Abstract
Inflammation and oxidative stress have been reported to play critical roles in the pathogenesis of neurodegenerative disease. Forsythiaside A, a phenylethanoside product isolated from air-dried fruits of Forsythia suspensa, has been reported to have anti-inflammatory and antioxidant effects. In this study, the anti-inflammatory effects of forsythiaside A on LPS-stimulated BV2 microglia cells and primary microglia cells were investigated. The production of inflammatory mediators TNF-α, IL-1β, NO and PGE2 were detected in this study. NF-κB, nuclear factor-erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1) expression were detected by western blot analysis. Our results showed that forsythiaside A significantly inhibited LPS-induced inflammatory mediators TNF-α, IL-1β, NO and PGE2 production. LPS-induced NF-κB activation was suppressed by forsythiaside A. Furthermore, forsythiaside A was found to up-regulate the expression of Nrf2 and HO-1. In conclusion, this study demonstrates that forsythiaside A inhibits LPS-induced inflammatory responses in BV2 microglia cells and primary microglia cells through inhibition of NF-κB activation and activation of Nrf2/HO-1 signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that neuroinflammation and oxidative stress are implicated in the development of neurodegenerative disease, such as Parkinson’s disease and Alzheimer’s disease [1, 2]. Microglia, one of the most important cells in the central nervous system (CNS), plays a critical role in the progress of neuroinflammation [3]. Stimulating of microglia by LPS induces NF-κB activation and inflammatory mediators production [4]. These inflammatory mediators such as TNF-α, IL-1β, NO, and PGE2 are known to be implicated in the pathogenesis of neurodegenerative diseases [5]. Recently studies showed that pharmacological or nutritional intervention to attenuate microglial activation could attenuate the severity of neurodegenerative diseases [6, 7].
Forsythiaside A, one of the main active ingredients isolated from air-dried fruits of Forsythia suspensa, has been reported to have anti-inflammatory and antioxidant effects [8]. Forsythiaside A was found to protect against transient cerebral global ischemia in gerbil [9]. Forsythiaside A was also found to inhibit hydrogen peroxide-induced oxidative stress in PC12 cell [10]. Previous studies showed that forsythiaside A inhibited LPS-induced injury on the bursa of Fabricius (BF) of chickens [11]. In addition, forsythiaside A has been reported to protect against LPS/d-galactosamine-induced liver injury [12] and LPS-induced acute lung injury in mice [13]. However, the anti-inflammatory effects of forsythiaside A on LPS-stimulated BV2 microglia cells have not been reported. Therefore, this study aimed to investigate the anti-inflammatory effects and mechanisms of forsythiaside A on LPS-stimulated BV2 microglia cells.
Materials and Methods
Materials
Forsythiaside (purity > 98 %) was purchased from the National Institutes for Food and Drug Control (China). LPS (Escherichia coli O55:B5) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma (St. Louis, MO, USA). ELISA kits of PGE2, TNF-α, and IL-1β were purchased from R&D Systems (Minneapolis, MN, USA). Rabbit anti-human NF-κB p65, IκBα, Nrf2, HO-1, and β-actin monoclonal antibodies were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA, USA). All other reagents were of analytical grade.
Cell Culture and Viability
BV2 microglia cells were purchased from the Institute of Basic Medical Sciences of the China Science Academy. The cells were cultured in DMEM supplemented with 10 % FBS, 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C. Primary rat microglia cells were cultured as reported elsewhere [14]. In brief, whole brains of 1-day-old neonatal Wistar rats were dissociated into individual cells that were cultured for 11 or 14 days as mixed glial cultures with 10 % fetal calf serum (FCS)-supplemented Dulbecco’s Modified Eagle’s Medium (DMEM; Wako, Osaka, Japan). For the analysis of cell viability, the cells (2 × 105 cells/ml) were treated with different concentrations of forsythiaside A in the presence or absence of LPS (0.5 μg/ml) for 24 h. Cell viability was detected by MTT assay.
Cytokine Assays
The levels of TNF-α, IL-1β, and PGE2 in the culture supernatant were determined using a commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacture’s protocol. The absorbance at 450 nm was measured using a microplate leader.
Nitrite Quantification
The generation of NO was assessed by measuring the nitrite accumulation in the medium using the Griess reaction. Briefly, BV2 microglia cells or primary microglia cells were pretreated with forsythiaside A and stimulated with LPS for 24 h. 100 μl of the cell media was mixed with an equal amount of Griess reagent. Light absorbance was read at 540 nm.
