosci Methods 179:1–8
Lobsiger CS, Cleveland DW (2007) Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci 10:1355–1360
Ma Y, Feng Q, Ma J, Feng Z, Zhan M, Ouyang L, Mu S, Liu B, Jiang Z, Jia Y, Li Y, Lei W (2013) Melatonin ameliorates injury and specific responses of ischemic striatal neurons in rats. J Histochem Cytochem 61:591–605
Manning NW, Campbell BC, Oxley TJ, Chapot R (2014) Acute ischemic stroke: time, penumbra, and reperfusion. Stroke 45:640–644
Marin-Teva JL, Cuadros MA, Martin-Oliva D, Navascues J (2011) Microglia and neuronal cell death. Neuron Glia Biol 7:25–40
Mu S, Lin E, Liu B, Ma Y, OuYang L, Li Y, Chen S, Zhang J, Lei W (2014) Melatonin reduces projection neuronal injury induced by 3-nitropropionic acid in the rat striatum. Neuro Degener Dis 14:139–150
Mu S, Ouyang L, Liu B, Qu H, Zhu Y, Li K, Lei W (2011) Relationship between inflammatory reaction and ischemic injury of caudate-putamen in rats: inflammatory reaction and brain ischemia. Anatomical Sci Int 86:86–97
Mu S, OuYang L, Liu B, Zhu Y, Li K, Zhan M, Liu Z, Jia Y, Lei W (2011) Protective effect of melatonin on 3-NP induced striatal interneuron injury in rats. Neurochem Int 59:224–234
Mu S, OuYang L, Liu B, Zhu Y, Li K, Zhan M, Liu Z, Jia Y, Lei W, Reiner A (2011) Preferential interneuron survival in the transition zone of 3-NP-induced striatal injury in rats. J Neurosci Res 89:744–754
Nishino H, Koide K, Aihara N, Kumazaki M, Sakurai T, Nagai H (1993) Striatal grafts in the ischemic striatum improve pallidal GABA release and passive avoidance. Brain Res Bull 32:517–520
Nowicka D, Rogozinska K, Aleksy M, Witte OW, Skangiel-Kramska J (2008) Spatiotemporal dynamics of astroglial and microglial responses after photothrombotic stroke in the rat brain. Acta Neurobiol Exp (Wars) 68:155–168
Papa M, De Luca C, Petta F, Alberghina L, Cirillo G (2014) Astrocyte-neuron interplay in maladaptive plasticity. Neurosci Biobehav Rev 42:35–54
Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho) physiology. J Neurochem 121:4–27
Paschen W, Mies G, Hossmann KA (1992) Threshold relationship between cerebral blood flow, glucose utilization, and energy metabolites during development of stroke in gerbils. Exp Neurol 117:325–333
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Ryu JK, Nagai A, Kim J, Lee MC, McLarnon JG, Kim SU (2003) Microglial activation and cell death induced by the mitochondrial toxin 3-nitropropionic acid: in vitro and in vivo studies. Neurobiol Dis 12:121–132
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Teunissen CE, Steinbusch HW, Angevaren M, Appels M, de Bruijn C, Prickaerts J, de Vente J (2001) Behavioural correlates of striatal glial fibrillary acidic protein in the 3-nitropropionic acid rat model: disturbed walking pattern and spatial orientation. Neuroscience 105:153–167
Vernadakis A (1996) Glia-neuron intercommunications and synaptic plasticity. Prog Neurobiol 49:185–214
Vis JC, Verbeek MM, De Waal RM, Ten Donkelaar HJ, Kremer HP (1999) 3-Nitropropionic acid induces a spectrum of Huntington’s disease-like neuropathology in rat striatum. Neuropathol Appl Neurobiol 25:513–521
Acknowledgments
This work was supported by the National Science Foundation of China (Nos. 81471288, 81301063 and 31070941) and by the Major State Basic Research Development Program of China (973 Program, No. 2010CB530004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shuhua Mu and Bingbing Liu have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Mu, S., Liu, B., Ouyang, L. et al. Characteristic Changes of Astrocyte and Microglia in Rat Striatum Induced by 3-NP and MCAO. Neurochem Res 41, 707–714 (2016). https://doi.org/10.1007/s11064-015-1739-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1739-2