Western Blot Analysis
Nuclear and cytoplasmic proteins of cells were extracted using NE-PER protein extraction reagents (Pierce, Rock ford, IL, USA). Protein concentration was determined by the Bradford method (Bio-rad, USA). Equal amounts of protein were separated on 10 % SDS-PAGE and then transferred to nitrocellulose membranes (Amersham, Arlington Heights, IL). After blocking with 5 % skim milk for 1 h at room temperature, the membrane was incubated with primary antibodies against NF-κB, IκBα, Nrf2, and HO-1. After washing three times, the membranes were probed with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive proteins were visualized using the ECL Prime Western blotting detection reagents (GE Healthcare, NJ, USA).
Nrf2 siRNA Transfections
BV2 microglia cells were transfected with Nrf2 siRNA (100 nM) or control siRNA (100 nM) using FuGENE HD transfection reagents (Promega, USA). 36 h later, the cells were treated with linalool and LPS. The production of TNF-α, IL-1β, NO and PGE2 were detected 24 h after LPS treatment.
Statistical Analysis
The values are expressed as mean ± SEM. Significance of the differences between groups were analyzed using one-way ANOVA followed by the Bonferroni test. Statistical significance was accepted p < 0.05 or p < 0.01.
Results
Effects of Forsythiaside A on Cell Viability
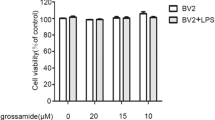
The effects of forsythiaside A on the viability of BV2 microglia cells or primary microglia cells were detected by MTT assay. As shown in Fig. 1, the results showed that forsythiaside A at the concentration of 2.5, 5, 10 μg/ml exerted no significant cytotoxicity on BV-2 microglial cells (Fig. 1a) or primary microglia cells (Fig. 1b).
Effects of forsythiaside A on the cell viability of BV-2 microglial cells (a) or primary microglia cells (b). Cells were cultured with different concentrations of PA (2.5, 5, 10 μg/ml) in the absence or presence of 0.5 μg/ml LPS for 24 h. The cell viability was determined by MTT assay. The values presented are the mean ± SEM of three independent experiments
Forsythiaside A Inhibits LPS-Induced TNF-α, IL-1β, NO, and PGE2 Production
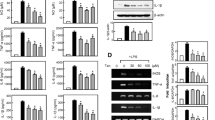
To evaluate the anti-inflammatory effects of forsythiaside A, the inhibitory effects of forsythiaside A on inflammatory mediators production were detected. Our results showed that LPS challenge significantly increased the production of inflammatory mediators TNF-α, IL-1β, NO and PGE2 production in BV2 microglia cells or primary microglia cells. However, treatment of forsythiaside A markedly attenuated LPS-induced TNF-α, IL-1β, NO and PGE2 production in BV2 microglia cells (Fig. 2a) or primary microglia cells (Fig. 2b).
Effects of forsythiaside A on LPS-induced TNF-α, IL-1β, NO and PGE2 production in BV-2 microglial cells (a) or primary microglia cells (b). The data presented are the mean ± SEM of three independent experiments and differences between mean values were assessed by Students’s t test. # p < 0.05 versus control group; *p < 0.05, **p < 0.01 versus. LPS group
Forsythiaside A Inhibits LPS-Induced NF-κB Activation
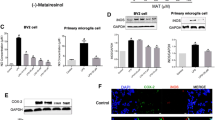
NF-κB is the main regulator of inflammatory mediators production. Therefore, the effects of forsythiaside A on LPS-induced NF-κB activation were detected in this study. As shown in Fig. 3, LPS challenge significantly increased NF-κB p65 translocation from cytoplasm to nuclear and IκBα degradation. However, LPS-induced NF-κB activation was significantly inhibited by treatment of forsythiaside A.
Forsythiaside A Induces Nrf2 Nuclear Translocation and HO-1 Expression
Nrf2/HO-1 signaling pathway has been reported to play a critical role in regulation of NF-κB activation. In the present study, the effects of forsythiaside A on Nrf2 nuclear translocation and HO-1 expression were detected in this study. The results showed that forsythiaside A could induce the translocation of Nrf2 from cytoplasm to nuclear and up-regulated the expression of HO-1 (Fig. 4).
Forsythiaside A Inhibits LPS-Induced Inflammatory Mediators Through Activating Nrf2
To investigate whether activation of Nrf2 is responsible for the anti-inflammatory effects of forsythiaside A, Nrf2 was knockdown by siRNA. Our results showed that Nrf2 knockdown significantly reversed the inhibition of NF-α, IL-1β, NO and PGE2 production by forsythiaside A (Fig. 5).
Discussion
Forsythiaside A, a phenylethanoside product isolated from air-dried fruits of Forsythia suspensa, has been reported to have anti-inflammatory effects. In the present study, the anti-inflammatory effects of forsythiaside A on LPS-stimulated BV2 microglia cells and primary microglia cells were detected. Our results showed that forsythiaside A significantly inhibited LPS-induced inflammatory mediators production. Forsythiaside A may be a therapeutic agent for neurodegenerative diseases.
Microglia are resident immune cells that play important roles in the progress of neuroinflammation [15]. During bacterial infection, the uncontrolled activation of microglia can cause neuronal injury [16]. LPS, a constituent of the cell well of Gram-negative bacteria, has been recognized as the primary factor that induces microglia activation [17, 18]. LPS significantly induced the production of inflammatory mediators including TNF-α, IL-1β, PGE2 and NO [19]. It has been reported the inflammatory mediators TNF-α, IL-1β, PGE2 and NO were increased in CNS-related diseases [20]. Overproduction of these inflammatory mediators could induce neuronal cell death [21]. In addition, TNF-α and IL-1β could induce the production of other inflammatory mediators such as PGE2 and NO to amplify the inflammatory responses [22]. In this study, the results showed that forsythiaside A inhibited LPS-induced TNF-α, IL-1β, PGE2 and NO production in a dose-dependent manner, suggesting forsythiaside A had the ability to attenuate neuroinflammation.
It is well known that NF-κB plays an important role in inflammatory mediators production [23]. During neuroinflammation, NF-κB regulates the production of TNF-α, IL-1β, PGE2 and NO [7]. Thus, in this study, the effects of forsythiaside A on LPS-induced NF-κB activation were detected. The results showed that treatment of forsythiaside A significantly inhibited NF-κB activation and IκBα degradation. Previous studies showed that HO-1 played important anti-inflammatory roles in LPS-stimulated BV2 microglia cells [24]. Activating HO-1 attenuated LPS-induced inflammatory mediators production [25]. Nrf2, an important transcription factor, has been shown to be involved in inflammatory and antioxidant responses [26]. Activation of Nrf2 could regulate the transcription of antioxidant enzymes such as HO-1 [27]. In this study, our results showed that forsythiaside A dose-dependently up-regulated the expression of Nrf2 and HO-1. Furthermore, our results showed that Nrf2 knockdown significantly reversed the anti-inflammatory effects of forsythiaside A. These results suggested that forsythiaside A inhibited LPS-induced inflammatory response by inhibiting Nrf2 signaling pathway.
In conclusion, the present study demonstrates that forsythiaside A inhibits TNF-α, IL-1β, PGE2 and NO production in LPS-stimulated BV2 microglia cells. The promising anti-inflammatory mechanism of forsythiaside A occurs by inhibiting NF-κB activation and activating of Nrf2/HO-1 signaling pathway. These findings suggest that forsythiaside A may be a possible agent in the treatment of neurodegenerative diseases.
References
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397
Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K et al (2006) Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin Neurosci Res 6:261–281
Liu B, Hong JS (2003) Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther 304:1–7
Park HY, Han MH, Park C, Jin CY, Kim GY, Choi IW et al (2011) Anti-inflammatory effects of fucoidan through inhibition of NF-kappa B, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol 49:1745–1752
Zhong LM, Zong Y, Sun L, Guo JZ, Zhang W, He Y et al (2012) Resveratrol inhibits inflammatory responses via the mammalian target of rapamycin signaling pathway in cultured LPS-stimulated microglial cells. Plos One 7:e32195
Yu Z, Tang L, Chen L, Li J, Wu W, Hu C (2015) Capillarisin suppresses lipopolysaccharide-induced inflammatory mediators in BV2 microglial cells by suppressing TLR4-mediated NF-kappaB and MAPKs signaling pathway. Neurochem Res 40:1095–1101
Zhang Y, Chen WA (2015) Biochanin A inhibits lipopolysaccharide-induced inflammatory cytokines and mediators production in BV2 microglia. Neurochem Res 40:165–171
Qu H, Zhang Y, Wang Y, Li B, Sun W (2008) Antioxidant and antibacterial activity of two compounds (forsythiaside and forsythin) isolated from Forsythia suspensa. J Pharm Pharmacol 60:261–266
Kim JM, Kim S, Kim DH, Lee CH, Park SJ, Jung JW et al (2011) Neuroprotective effect of forsythiaside against transient cerebral global ischemia in gerbil. Eur J Pharmacol 660:326–333
Huang C, Lin Y, Su H, Ye D (2015) Forsythiaside protects against hydrogen peroxide-induced oxidative stress and apoptosis in PC12 cell. Neurochem Res 40:27–35
Cheng G, Zhao Y, Li H, Wu Y, Li X, Han Q et al (2014) Forsythiaside attenuates lipopolysaccharide-induced inflammatory responses in the bursa of Fabricius of chickens by downregulating the NF-κB signaling pathway. Exp Ther Med 7:179–184
Pan C-W, Zhou G-Y, Chen W-L, Zhuge L, Jin L-X, Zheng Y et al (2015) Protective effect of forsythiaside A on lipopolysaccharide/d-galactosamine-induced liver injury. Int Immunopharmacol 26:80–85
Zhou L, Yang H, Ai Y, Xie Y, Fu Y (2014) Protective effect of forsythiaside A on acute lung injure induced by lipopolysaccharide in mice. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 30:151–154
Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS et al (2015) Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci Lett 584:191–196
Monji A, Kato TA, Mizoguchi Y, Horikawa H, Seki Y, Kasai M et al (2013) Neuroinflammation in schizophrenia especially focused on the role of microglia. Prog Neuropsychopharmacol Biol Psychiatry 42:115–121
Scheld WM, Koedel U, Nathan B, Pfister H-W (2002) Pathophysiology of bacterial meningitis: mechanism (s) of neuronal injury. J Infect Dis 186:S225–S233
Qin L, Li G, Qian X, Liu Y, Wu X, Liu B et al (2005) Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia 52:78–84
Wang AL, Albert C, Lau LT, Lee C, Tso MO (2005) Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int 47:152–158
Im NK, Zhou W, Na M, Jeong GS (2015) Pierisformoside B exhibits neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells via the HO-1/Nrf2-mediated pathway. Int Immunopharmacol 24:353–360
Munoz L, Ammit AJ (2010) Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology 58:561–568
Wang CX, Shuaib A (2002) Involvement of inflammatory cytokines in central nervous system injury. Prog Neurobiol 67:161–172
Shakibaei M, John T, Seifarth C, Mobasheri A (2007) Resveratrol inhibits IL-1β-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann N Y Acad Sci 1095:554–563
Sanz-Rosa D, Oubina MP, Cediel E, de las Heras N, Vegazo O, Jimenez J et al (2005) Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-κB/IκB system. Am J Physiol Heart Circ Physiol 288:H111–H115
Lee I-S, Lim J, Gal J, Kang JC, Kim HJ, Kang BY et al (2011) Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int 58:153–160
Lee S, Suk K (2007) Heme oxygenase-1 mediates cytoprotective effects of immunostimulation in microglia. Biochem Pharmacol 74:723–729
Li WG, Khor TO, Xu CJ, Shen GX, Jeong WS, Yu S et al (2008) Activation of Nrf2-antioxidant signaling attenuates NF kappa B-inflammatory response and elicits apoptosis. Biochem Pharmacol 76:1485–1489
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y et al (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275:16023–16029
Acknowledgments
This study was supported by Grants from the ‘Neuroprotective effects of microRNA-124 on dopaminergic neurons in Parkinson’s disease and its related mechanism (81371397)’ and The Guangdong Provincial Clinical Medical Centre for Neurosurgery (No. 2013B0204000 05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhao, H., Lin, C. et al. Forsythiaside A Exhibits Anti-inflammatory Effects in LPS-Stimulated BV2 Microglia Cells Through Activation of Nrf2/HO-1 Signaling Pathway. Neurochem Res 41, 659–665 (2016). https://doi.org/10.1007/s11064-015-1731-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1731-x